Population Dynamics and Epidemiology of Diaphorina citri in Relation to Yield Losses Caused by Citrus Greening
Population Dynamics and Epidemiology of Diaphorina citri in Relation to Yield Losses Caused by Citrus Greening
Salman Ahmad1*, Muhammad Asim1, Muhammad Zeeshan Majeed2, Muhammad Atiq3, Yasir Ali4, Hafiz Bashir Ahmad5, Malik Abdul Rehman6 and Naeem Akhtar7
1Department of Plant Pathology, College of Agriculture, University of Sargodha, Sargodha, Pakistan
2Department of Entomology, College of Agriculture, University of Sargodha, Sargodha, Pakistan
3Department of Plant Pathology, University of Agriculture, Faisalabad, Pakistan
4College of Agriculture, University of Layyah 31200, Layyah, Pakistan
5Department of Forestry, College of Agriculture, University of Sargodha, Sargodha, Pakistan
6Citrus Research Institute, Sargodha, Punjab, Pakistan
7Department of Plant Breeding and Genetics, College of Agriculture, University of Sargodha, Sargodha, Pakistan
Abstract | Citrus greening (CG) or huanglongbing (HLB) is the major cause of citrus decline. The disease is currently prevailing in all districts of Punjab and posing severe threat to citrus production and export. The current research was designed with two objectives; (1) to study the population dynamics of Diaphorina citri, the vector of CG, and (2) to record the incidence of CG in different citrus growing areas of Punjab and estimation of losses caused by this disease. To study the dynamics of Asian citrus psyllid (ACP), a comprehensive survey of kinnow (Citrus reticulate cv. kinnow), musambi (Citrus sinensis cv. musambi) and feutrell’s early (Citrus reticulate cv. feutrell’s early) orchards was conducted in tehsil Sargodha during 2017 and 2018. Correlation and regression analysis were used to determine the relationship of ACP with CG, and the relationship of environmental factors with ACP. Surveys were conducted to record the incidence of CG and estimate the yield losses caused by it. The study of population dynamics of ACP revealed that ACP population was higher between 7th to 14th April and 11th to 18th September, during 2017 and 2018. While, there was less ACP population between February 10th to 2nd March and 10th July to 30th July, during 2017 and 2018, respectively. There was significant and positive correlation between ACP population and CG on kinnow, musambi and feutrell’s early cultivars. Maximum and minimum temperatures and rainfall also showed positive and significant correlation with CG, while relative humidity showed not significant correlation with CG. During current study, maximum temperature, minimum temperature and rainfall explained maximum, i.e., 79, 76 and 83% variability in ACP population, respectively. The incidence of CG was observed higher (45%) in Faisalabad while lower (21.67%) in Multan. Similarly, yield losses due to CG were recorded maximum (33.5%) in Faisalabad and lowest (15.5%) in Multan districts. The current findings will help in the effective control of CG in Punjab by the in time management of its vector.
Novelty Statement | The epidemiology of D. citri and yield losses caused by CG have been studied first time in citrus belt of Punjab, Pakistan, during this research.
Article History
Received: February 16, 2023
Revised: April 20, 2023
Accepted: May 17, 2023
Published: June 28, 2023
Authors’ Contributions
SA conceived the research idea. MA conducted research. MZM and MA wrote original draft. YA and HBA proof-read the manuscript. MAR and NA analyzed the research data.
Keywords
Diseases prevalence, Huanglongbing, Citrus psyllid, Kinnow, Musambi, Feutrell’s early
Copyright 2023 by the authors. Licensee ResearchersLinks Ltd, England, UK. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Corresponding author: Salman Ahmad
salman.ahmad@uos.edu.pk
To cite this article: Ahmad, S., Asim, M., Majeed, M.Z., Atiq, M., Ali, Y., Ahmad, H.B., Rehman, M.A. and Akhtar, N., 2023. Population dynamics and epidemiology of Diaphorina citri in relation to yield losses caused by citrus greening. Punjab Univ. J. Zool., 38(1): 81-88. https://dx.doi.org/10.17582/journal.pujz/2023.38.1.81.88
Introduction
Citrus greening (CG) is the most notorious disease of citrus and significantly affecting its yield (Ma et al., 2022). This disease has been in India and China since many years (Cui et al., 2022). CG is associated with a fastidious bacterium Candidatus laberibacter and spread through two species of psyllid Trioza erytreae and Diaphorina citri. Three species of C. laberibacter, i.e., africanus, asiaticus and americanus, are reported to be linked with CG. Among these species, the most widely and virulent species is asiaticus (Ramadugu et al., 2016). CG is associated with citrus decline and is prevalent in most parts of the world. CG has the ability to affect all cultivars of citrus, and is causing huge losses by decreasing the productive life span of trees. This disease has been reported to cause huge losses in Africa and Asia.
During the attack of CG, the infected trees produce blotchy and yellow shoots, mottled leaves, and their leaves veins turned yellow (Bové, 2006). During severe attack, the canopy of the infected trees gradually becomes chlorotic, leaves size gets smaller, tree growth gets slow and leaves tips turn necrotic (Gottwald et al., 2007). CG produces sectored types symptoms in which some parts of trees remain healthy while others show symptoms. The other prominent symptoms are thickening of leaves, enlargement of veins and at the end corky appearance of veins. Infected leaves show typical symptoms of Zn deficiency and later on fallen off from infected twigs (Gottwald et al., 2007).
D. citri, Asian citrus psyllid (ACP), is the vector of CG, has a wide geographical distribution and is present in Middle East, Asia, America and Mauritius (Halbert et al., 2010). D. citri affects all grown cultivars of citrus and could be found on other species of family Rutaceae (Halbert and Manjunah, 2004). Among the Rutaceae species, the leading host is the mostly planted ornamental plant Murraya exotica (Bergmann et al., 1994), therefore, in CG management program; this plant is not recommended for landscaping. Efficient control of D. citri requires a detailed knowledge of their dispersion behavior and population dynamics. Thus far, very little research related to population dynamics of D. citri has been published. The overall objectives of this study were; (1) to study the population dynamics of D. citri, the vector of CG, and (2) to record incidence of CG in different citrus growing areas of Punjab and estimation of losses caused by this disease.
Materials and Methods
Population dynamics of ACP and role of environmental factors in its development
For population dynamics of ACP, a comprehensive survey of kinnow, musambi and feutrell’s early orchards was conducted in tehsil Sargodha during 2017 and 2018. Psyllid data was recorded during two seasons, February to April and July to September, during 2017 and 2018. The data was recorded after ten-days interval. Population of ACP was monitored by selecting trees randomly from center and four corners of each orchard. Population of ACP was recorded from top, middle and bottom portions of each selected tree and average number of adults per leaf was calculated (Zeb et al., 2011). Means were compared using Duncan’s Multiple Range test (DMRT) (Steel et al., 1997). Line graphs were plotted to represent the data of ACP population at different time intervals.
To determine the effect of environmental factors on ACP population, correlation and regression analysis were used (Steel et al., 1997). For this, the population data of ACP, recorded on three cultivars (kinnow, musambi and feutrell’s early) for two years 2017 and 2018, was used. The population data of ACP served as dependent variable while environmental data served as independent variable. Environmental data of Sargodha was collected from Distt. Meteorological Department, Sargodha.
Monitoring of CG disease in citrus growing areas of Punjab and estimation of yield losses caused by this disease
A comprehensive survey was conducted in all citrus growing areas of Punjab. Total 120 orchards were selected in all citrus growing areas of Punjab for monitoring of CG disease and estimation of yield losses caused by CG. During survey of each orchard, four corners and middle of each orchard were selected. The trees were characterized as decline and partially decline by modifying Kamble et al. (2017) rating scale (Table 1).
Table 1: Modified rating scale for scoring citrus decline.
|
Point scale |
Illustration |
Reaction |
Characterization |
|
0 |
Zero Infection |
HR or I |
Healthy |
|
1 |
> 10% incidence |
R |
Healthy |
|
2 |
10-20% infected leaf area |
MR |
Healthy |
|
3 |
20-40% infected leaf area |
MS |
Partially diseased |
|
4 |
40-60% infected leaf area |
S |
Partially diseased |
|
5 |
> 60% infected leaf area |
HS |
Fully diseased |
The CG disease incidence was recorded using the following formula.
CG disease incidence (%) = Number of infected Plants/Total number of plants x 100
For yield losses estimation caused by CG, 600 trees were selected in 120 orchards. Yield losses were computed by counting the number of infected fruits (showing clear greening symptoms) on fully and partially CG infected trees. The yield loss was determined using following formula (Cooke, 2006).
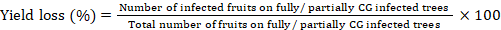
Results and Discussion
Population dynamics of ACP during 2017and 2018
Mean number of adults of ACP at ten days interval showed significant (P > 0.05) variation during 2017 and 2018 on kinnow, feutrell’s early and musambi cultivars in different orchards of Sargodha (Figures 1-4). Mean number of ACP were recorded higher during both years on three cultivars between 7th to 14th April and 11th to 18th September. While, least number of psyllids were recorded between February 10th to 2nd March and 10th July to 30th July, during both years on three cultivars. However, psyllid population declined after 21st April and 18 September during 2017 and 2018. Further, musambi showed higher psyllid population compared to kinnow and feutrell’s early (Figures 1-4).
Correlation of ACP population with CG during years 2017 and 2018
ACP population significantly affected the CG incidence on three cultivars. There was significant correlation between ACP population and incidence of CG on three cultivars (Table 2).
Table 2: Correlation of ACP population with CG.
|
Year |
Kinnow |
Musambi |
Feutrell’s early |
|
2017 |
0.722* 0.001 |
0.773* 0.001 |
0.756* 0.001 |
|
2018 |
0.744* 0.001 |
0.765* 0.001 |
0.691* 0.001 |
Upper values indicate Pearson’s correlation coefficient. Lower values indicate level of probability at P = 0.05
Relationship of ACP population with CG on three citrus cultivars during year 2017 and 2018
ACP population significantly influenced the incidence of CG on three citrus cultivars during year 2017 and 2018. The relationship between ACP population and CG incidence on three cultivars was linear and positive, i.e., with the increase of psyllid population CG incidence also increased. Linear regression models explained significant contribution of psyllid population in the development of CG as shown by high R2 value, kinnow 0.52 and 0.55, feutrell’s early 0.57 and 0.48, and musambi 0.59 and 0.58, respectively, during years 2017 and 2018 (Figure 5-7).
Correlation of environmental variables with ACP population during year 2017 and 2018
Significant correlation was found between three environmental variables (i.e., maximum and minimum temperatures and rainfall) and ACP population, while there was not significant correlation found between relative humidity and psyllid population (Table 3). Maximum and minimum temperatures and rainfall were positively correlated with psyllid population during year 2017 and 2018. However, the correlation of relative humidity with psyllid population was positive in year 2017 while negative in year 2018 (Table 3).
Table 3: Correlation of environmental variables with ACP population during year 2017 and 2018.
|
Environmental variables |
Kinnow |
Feutrell’s early |
Musambi |
|
|
Year-2017 |
Max. temp. |
0.834* |
0.843* |
0.816* |
|
Min. temp. |
0.803* |
0.768* |
0.780* |
|
|
Relative humidity |
0.156ns |
0.157ns |
0.171ns |
|
|
Rainfall |
0.809* |
0.821* |
0.835* |
|
|
Year-2018 |
Max. temp. |
0.890* |
0.827* |
0.751* |
|
Min. temp. |
0.827* |
0.838* |
0.873* |
|
|
Relative humidity |
-0.225ns |
-0.269ns |
-0.295ns |
|
|
Rainfall |
0.761* |
0.659* |
0.791* |
Upper values indicate Pearson’s correlation coefficient. Lower values indicate level of probability at P = 0.05
Effect of environmental variables on ACP population during year 2017 and 2018
The effect of three environmental variables, except relative humidity, was significant on three citrus cultivars in the development of ACP population as shown by high R2 values during year 2017 and 2018 (Tables 4-6). On kinnnow cultivar, regression models explained maximum variability in psyllid population due to maximum temperature 66% and 79%, respectively, during year 2017 and 2018, while second maximum variability was due to minimum temperature 64% and 68%, respectively, during the same years (Table 4). On musambi cultivar, maximum variability (83%) in psyllid population was due to rainfall during year 2018, while second maximum variability 76% was due to minimum temperature during the same year. On musambi, during 2017, maximum variability 71% was due to maximum temperature followed by rainfall 69% (Table 5). On cultivar feutrell’s early, the regression models explained maximum variability 70% in psyllid population due to minimum temperature in year 2018 and second maximum variability 67% was recorded in year 2017 due to rainfall (Table 6).
Table 4: Regression models for the effect of environmental variables on ACP population on kinnow cultivar during year 2017 and 2018.
|
Environmental variables |
Kinnow |
|
|
Regression equation |
R square |
|
|
Year-2017 |
||
|
Max. temperature (°C) |
*y = - 14.56+0.83 Max. temp. |
0.66 |
|
Min. temperature (°C) |
y = - 7.888+0.90 Min. temp. |
0.64 |
|
Relative humidity (%) |
y = + 4.981+0.060 Rh. |
0.24 |
|
Rainfall (mm) |
y = + 4.7546+0.59 RF. |
0.65 |
|
Year-2018 |
||
|
Max. temperature (°C) |
y = - 37.14+1.67 Max. temp |
0.79 |
|
Min. temperature (°C) |
y = - 11.40+1.26 Min. temp. |
0.68 |
|
Relative humidity (%) |
y = 19.52 -0.21 Rh. |
0.05 |
|
Rainfall (mm) |
y = -0.71+19.52 RF. |
0.61 |
*y = Citrus psyllid population.
Table 5: Regression models for the effect of environmental variables on ACP population on musambi cultivar during year 2017 and 2018.
|
Environmental variables |
Musambi |
|
|
Regression equation |
R square |
|
|
Year-2017 |
||
|
Max. temperature (°C) |
*y = -13.24+0.76 Max. temp. |
0.71 |
|
Min. temperature (°C) |
y = -8.27+0.96 Min. temp. |
0.60 |
|
Relative humidity (%) |
y = +5.06+0.06 Rh. |
0.02 |
|
Rainfall (mm) |
y = +4.95+0.67 RF. |
0.69 |
|
Year-2018 |
||
|
Max. temperature (°C) |
y = -26.95+1.34 Max. temp |
0.67 |
|
Min. temperature (°C) |
y = -12.03+1.44 Min. temp. |
0.76 |
|
Relative humidity (%) |
y = 26.49 -0. 30 Rh. |
0.08 |
|
Rainfall (mm) |
y = -1.31+1.27 RF. |
0.83 |
*y = Citrus psyllid population.
Table 6: Regression models for the effect of environmental variables on ACP population on feutrell’s early cultivar during year 2017 and 2018.
|
Environmental variables |
Feutrell’s early |
|
|
Regression equation |
R square |
|
|
Year-2017 |
||
|
Max. temperature (°C) |
*y= -15.82+0.85 Max. temp. |
0.65 |
|
Min. temperature (°C) |
y= -8.79+0.95 Min. temp. |
0.58 |
|
Relative humidity (%) |
y= +4.50+0.06 Rh. |
0.04 |
|
Rainfall (mm) |
y= +4.84+0.68 RF. |
0.67 |
|
Year-2018 |
||
|
Max. temperature (°C) |
y= -34.78+1.63 Max. temp |
0.61 |
|
Min. temperature (°C) |
y= -12.13+1.40 Min. temp. |
0.70 |
|
Relative humidity (%) |
y = 24.43 - 0.28 Rh. |
0.07 |
|
Rainfall (mm) |
y = 4.41 + 0.93 RF. |
0.51 |
*y = Citrus psyllid population.
Incidence of CG in different citrus growing areas of Punjab and estimation of yield losses caused by CG
CG incidence and the yield losses caused by it were significantly different (P<0.05) in different citrus growing areas of Punjab. Maximum CG incidence was observed in Faisalabad (45%), followed by Kotmomin (36.66 %) and Bhalwal (33.33%), respectively. Minimum CG incidence was observed in Multan (21.67 %) (Table 7). Yield losses due to CG were maximum in Faisalabad (33.56 %) followed by kot momin (28.22 %) and Bhalwal (24 %), respectively. Minimum yield losses were recorded in Multan (15.5 %) (Table 7).
Table 7: Incidence of CG and yield losses due to CG in different citrus growing areas of Punjab.
|
Location |
Disease incidence (%) |
Yield losses (%) |
|
Kot momin |
36.66 a* |
28.22 ab |
|
Bhalwal |
33.33 ab |
24.00 bc |
|
Sargodha |
31.67 ab |
18.44 cd |
|
Shahpur |
25.00 b |
20.44 cd |
|
Silanwali |
31.67 ab |
21.33 bcd |
|
Sahiwal |
30.00 ab |
22.44 bcd |
|
Multan |
21.67 b |
15. 56 d |
|
Faisalabad |
45.00 a |
33. 56 a |
* Means with similar letters in a row are not significantly different at P = 0.05
CG is very destructive disease throughout the world (Albrecht and Bowman, 2012; Fiaz et al., 2018). The current study revealed that correlation of ACP with CG was significant and positive during both years. It was observed that with the increase of ACP, CG incidence on three varieties also increased. This is consistent with the findings of Hall and Hentz (2019). They found strong correlation between ACP and CG. They further reported positive correlation between ACP and CG. ACP is in fact the vector of CG, its both high and low populations, having the ability to transmit the bacterium of CG (Ukuda-Hosokawa et al., 2015). However, the transmission of L. candidatus through ACP may be slow due to low population of ACP (Ukuda-Hosokawa et al., 2015). A single adult of ACP may transfer 8.5% CG disease to number of seedlings in one week. Further, the transmission of CG depends on the stylet length and width of maxillary salivary canal of psyllid. Higher stylet length and wider maxillary salivary canal, increase the transmission rate of CG to citrus plants (Hall et al., 2013). There are several researches which show that nymphs of ACP are more efficient in acquiring CLas bacterium from diseased citrus trees than adults (Inoue et al., 2009; Pelz-Stelinski et al., 2010), whereas those adults which acquire CLas bacterium at the stage of nymph, are also highly important for disseminating CLas bacterium from diseased trees to healthy trees over long and short distances (Garzo et al., 2012). The strong correlation between ACP and CG confirmed that transmission of CLas in our conditions is due to this insect vector.
The current research revealed that maximum and minimum temperatures and rainfall significantly contributed in the development of ACP population; however, the influence of relative humidity was not significant. This is in line with the findings of Fiaz et al. (2018) and Devi and Sharma (2014). The significant relationship of three environmental factors with psyllid explains that ACP population depends on these three environmental factors. Temperature significantly affects the increase or decrease of psyllid population (Bayles et al., 2017). The pattern of spread of psyllid population is driven by fluctuation in temperature (Bayles et al., 2017). Temperature has significant role in oviposition of ACP. Females of ACP can live for 300 days at 16 °C, but do not lay eggs (Fung and Chen, 2006); however, lay eggs when temperature is 26°C (Liu and Tsai, 2000). Females of D. citri lays more eggs at 28°C-32°C (Fung and Chen, 2006; Liu and Tsai, 2000). Temperature also impacts the survival of psyllid. ACP can survive up to 45°C and -6°C. The development, reproduction and longevity of ACP is also influenced by temperature (Liu and Tsai, 2000).
The role of rainfall on the increase and decrease of ACP population was also significant. Sétamou et al. (2023) reported that heavy rainfall is linked with low population of psyllid, as this amount of rainfall washes out eggs and nymphs of psyllid, while low rainfall favours psyllid population (Devi and Sharma, 2014). The seasonal distribution of psyllid completely depends on the rainfall (Devi and Sharma, 2014).
Conclusions and Recommendations
The reason for studying the population dynamics of ACP is to take correct decisions for the effective management of this vector and CG disease in district Sargodha of Punjab province. The study revealed maximum population of ACP in first two weeks of April, and second and third weeks of September, hence, the farmers need to be vigilant during these two months and apply appropriate insecticides to control ACP. The current study provides the information of significant role of three environmental factors, viz., maximum and minimum temperatures and rainfall on the ACP population on three cultivars of citrus in Sargodha. However, maximum variability in the population of ACP is caused by rainfall, therefore, pest scouting is recommended during frequent rainfalls in the months of April and September in Sargodha.
Acknowledgments
The authors are thankful for the support provided by Higher Education Commission, Islamabad, Pakistan for funding the project (No. 20- 3559/NRPU/RandD/HEC/14/1156) for the Department of Plant Pathology, College of Agriculture, University of Sargodha, Sargodha.
Acknowledgments
Authors acknowledge the technical help and valuable advice given by Dr. Muhammad Usman Ghazanfar during the preparation and proofreading of the draft.
Availability of data and materials
All data generated or analyzed in this work are available in the published manuscript.
Consent for publication
Not applicable.
Ethics approval consent to participate
Authors declare that this study did not require ethical approval or considerations.
Conflict of interest
The authors have declared no conflict of interest.
References
Albrecht, U. and Bowman, K.D., 2011. Tolerance of the trifoliate citrus hybrid US-897 (Citrus reticulata Blanco× Poncirus trifoliata L. Raf.) to Huanglongbing. Hortic. Sci., 46: 16-22. https://doi.org/10.21273/HORTSCI.46.1.16
Albrecht, U. and Bowman, K.D., 2012. Tolerance of trifoliate citrus rootstock hybrids to Candidatus Liberibacter asiaticus. Sci. Hortic., 147: 71-80. https://doi.org/10.1016/j.scienta.2012.08.036
Batra, R.C., Uppal, D.K. and Sohi, B.S., 1970. Indexing the genetic stock of different species of citrus against citrus leaf miner and citrus psylla. Ind. J. Hortic., 27: 76-79.
Bayles, B.R., Thomas, S.M., Simmons, G.S., Grafton-Cardwell, E.E. and Daugherty, M.P., 2017. Spatiotemporal dynamics of the Southern California Asian citrus psyllid (Diaphorina citri) invasion. PLoS One, 12: e0173226. https://doi.org/10.1371/journal.pone.0173226
Bergmann, E.C., Fernandes, S.C.S. and De Faria, A.M., 1994. Surto de Diaphorina citri Kuwayama, 1908 (Homoptera: Psyllidae), em pomares cítricos do estado de São Paulo. Biol. São Paulo., 56: 22-25.
Bhagat, K.C. and Nehru, R.K., 2005. Screening of sweet orange cultivars Against Asian citrus psylla, Diaphorina citri. Ann. Pl. Prot. Sci., 13: 242-243.
Bové, J.M., 2006. Huanglongbing: A destructive, newly-emerging, century-old disease of citrus. J. Pl. Pathol., 58: 7-37.
Cooke, B.M., 2006. Disease assessment and yield loss. In: The epidemiology of plant diseases. Springer, Dordrecht. pp. 43-80. https://doi.org/10.1007/1-4020-4581-6_2
Cui, X., Liu, K., Huang, J., Fu, S., Chen, Q., Liu, X. and Wang, X., 2022. Population diversity of Candidatus Liberibacter asiaticus and Diaphorina citri in Sichuan: A case study for huanglongbing monitoring and interception. Pl. Dis., 106: PDIS-07. https://doi.org/10.1094/PDIS-07-21-1539-RE
Devi, H.S. and Sharma, D.R., 2014. Impact of abiotic factors on build-up of citrus psylla, Diaphorina citri Kuwayama population in Punjab, India. J. app. Nat. Sci., 6: 371-376. https://doi.org/10.31018/jans.v6i2.430
Fiaz, M., Afzal, M. and Majeed, M.Z., 2018. Influence of abiotic weather factors on population dynamics of asian citrus psyllid, Diaphorina citri Kuwayama (Hemiptera: Psyllidae) in central Punjab, Pakistan. J. Agric. Res., 56: 35-40.
Fung, Y.C. and Chen, C.N., 2006. Effects of temperature and host plant on population parameters of the citrus psyllid (Diaphorina citri Kuwayama). Formosan Entomol., 26: 109-123.
Garzo, E., Bonani, J.P., Lopes, J.R.S. and Fereres, A., 2012. Morphological description of the mouthparts of the Asian citrus psyllid, Diaphorina citri Kuwayama (Hemiptera: Psyllidae). Arthropod. Struct. Dev., 41: 79-86. https://doi.org/10.1016/j.asd.2011.07.005
Gottwald, T.R., Graça, J.V.D. and Bassanezi, R.B., 2007. Citrus huanglongbing: The pathogen and its impact. Pl. Hlth. Prog., 8: 1-36. https://doi.org/10.1094/PHP-2007-0906-01-RV
Halbert, S.E. and Manjunath, K.L., 2004. Asian citrus psyllids (Sternorrhyncha: Psyllidae) and greening disease of citrus: A literature review and assessment of risk in Florida. Florida Entomol., 87: 330-353. https://doi.org/10.1653/0015-4040(2004)087[0330:ACPSPA]2.0.CO;2
Halbert, S.E., Manjunath, K.L., Ramadugu, C., Brodie, M.W., Webb, S.E. and Lee, R.F., 2010. Trailers transporting oranges to processing plants move Asian citrus psyllids. Florida Entomol., 93: 33-39. https://doi.org/10.1653/024.093.0104
Hall, D.G. and Hentz, M.G., 2019. Influence of light on reproductive rates of Asian citrus psyllid (Hemiptera: Liviidae). J. Insect Sci., 19: 9. https://doi.org/10.1093/jisesa/iey141
Hall, D.G., Richardson, M.L., Ammar, E.D. and Halbert, S.E., 2013. Asian citrus psyllid, Diaphorina citri, vector of citrus huanglongbing disease. Entomol. Exp. Appl., 146: 207-223. https://doi.org/10.1111/eea.12025
Inoue, H., Ohnishi, J., Ito, T., Tomimura, K., Miyata, S., Iwanami, T. and Ashihara, W., 2009. Enhanced proliferation and efficient transmission of Candidatus Liberibacter asiaticus by adult Diaphorina citri after acquisition feeding in the nymphal stage. Ann. Appl. Biol., 155: 29-36. https://doi.org/10.1111/j.1744-7348.2009.00317.x
Kamble, S.G., Ghutukade, K.S., Patil, N.P., Yamgar, S.V. and Bulbule, S.V., 2017. Responses of different genotypes of citrus to Huanglongbing (citrus greening) under field condition. J. Pharmacogn. Phytochem., 6: 207-211. https://doi.org/10.20546/ijcmas.2017.611.365
Killiny, N. and Hijaz, F., 2016. Amino acids implicated in plant defense are higher in Candidatus liberibacter asiaticus-tolerant citrus varieties. Pl. Signal. Behav., 11: e1171449. https://doi.org/10.1080/15592324.2016.1171449
Liu, Y.H. and Tsai, J.H., 2000. Effects of temperature on biology and life table parameters of the Asian citrus psyllid, Diaphorina citri Kuwayama (Homoptera: Psyllidae). Ann. appl. Biol., 137: 201-206. https://doi.org/10.1111/j.1744-7348.2000.tb00060.x
Ma, W., Pang, Z., Huang, X., Xu, J., Pandey, S.S., Li, J. and Wang, N., 2022. Citrus Huanglongbing is a pathogen-triggered immune disease that can be mitigated with antioxidants and gibberellin. Nat. Commun., 130: 1-13. https://doi.org/10.1038/s41467-022-28189-9
Mabberley, D.J., 1997. A classification for edible Citrus (Rutaceae). Telopea, 7: 167-172. https://doi.org/10.7751/telopea19971007
Miyakawa, T., 1980. Experimentally-induced symptoms and host range of citrus likubin (greening disease). Jpn. J. Phytopathol., 46: 224-230. https://doi.org/10.3186/jjphytopath.46.224
Paudyal, K.P., 2015. Technological advances in huanglongbing (HLB) or citrus greening disease management. J. Nepal Agric. Res. Counc., 1: 41-50. https://doi.org/10.3126/jnarc.v1i0.15735
Pelz-Stelinski, K.S., Brlansky, R.H., Ebert, T.A. and Rogers, M.E., 2010. Transmission parameters for Candidatus Liberibacter asiaticus by Asian citrus psyllid (Hemiptera: Psyllidae). J. Econ. Entmol., 103: 1531-1541. https://doi.org/10.1603/EC10123
Puterka, G.J., Burd, J.D. and Burton, R.L., 1992. Biotypic variation in a worldwide collection of Russian wheat aphid (Homoptera: Aphididae). J. Econ. Entomol., 85: 1497-1506. https://doi.org/10.1093/jee/85.4.1497
Ramadugu, C., Keremane, M.L., Halbert, S.E., Duan, Y.P., Roose, M.L., Stover, E. and Lee, R.F., 2016. Long-term field evaluation reveals Huanglongbing resistance in Citrus relatives. Pl. Dis., 100: 1858-1869. https://doi.org/10.1094/PDIS-03-16-0271-RE
Richardson, M.L. and Hall, D.G., 2013. Resistance of poncirus and Citrus× Poncirus germplasm to the Asian citrus psyllid. Crop Sci., 53: 183-188. https://doi.org/10.2135/cropsci2012.02.0091
Schwartz, T., Nylinder, S., Ramadugu, C., Antonelli, A. and Pfeil, B.E., 2016. The origin of oranges: a multi-locus phylogeny of Rutaceae subfamily Aurantioideae. Syst. Bot., 40: 1053-1062. https://doi.org/10.1600/036364415X690067
Sétamou, M., Soto, Y.L., Tachin, M. and Alabi, O.J., 2023. Report on the first detection of Asian citrus psyllid Diaphorina citri Kuwayama (Hemiptera: Liviidae) in the Republic of Benin, West Africa. Sci. Rep., 13: 801. https://doi.org/10.1038/s41598-023-28030-3
Steel, R.G.D., Torrie, J.H. and Dickey, D.A., 1997. Principles and procedures of statistics: A biometrical approach, 3rd edn. (McGraw-Hill: New York).
Timmer, L.W., Roberts, P.D., Darhower, H.M., Bushong, P.M., Stover, E.W., Peever, T.L. and Ibáñez, A.M., 2000. Epidemiology and control of citrus greasy spot in different citrus-growing areas in Florida. Pl. Dis., 84: 1294-1298. https://doi.org/10.1094/PDIS.2000.84.12.1294
Ukuda-Hosokawa, R., Sadoyama, Y., Kishaba, M., Kuriwada, T., Anbutsu, H. and Fukatsu, T., 2015. Infection density dynamics of the citrus greening bacterium “Candidatus Liberibacter asiaticus” in field populations of the psyllid Diaphorina citri and its relevance to the efficiency of pathogen transmission to citrus plants. Appl. Environ. Microbiol., 81: 3728-3736. https://doi.org/10.1128/AEM.00707-15
Westbrook, C.J., Hall, D.G., Stover, E., Duan, Y.P. and Lee, R.F., 2011. Colonization of citrus and citrus-related germplasm by Diaphorina citri (Hemiptera: Psyllidae). Hortic. Sci., 46: 997-1005. https://doi.org/10.21273/HORTSCI.46.7.997
Zeb, Q., Khan, I., Inayatullah, M., Hayat, Y., Khan, M.A., Saljoqi, A.R. and Khan, M.A., 2011. Population dynamics of citrus whiteflies, aphids, citrus psylla leaf miner and their biocontrol agents in Khyber Pakhtunkhwa. Sarhad J. Agric., 27: 451-457.
To share on other social networks, click on any share button. What are these?








