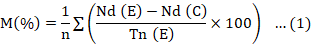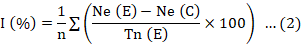Plant Phenology and Leaf Maturity Influences on the Nematicidal Activity of Castor Leaf Extracts Against Okra Root-Knot Nematodes
Plant Phenology and Leaf Maturity Influences on the Nematicidal Activity of Castor Leaf Extracts Against Okra Root-Knot Nematodes
Kouakou Yadom Yao François-Regis*, Kra Kouamé Daniel and Atta Diallo Hortense
Nangui Abrogoua University, Faculty of Natural Sciences, Plant Health Laboratory, Abidjan, Côte d’Ivoire, 02 BP 801 Abidjan 02.
Abstract | The nematicidal efficacy of castor plants was usually assessed using leaves, regardless of maturity or plant phenology. Under these conditions, the efficacy of each leaf type is unknown. This study aimed to identify the maturity level of castor leaves that exhibit the highest nematicidal activity against okra root-knot nematodes. Aqueous extracts of five types of castor leaves were prepared. These were immature (ILV) and mature (MLV) leaves from vegetative plants and immature (ILF) and mature (MLF) leaves from flowering plants. The fifth type (ATL), a mixture of all leaf types, was used as a control. Second-stage juveniles and eggs of M. incognita were exposed to extracts for 72 hours and 10 days, respectively. Egg-hatching inhibition and juvenile mortality were used to evaluate the in vitro efficacy of extracts. Castor leaf extracts were applied to okra soils infested with M. incognita. The gall index, gall reduction, and nematode reproduction were determined. The unhatched egg rate ranged from 49.50 to 81.60%, and the juvenile mortality varied from 73.10 to 100% depending on extracts. The immature leaf extract from flowering plants was the most effective, with 81.60% unhatched eggs and 100% juvenile mortality. Regardless of plant phenology, immature leaf extracts were the most effective in limiting gall development by approximately 50%. Immature castor leaf extracts have high nematicidal activity. Farmers could prefer immature leaves in order to develop nematicides for okra root-knot nematode management.
Received | July 24, 2024; Accepted | August 27, 2024; Published | September 14, 2024
*Correspondence | Kouakou Yadom Yao François-Regis, Nangui Abrogoua University, Faculty of Natural Sciences, Plant Health Laboratory, Abidjan, Côte d’Ivoire, 02 BP 801 Abidjan 02; Email: [email protected]
Citation | François-Regis, K.Y.Y., Daniel, K.K. and Hortense, A.D., 2024. Plant phenology and leaf maturity influences on the nematicidal activity of castor leaf extracts against okra root-knot nematodes. Pakistan Journal of Nematology, 42(2): 127-135.
DOI | https://dx.doi.org/10.17582/journal.pjn/2024/42.2.127.135
Keywords | Aqueous extract, Castor plant, Nematicidal activity, Plant phenology, Okra, Root-knot nematodes
Copyright: 2024 by the authors. Licensee ResearchersLinks Ltd, England, UK.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Introduction
Okra is one of the most popular vegetables in the world (Naveed et al., 2009). People cultivate okra for its immature and edible pods (Ngbede et al., 2014). Okra cultivation provides a source of food (Kumar et al., 2010; Yaseen and Mukhtar, 2024). It serves as a significant source of income for farmers (Ekunwe et al., 2018). The stem of the okra plant is utilized by the paper industry (Yaseen and Mukhtar, 2024). Côte d’Ivoire ranks 4th among African Okra producers, behind Nigeria, Mali, and Sudan, with 183.8 thousand tons of immature pods produced in 2022 (FAOSTAT, 2024). The main okra species cultivated are Abelmoschus esculentus and A. caillei (Fondio et al., 2007). Abelmoschus esculentus pods have an octagonal section. However, Abelmoschus caillei pods describe a circular section. Côte d’Ivoire widely cultivates okra in its rural, urban, and peri-urban areas (Fondio et al., 2007). Farmers cultivate okra in all of the country’s agroecological zones.
Okra production, like all vegetable crops, is prone to infection by root-knot nematodes (Sikora and Fernandez, 2005). Root-knot nematodes are microscopic soil-dwelling roundworms that parasitize plant roots, causing damage and reducing crop yields (Jones et al., 2013; Shahid et al., 2023). The main root-knot nematode species in the tropics are Meloidogyne arenaria, M. javanica, and M. incognita (Moens et al., 2009). Meloidogyne incognita is considered the most devastating species, with high damage rates (Trudgill et al., 2000). It has a wide range of host plants (Jones et al., 2013), including okra. Second-stage juveniles penetrate the roots, causing the formation of galls, which impede the flow of water and nutrients to the rest of the plant (Shahid et al., 2023). Gall formation disrupts water and nutrient uptake. This leads to wilting, stunting, leaf chlorosis, slow growth, and root reduction (Ayub et al., 2024). Ultimately, these symptoms lead to a poor harvest and reduced yield (Asghar et al., 2020).
Farmers, faced with this situation, use chemical nematicides besides crop rotation and organic soil amendments in order to limit yield losses. Chemical nematicides are effective against root-knot nematodes in vegetable crops (Cadet, 1990). Farmers spread them on the soil surface, incorporate them into the soil before seed planting, or apply them in strips in the plant’s furrow (Sikora and Fernandez, 2005). Due to the high prevalence of parasitic nematodes, farmers tend to excessively rely on chemical nematicides. Frequent use of chemical nematicides can lead to resistance in nematode populations, environmental pollution, and ecological disruption (Shahid et al., 2023).
Currently, the search for plant-based product development alternatives to chemical nematicides is booming. In this context, Kouakou et al. (2017) investigated the nematicidal potential of castor leaf extract against yam nematodes. This extract was a mixture of all-type leaves from vegetative and flowering plants. Under these conditions, the effectiveness of each type of leaf is unknown. The study aimed to identify the maturity level of castor leaves with the highest nematicidal activity against okra root-knot nematodes.
Materials and Methods
In vitro evaluation of castor leaf extracts on M. incognita
Preparation of castor leaf extracts: Five types of castor leaves were sampled during this study in the suburbs of the District of Abidjan in southern Côte d’Ivoire. There were immature leaves from vegetative (ILV) and flowering (ILF) plants, as well as mature leaves from vegetative (MLV) and flowering (MLF) plants. Plants have immature leaves at the apical end mature leaves at the median and basal ends. The fifth type of leaf (ATL) was a mixture of all leaf types. In the current study, ATL treatment served as a control. One kilogram of each castor leaf type was sampled. One kilogram of the ATL sample consisted of 250 g of each leaf type. Leaf samples were washed with tap water and dried in an incubator at 35 °C for 72 hours. Dried leaves were powdered using a mixer.
A concentrated extract (100 g/l) was prepared for each type of castor leaf. To achieve this, 100 g of each leaf powder were macerated in 1 L of distilled water for 72 hours. The crude extracts were filtered three times through a hydrophilic cotton column in a 60-ml syringe. Oxamyl, a synthetic nematicide, was also used as a control. It was used at the same concentration (100 g/l). Thus, 100 g of oxamyl in the granule formulation was dissolved in 1 L of distilled water. The oxamyl solution was filtered under the same conditions as before. Both the castor extract and the oxamyl solution were used against okra root-knot nematodes.
Preparation of nematode inoculum
Reared individuals of M. incognita from the okra plants (cultivar Hiré) were used as an inoculum. The okra plants were uprooted and washed with tap water. The roots were cut into explants approximately 5 mm in size in order to extract the root-knot nematode eggs using the method of Hussey and Barker (1973). The egg suspension was incubated at 26 °C for 72 hours in an incubator. Second-stage juveniles from the hatched eggs were concentrated approximately at 100 individuals per 100-µl suspension.
Exposure of nematodes to castor extracts
The nematicidal efficacy of castor leaf extract was evaluated on eggs and second-stage juveniles of M. incognita. A 5 ml sample of extract from each type of castor leaf was transferred into test tubes with 10 replicates per extract. Aliquots of 100 µl containing approximately 100 individuals were added to extracts in test tubes. Control juveniles were exposed to 5 ml of distilled water. Moreover, 100 µl aliquots containing approximately 100 eggs were added to extracts in other test tubes under similar conditions, with 10 replicates per extract. Control eggs were exposed to 5 ml of distilled water. Juvenile and egg cultures were incubated at room temperature for 72 hours and 10 days, respectively.
Determination of juvenile mortality
Second-stage juveniles were transferred to sterile distilled water and incubated for 24 hours under the same conditions. Stimulation allowed for the identification and counting of dead individuals. An individual was judged dead if it did not move after being pricked with a needle (Cayrol et al., 1989). The mean mortality of second-stage juveniles was calculated based on the extracts (Equation 1).
M (%): mortality of juveniles in the extract; Nd (E): number of dead juveniles in the extract; Nd (C): number of dead juveniles in the control; Tn (E): total number of juveniles in the extract; n: number of replicates per extract (10 tubes).
Determination of egg-hatching inhibition
Egg-hatching inhibition was determined after the eggs were exposed to castor extracts for 10 days (Equation 2).
I (%): egg-hatching inhibition; Ne (E): number of unhatched eggs in the extract; Ne (C): number of unhatched eggs in the control; Tn (E): total number of eggs in the extract; n: number of replicates per extract (10 tubes).
In vivo evaluation of castor leaf extracts on M. incognita
Acquisition of okra plants: A soil sample was used as a substrate for okra plant cultivation. The soil sample was autoclaved twice at 121 °C for 30 minutes and distributed in perforated plastic bags with 900 g of soil (approximately 1 L of soil) per bag. Okra seeds (cultivar Hiré), from a local seed company, were planted on the bagged soil with three seeds per bag. The seedlings were watered every 48 hours. Stunted okra seedlings were removed 7 days after emergence to conserve one vigorous seedling per bag.
Inoculation of okra plants
Castor leaf extracts and second-stage juveniles of M. incognita were prepared as previously. Oxamyl was used as the reference chemical nematicide to evaluate the nematicidal efficacy of castor leaf extracts. There were eight treatments, including T0: uninoculated and untreated okra plants; T1: inoculated and untreated okra plants; Oxamyl: okra plants inoculated and treated with oxamyl; ILV: okra plants inoculated and treated with immature leaf extract from vegetative plants; MLV: okra plants inoculated and treated with mature leaf extract from vegetative plants; ILF: okra plants inoculated and treated with immature leaf extract from flowering plants; MLF: okra plants inoculated and treated with mature leaf extract from flowering plants; ATL: okra plants inoculated and treated with all leaf-type extract.
Four holes, approximately 5 cm deep, were made in the soil around each 21-day-old okra plant. A 1 ml aliquot containing 500 second-stage juveniles of M. incognita was distributed through the holes. Ten okra plants were used per treatment.
Application of extracts
Okra plants were watered with water or castor leaf extract, depending on the treatment. Every 48 hours, 100 ml of castor leaf extract and 100 ml of water were alternately applied around the base of the okra plants. The extract of each type of castor leaf was applied for 30 days. In the second month, the plants were watered with water only. For plants treated with oxamyl, 4 g of product were placed around each plant. The plants were watered every 48 hours using water only. Plants from control treatments (T0 and T1) were watered only with 100 ml of water every 48 hours.
Determination of pathological parameters
The gall index, gall reduction rate, final density, and reproductive factor of M. incognita were used to evaluate the effects of treatments on gall development. Okra plants were uprooted and grouped by treatment. Using the Bridge and Page (1980) scale, the severity of galls in okra plant root systems was assessed after they were rinsed with tap water. Zewain’s (2014) formula was used to calculate the gall index. The gall reduction rate was computed using the Mahdy et al. (2014) formula.
The roots of okra plants were weighted using an electronic balance and chopped using a pair of scissors. The Hussey and Barker (1973) method was used to collect eggs and swollen individuals. Second-stage juveniles and adult males were collected from the root shred using the maceration method (Coyne et al., 2010). Individuals from soil samples of each plant were extracted using Whitehead’s method (Coyne et al., 2010). Following the three extraction methods, the final density of M. incognita per plant was calculated as the sum of all individuals (eggs, juveniles, adult males, and swollen stages) collected per root system and soil samples. The reproductive factor was calculated using Rivoal et al. (2001) formula.
Statistical analyses
Juvenile mortality, egg-hatching inhibition, and gall reduction were normalized using the arcsin√(p/100) function, where p is the determined parameter. The final density of M. incognita was normalized by the Log10 (x+1) function, where x is the determined density. Levene’s test was used to assess the homoscedasticity among treatments at the 5% level. Because the homoscedasticity occurred, the data were subjected to one-way analysis using Statistica 7.1 software. If a significant difference occurred at the 5% level, the Dunnett’s test (including control) was performed in order to identify the effective treatment.
Results and Discussion
In vitro nematicidal efficacy of castor leaf extracts on Meloidogyne incognita
Effects of leaf maturity from vegetative plants: The mortality rate of M. incognita juveniles varied between 73.10 and 99.80%, depending on leaf maturity from vegetative plants. Meanwhile, egg-hatching inhibition varied from 49.50 to 86.60% (Table 1). Statistical analysis revealed a highly significant difference between the extracts based on dead juveniles and unhatched egg rates (P < 0.001). Oxamyl was more effective than immature and mature leaf extracts against M. incognita. Oxamyl was responsible for 99.80% of the dead juveniles and 86.60% of the unhatched eggs. Immature leaf extract was more effective than mature leaf extract. The immature leaf extract resulted in 86% of dead juveniles and 74.80% of unhatched eggs. However, mature leaf extract produced 75.80% of dead juveniles and 52.60% of unhatched eggs. In addition, the mature leaf extract from vegetative plants had a statistically similar nematicidal potential to that of the all-leaf type extract (73.10% dead juveniles and 49.50% unhatched eggs).
Table 1: Juvenile mortality and egg-hatching inhibition of Meloidogyne incognita based on the castor leaf maturity extracts.
|
Castor leaves |
Juvenile mortality (%) |
Unhatched eggs (%) |
|
Oxamyl |
99.80 ± 0.41a |
86.60 ± 2.50a |
|
ATL |
73.10 ± 2.21c |
49.50 ± 3.66c |
|
ILV |
86.00 ± 9.35b |
74.80 ± 4.16b |
|
MLV |
75.80 ± 1.37c |
52.60 ± 4.37c |
|
P |
0.000 |
0.000 |
Values marked with the same letter in each column are statistically identical based on Dunnett’s test at the 5% level. P: Probability value; Oxamyl: solution of Oxamyl; ATL: all leaf type extract; ILV: immature leaf extract from vegetative plants; MLV: mature leaf extract from vegetative plants.
Table 2: Juvenile mortality and egg-hatching inhibition of Meloidogyne incognita based on the castor leaf maturity extracts.
|
Castor leaves |
Juvenile mortality (%) |
Unhatched eggs (%) |
|
Oxamyl |
99.80 ± 0.41a |
86.60 ± 2.50a |
|
ATL |
73.10 ± 2.21c |
49.50 ± 3.66d |
|
ILF |
100.0 ± 0.00a |
81.60 ± 4.42b |
|
MLF |
86.60 ± 5.05b |
65.20 ± 5.65c |
|
P |
0.000 |
0.000 |
Values marked with the same letter in each column are statistically identical based on Dunnett’s test at the 5% level. P: Probability value; Oxamyl: solution of Oxamyl; ATL: extract of all types of castor leaves; ILF: immature leaf extract from flowering plants; MLF: mature leaf extract from flowering plants.
Effects of leaf maturity from flowering plants
Dead juvenile and unhatched egg rates ranged from 73.10 to 100% and 49.50 to 86.60%, respectively, according to the treatments (Table 2). Statistical analysis showed a highly significant difference between castor extracts in terms of dead juvenile and unhatched egg rates (P < 0.001). As a result, immature leaf extract from flowering plants was as effective as the oxamyl solution on M. incognita, with 100% of dead juveniles and 81.60% of unhatched eggs. Furthermore, immature castor leaf extract was more effective than mature leaf extract and all leaf type extract. However, the mature leaf extract from flowering plants (86.60% dead juveniles and 65.20% unhatched eggs) was statistically more effective than the all-leaf type extract (73.10% dead juveniles and 49.50% unhatched eggs).
Effects of plant phenology
The dead juvenile (73.10-100%) and unhatched egg (49.50-86.60%) rates varied according to the castor plant phenology (Table 3). There was a highly significant difference between immature leaf extracts regarding dead juvenile and unhatched egg rates (P < 0.001). Oxamyl (99.80% dead juveniles and 86.60% unhatched eggs) and immature leaf extract from flowering plants (100% dead juveniles and 81.60% unhatched eggs) had similar nematicidal efficacy on M. incognita. The efficacy of oxamyl and immature leaf extract from flowering plants was greater than that from vegetative plants and all leaf-type extract.
Table 3: Juvenile mortality and egg-hatching inhibition of Meloidogyne incognita based on the castor phenology.
|
Castor leaves |
Juvenile mortality (%) |
Unhatched eggs (%) |
|
Oxamyl |
99.80 ± 0.41a |
86.60 ± 2.50a |
|
ATL |
73.10 ± 2.21c |
49.50 ± 3.66d |
|
ILV |
86.00 ± 9.35b |
74.80 ± 4.16c |
|
ILF |
100.0 ± 0.00a |
81.60 ± 4.42b |
|
P |
0.000 |
0.000 |
Values marked with the same letter in each column are statistically identical based on Dunnett’s test at the 5% level. P: Probability value; Oxamyl: solution of Oxamyl; ATL: all leaf type extract; ILV: immature leaf extract from vegetative plants; ILF: immature leaf extract from flowering plants.
In vivo nematicidal efficacy of castor leaf extracts on Meloidogyne incognita
Gall development: An anatomical modification of okra roots was observed after two months of cultivation on M. incognita-infested soils. However, the roots of control plants (on non-infested soils) did not develop any anatomical changes (Figure 1A). The anatomical modification is characterized by round protuberances along the okra’s roots, ranging from 4 to 10 mm in diameter. This type of anatomical change is characteristic of root galls (Figure 1B). Galls were stacked along pivoting and axillary roots. The number of galls per root system varied depending on the okra plant and treatment.
Effects of extracts on the gall development
The gall index, gall reduction, final density, and reproductive factor of M. incognita varied according to the treatments (Table 4). The uninoculated and untreated okra plants (Treatment T0) showed no galls or M. incognita individuals. However, the gall index varied from 2.20 to 4.60, depending on the other treatments. Gall reduction ranged from 23 to 53.30%. The final density of M. incognita varied between 103 and 734 individuals, while the reproductive factor varied between 0.21 and 1.47, depending on the treatments. Statistical analysis revealed a highly significant difference between treatments for each pathological parameter (P < 0.001). The oxamyl, ILF, and ILV treatments had the lowest gall index (2.10 to 2.50), final density (103 to 117 individuals), and reproductive factor (0.21 to 0.23). As expected, gall reduction was the highest (45.30 to 53.30%) in plants treated with oxamyl and immature leaf extracts. Thus, immature leaf extracts, irrespective of castor phenology, were more effective than mature leaf extracts on M. incognita.
Table 4: Pathological parameters of Meloidogyne incognita on okra plants.
|
Treatments |
Gall index |
Gall reduction |
Final density |
Reproductive factor |
|
T0 |
0 |
- |
0 |
0 |
|
T1 |
4.60±0.70a |
- |
734±123a |
1.47±0.58a |
|
Oxamyl |
2.20±0.79c |
52.60±4.33a |
110±26c |
0.22±0.09c |
|
ATL |
3.50±1.02b |
23.90±4.27c |
533±31b |
1.07±0.18b |
|
ILV |
2.50±1.08c |
45.30±6.64b |
117±26c |
0.23±0.09c |
|
MLV |
3.60±1.17b |
23.00±7.90c |
521±69b |
1.04±0.12b |
|
ILF |
2.10±0.74c |
53.30±5.25a |
103±18c |
0.21±0.08c |
|
MLF |
3.40±1.51b |
27.60±8.97c |
429±72b |
0.86±0.11b |
|
P |
0.000 |
0.004 |
0.000 |
0.000 |
Values marked with the same letter in each column are statistically identical based on Dunnett’s test at the 5% level. P: Probability value; T0: uninoculated and untreated okra plants; T1: inoculated and untreated okra plants; Oxamyl: okra plants inoculated and treated with oxamyl; ILV: okra plants inoculated and treated with immature leaf extract from vegetative plants; MLV: okra plants inoculated and treated with mature leaf extract from vegetative plants; ILF: okra plants inoculated and treated with immature leaf extract from flowering plants; MLF: okra plants inoculated and treated with mature leaf extract from flowering plants; ATL: okra plants inoculated and treated with all leaf-type extract.
Root-knot nematodes are obligatory plant parasites that cause significant losses in agricultural production (Shahid et al., 2023). They represent the greatest challenge in agricultural production, as they attack over five thousand plants, including okra (Abelmoschus spp.). Root-knot nematodes embed inside the root tissue during the plant’s life span (Alam and El-Nuby, 2022). Nowadays, chemical nematicide usage has declined globally due to their wide spectrum toxicity (El-Nuby and Alam, 2020). Consequently, researchers are exploring alternatives. These include plant-based product development to manage plant-parasitic nematodes and conserve natural resource sustainability (Alam and El-Nuby, 2022). In this context, Kouakou et al. (2017) demonstrated the nematicidal potential of aqueous castor leaf extract on yam nematodes. All leaf-type mixtures, regardless of their maturity and plant phenology, yielded this extract. The aim of this study was to identify the maturity level of castor leaves with the highest nematicidal activity against okra root-knot nematodes.
This study revealed that each type leaf extract possessed a nematicidal potential against M. incognita. This is because the extracts contain phytochemicals with nematicidal properties. Indeed, plant extracts contain oxygenated compounds possessing lipophilic properties. Due to their lipophilic attributes, oxygenated compounds could dissolve the cytoplasmic membrane of cells of nematodes (Knobloch et al., 1989). Additionally, the functional groups facilitate oxygenated compound accumulation in the vital organs of nematodes. This could then change the enzymatic protein structure (Knobloch et al., 1989) and disrupt nematode organ function. Koul (2008) attributed the nematicidal potential of aqueous castor leaf extracts to phenolic compounds. Ladi et al. (2019) suggested that inhibiting M. incognita egg hatching may be due to phytochemicals such as flavonoids, alkaloids, amides, and ketones.
Whatever the castor plant phenology, immature leaf extracts possessed the greatest larvicidal and ovicidal activity on M. incognita juveniles and eggs. This aligns with the findings of Beesa et al. (2023, 2024). They found that the cavalcade (Centrosema pascuorum) with the younger leaf appeared to be more potent, exhibiting higher inhibitory effects on the nematode. This suggests an uneven distribution of oxygenated compounds in castor plant parts. In fact, immature leaves would have a higher phenolic compound content than mature leaves. Nor and Mohd (2013) conducted a study on green tea (Camellia sinensis). They discovered that the buds had the highest content of polyphenols and flavonoids, followed by immature and mature leaves. Additionally, they discovered that buds had the highest antioxidant activity of phenolic compounds, followed by immature and mature leaves. Nadeem and Zeb (2018) also showed that phenolic compound content increased with age in fig (Ficus carica) leaves. This trend is especially contrary to that of Jolkili et al. (2018). They found phenolic compound content increased with age in Cassia alata leaves. Thus, the increase or decrease in phenolic compound content with leaf age should depend on the plant species.
From a phenological perspective, the immature castor leaves from flowering plants had more nematicidal potential than those from vegetative plants. This result opposed that of Bar-Eyal et al. (2006) on Chrysanthemum coronarium. They found that plant parts (before flowering) were more effective than flowering plants. This is due to a variation in the content of oxygenated compounds with nematicidal activity during castor plant development. Immature castor leaves from flowering plants would have a higher content of oxygenated compounds than immature castor leaves from vegetative plants. Salem et al. (2018) showed that oxygenated compound contents in Mentha pulegium leaves depended on the plant phenology. They discovered that M. pulegium leaves had the highest oxygenated compound contents among flowering plants.
Under greenhouse conditions, immature leaf extract application reduced gall development on okra roots. Dura et al. (2018) found that using castor leaves as a soil amendment against M. incognita significantly reduced nematode populations and root galling by 80%. Gall reduction is due to the compounds with nematicidal activity in the extract of immature leaves. Indeed, juveniles were exposed to the extract in the soil interstices. Repeated application of that extract would have created an unfavorable environment for M. incognita development, resulting in a significant impact on its pathological activity. This situation would result in low gall development and improved okra development. The greenhouse trial’s results confirmed those obtained during the in vitro trials.
Conclusions and Recommendations
The current study reveals the larvicidal and ovicidal activities of castor leaf extracts against okra root-knot nematodes. Castor plant phenology and leaf maturity affect the larvicidal and ovicidal activities of leaf extracts. Under greenhouse conditions, immature leaf extracts, regardless of plant phenology, possessed the greatest effect on limiting gall development. In okra cultivation, immature leaf extracts help to control root-knot nematodes. When sampling castor leaves, farmers may prefer immature leaves in order to develop nematicides effective against root-knot nematodes. However, more research is required to do a phytochemical screening of extracts and assess their effectiveness under field conditions.
Novelty Statement
This is one of the first studies to evaluate the effect of plant phenology and leaf maturity on the nematicidal efficacy of castor oil extracts. It recommends that farmers give preference to immature castor leaves in order to develop effective products against okra root-knot nematodes.
Author’s Contribution
Kouakou Y.Y.F.R. designed the study, conducted trials, performed the statistical analysis, and wrote the manuscript. “Kra K.D. and Atta D.H. assisted, supervised, and reviewed the manuscript.
Conflict of interest
The authors have declared no conflict of interest.
References
Alam, E.A.A.H. and El-Nuby, A.S.M., 2022. Phytochemical and nematicidal screening on some extracts of different plant parts of Egyptian Moringa oleifera L. Pak. J. Phytopathol., 34(2): 293-306. https://doi.org/10.33866/phytopathol.034.02.0818
Asghar, A., Mukhtar, T., Raja, M.U. and Gulzar, A., 2020. Interaction between Meloidogyne javanica and Ralstonia solanacearum in chili. Pak. J. Zool., 52(4): 1525-1530. https://doi.org/10.17582/journal.pjz/20190501030529
Ayub, H.M., Akram, M., Mehak, A., Ajmal, M., Ilyas, I., Seerat, W., Tatar, M., Ali, A., Sarwar, R., Abbasi, M. and Rahman, A., 2024. A preliminary study on the interaction between Meloidogyne incognita and some strains of Pseudomonas spp. on growth performance of tomato under greenhouse conditions. Plant Prot., 8(2): 341-350.
Bar-Eyal, M., Sharon E. and Spiegel, Y., 2006. Nematicidal activity of Chrysanthemum coronarium. Eur. J. Plant Pathol., 114: 427–433. https://doi.org/10.1007/s10658-006-0011-7
Beesa, N., Chinnasri, B., Sasnarukkit, A., Dethoup, T., Jindapunnapat, K., Kiriga, W.A., Saikai, K., Haukeland, S. and Coyne, D., 2023. Potential of cavalcade, Centrosema pascuorum, leaves for controlling Meloidogyne javanica and quantification of active phytochemicals. Nematology, 25: 1019-1032. https://doi.org/10.1163/15685411-bja10273
Beesa, N., Nattakorn K., Tida, D., Kansiree J., Anongnuch, S. and Buncha, C., 2024. Identification of bioactive compounds in cavalcade leaves for nematicidal activity against Hirschmanniella mucronate and Meloidogyne graminicola using LC-QTOF-MS. Crop Prot., 185: 106875. https://doi.org/10.1016/j.cropro.2024.106875
Bridge, J. and Page, S.L.J., 1980. Estimation of root-knot nematode infestation levels on roots using a rating chart. Trop. Pest Manage., 26: 296-298. https://doi.org/10.1080/09670878009414416
Cadet, P., 1990. Influence de quelques nématicides sur les rendements et la multiplication des nematodes parasites et la tomate à la Martinique. Bull. Agron. Antilles Guyane, 10: 42-48.
Cayrol, J.C., Djian, C. and Pijarowski, L., 1989. Study of the nematicidal properties of the culture filtrate of the nematophagous fungus Paecilomyces lilacinus. Rev. Nématol., 12: 331-336.
Coyne, D.L., Nicol, J.M. and Claudius-Cole, B., 2010. Practical plant nematology: A field and laboratory guide. SP-IPM Secretariat, International Institute of Tropical Agriculture (IITA), Cotonou, Benin.
Dura, O., Sönmez, İ., Çelik, Y.N., Kurtuldu, H.M., Dura, S. and Kepenekci, İ., 2018. Effect of castor bean [Ricinus communis L. (Euphorbiaceae)] and dieffenbachia [Dieffenbachia maculata (Araceae)] of root-knot nematode (Meloidogyne incognita) on greenhouse tomatoes. Munis Entomol. Zool. J., 13(2): 566-73.
Ekunwe, P.A., Alufohai, G. and Adolue, C.F., 2018. Economic viability of okra (Abelmoschus esculentus) production in Ika south and north east local government areas of Delta State, Nigeria. J. Trop. Agric. Food Environ. Ext., 17(1): 57-62. https://doi.org/10.4314/as.v17i1.8
El-Nuby, A.S.M. and Alam, E.A.A.H., 2020. Phytochemical and nematicidal activity studies of some extracts of different plant parts of Leucaena leucocephala against Meloidogyne incognita. Int. J. Chem. Pharma. Sci., 11(1): 1-17.
FAOSTAT, 2024. Available at: http://www.fao.org/faostat/fr/#data/QC (accessed 19 Jan 2024).
Fondio, L., Djidji, A.H., Kouamé, C., Aïdara, S. and Hala, N., 2007. Bien cultiver le gombo en Côte d’Ivoire. Fiche technique, CNRA.
Hussey, R.S. and Barker, K.R., 1973. A comparison of methods of collecting inocula of Meloidogyne spp. including a new technique. Plant Dis. Rep., 57: 1025-1028.
Jolkili, M., Shaari, A.R. and Razak, N.A., 2018. Effect of leaf maturity and drying temperature on total phenolic content of Cassia alata. AIP Conf. Proc., 2030: 1-4. https://doi.org/10.1063/1.5066762
Jones, J.T., Haegeman, A., Danchin, E.G.J., Gaur, H.S., Helder, J., Jones, M.G.K., Kikuchi, T., Manzanilla-López, R., Palomares-Rius, J.E., Wesemael, W.M.L. and Perry, R.N., 2013. Top 10 plant-parasitic nematodes in molecular plant pathology. Mol. Plant Pathol., 14: 946-961. https://doi.org/10.1111/mpp.12057
Knobloch, K., Pauli, A., Iberl, N., Weigand, N. and Weis, H.M., 1989. Antibacterial and antifungal properties of essential oil components. J. Essential Oil Res., 1: 119-128. https://doi.org/10.1080/10412905.1989.9697767
Kouakou, Y.Y.F.R., Kra, K.D., Kouamé, A.P. and Diallo, A.H., 2017. Nematicidal effectiveness of products stemming from dried leaves of castor-oil plant (Ricinus communis L.) on Meloidogyne and Pratylenchus, yam pathogenic nematodes in Côte d’Ivoire. Int. J. Agron. Agric. Res., 11(5): 57-68.
Koul, O., 2008. Phytochemicals and insect control: An antifeedant approach. Crit. Rev. Plant Sci., 27: 1-24. https://doi.org/10.1080/07352680802053908
Kumar, S., Dagnoko, S., Haougui, A., Ratnadass, A., Pasternak, D. and Kouame, C., 2010. Okra (Abelmoschus spp.) in West and Central Africa: Potential and progress on its improvement. Afr. J. Agric. Res., 5(25): 3590-3598.
Ladi, B.Y., Muhammad, A.K., Joy, B., Alake, N.M. and Jumoke, S.O., 2019. Inhibitory effect of neem (Azadirachta indica) and Moringa (Moringa oleifera) leaf extracts on egg hatch of root knot nematode Meloidogyne incognita. World J. Adv. Res. Rev., 1: 28-33. https://doi.org/10.30574/wjarr.2019.1.2.0001
Mahdy, M.E., Mousa, E.M., Sanaa, E. and Abeer, A.E., 2014. Effect of fresh and dry neem leaves applications on root-knot nematode Meloidogyne javanica on tomato plants. Egypt. J. Plant Prot., 9: 12-20. https://doi.org/10.21608/ejcp.2014.239260
Moens, M., Perry, R.N. and Starr, J.L., 2009. Meloidogyne species-a diverse group of novel and important plant parasites. In: (eds. R.N. Perry, M. Moens and J.L. Starr), root-knot nematodes. pp.1-17. CABI, UK. https://doi.org/10.1079/9781845934927.0001
Nadeem, M. and Zeb, A., 2018. Impact of maturity on phenolic composition and antioxidant activity of medicinally important leaves of Ficus carica L. Physiol. Mol. Biol. Plants, 24(5): 881-887. https://doi.org/10.1007/s12298-018-0550-3
Naveed, A., Khan, A.A. and Khan, I.A., 2009. Generation mean analysis of water stress tolerance in okra (Abelmoschus esculentus L.). Pak. J. Bot., 41: 195-205.
Ngbede, S.O., Onyegbule, U.N., Ibekwe, H.N., Uwalaka, O.A. and Okpara, S.C., 2014. Economic analysis of okra (Abelmoschus esculentus L. Moench) production under different rates of organic manure in Okigwe, Southern Nigeria. Asian J. Agric. Food Sci., 2(2): 96-99.
Nor, Q.I.M.N. and Mohd, F.A.B., 2013. Phytochemicals and antioxidant properties of different parts of Camellia sinensis leaves from Sabah Tea Plantation in Sabah, Malaysia. Int. Food Res. J., 20(1): 307-312.
Rivoal, R., Bekal, S., Valette, S., Gauthier, J.P., Bel, H.F.M., Mokabli, A., Jahier, J., Nicol, J. and Yahyaoui, A. 2001. Variation in reproductive capacity and virulence on different genotypes and resistance genes of Triticeae, in the cereal cyst nematode species complex. Nematology, 3(6): 581-593. https://doi.org/10.1163/156854101753389194
Salem, N., Sriti, J., Bachrouch, O., Msaada, K., Khammassi, S., Hammami, M., Selmi, S., Boushih, E., Ouertani, M., Hachani, N., Abderraba, M., Marzouk, B., Liman, F., Mediouni, J. and Jemaa, B., 2018. Phenological stage effect on phenolic composition and repellent potential of Mentha pulegium against Tribolium castaneum and Lasioderma serricorne. Asian Pac. J. Trop. Biomed., 8(4): 207-216. https://doi.org/10.4103/2221-1691.231283
Shahid, M., Gowen, S.R., Burhan, M., Niaz, Z. and Haq, A., 2023. Studies on the efficacy of heterogeneously produced Pasteuria penetrans (PP3) isolate over individual Pasteuria isolates in the spore attachment, and pathogenic potential on three Meloidogyne species. Plant Prot., 7: 9-16. https://doi.org/10.33804/pp.007.01.4529
Sikora, R.A. and Fernandez, E., 2005. Nematodes parasites of vegetables. In: (eds. M. Luc, R.A. Sikora and J. Bridge), Plant parasitic nematodes in subtropical and tropical agriculture. 2nd edn, pp. 319-392. CABI, UK. https://doi.org/10.1079/9780851997278.0319
Trudgill, D.L., Bala, G., Blok, V.C., Daudi, A., Davies, K.G. and Gowen, S.R., 2000. The importance of tropical root-knot nematodes (Meloidogyne spp.) and factors MLFecting the utility of Pasteuria penetrans as a biological agent. Nematology, 2: 823-845. https://doi.org/10.1163/156854100750112789
Yaseen, I. and Mukhtar, T., 2024. Impact of sequential and concurrent inoculations of Meloidogyne incognita and Fusarium oxysporum f.sp. vasinfectum on the growth performance of diverse okra cultivars. Plant Prot., 8(2): 303-313. https://doi.org/10.1590/1678-4499.20230266
Zewain, Q.K., 2014. Evaluation of some chemical nematicides and organic formulations in management of root knot nematode Meloidogyne on eggplant. J. Tikrit Univ. Agric. Sci., 14(0): 8-14.
To share on other social networks, click on any share button. What are these?