Plant-Derived Oils Enhance the Effectiveness of Entomopathogenic Fungi in Controlling Melon Fruit Fly Maggots
Research Article
Plant-Derived Oils Enhance the Effectiveness of Entomopathogenic Fungi in Controlling Melon Fruit Fly Maggots
Shahbaz Ahmad1*, Mubashar Iqbal1, Arshad Javaid2, Muhammad Bilal Chattha3, Muhammad Ashfaq4, Tajamal Hussain5 and Sumra Ashraf1
1Department of Entomology, Faculty of Agricultural Sciences, University of the Punjab, Lahore 54590, Pakistan; 2Department of Plant Pathology, Faculty of Agricultural Sciences, University of the Punjab, Lahore 54590, Pakistan; 3Department of Agronomy, Faculty of Agricultural Sciences, University of the Punjab, Lahore 54590, Pakistan; 4Department of Plant Breeding and Genetics, Faculty of Agricultural Sciences, University of the Punjab, Lahore 54590, Pakistan; 5School of Chemistry, University of the Punjab, Lahore 54590, Pakistan.
Abstract | The present study was carried out to investigate the sensitivity of nine entomopathogenic fungal (EPF) strains to three plant-derived oils viz. coriander-oil (Coriandrum sativum), caster-oil (Ricinus communis) and taramira-oil (Eruca Sativa) and to assess the impact of these oils on the pathogenicity of EPF strains against melon fruit fly (Bactrocera cucurbitae Coquillett) maggots. Under the laboratory conditions, EPF strains namely Metarhizium anisopliae (F 52), Metarhizium pinghaense (MBC 709), Isaria cateniammulata (MBC 289), Isaria javanica (MBC 524), Isaria farinose (MBC 389), Isaria fumosorosea (MBC 053), Lecanicillium attenuatum (MBC 807), Beauveria brongniartii (MBC 397) and Beauveria bassiana (MBC 076) were assessed. Five conidial/spore concentrations (1×105, 1×106, 1×107, 1×108, and 1×109 conidia mL-1) of each EPF were prepared in different concentrations of essential oils (5, 10, 15, 20, 25, 30 and 35%) in tween-20. The virulence and mortality of each strain was estimated after 3, 5, 7, 14 and 21 days. Result revealed that up to 87% of M. pinghaense, 90% of I. javanica, 84% of I. fumosorosea, 87% of L. attenuatum, 95% of M. anisopliae, 80% of I. cateniammulata, and 81% of B. brongniartii germination of EPF was observed at the lowest concentration (5%) of the oils. The germination of EPF conidia/spores with different oils, namely coriander, taramira, and castor, were 70%, 76%, and 82%, respectively. EPF strain MBC 389 demonstrated the least LC50 value (4.32 × 108 conidia mL-1) and the strains MBC 709, MBC 053, MBC 807, F 52, MBC 076, MBC 289 and MBC 397 exhibited 8.13 × 109, 3.46 × 109, 1.72×1010, 1.07×109, 5.40×108, 1.00×109 and 1.25×109 conidia mL-1 LC50, respectively. Maggots of B. cucurbitae showed the highest mortality of 50% after 21 days of application of the treatments and minimum mortality (18%) was recorded after 3 days of application. Likewise, 23% mortality was recorded after 5 days, 32% mortality after 7 days and 39%. Mortality was recorded after 14 days of application of the treatments. The order of pathogenicity of the tested EPF strains was MBC 389 > MBC 397 > MBC 076 > MBC289 > F52 > MBC709 > MBC524 > MBC053 > MBC807. B. cucurbitae maggots treated with different strains (MBC709, MBC524, MBC053, MBC807, F52, MBC389, MBC076, MBC289 and MBC397) at 1 × 109 conidia mL-1 showed 52%, 44%, 52%, 39%, 55%, 58%, 58%, 569% and 60% mortality, respectively. Entomopathogenic fungus (B. brongniartii MBC397) can be utilized as a cost-effective bio-insecticide in integrated pest management programs to control B. cucurbitae. This study provides valuable insights for managing this pest through sustainable biological control methods.
Received | November 07, 2024; Accepted | December 18, 2024; Published | December 28, 2024
*Correspondence | Shahbaz Ahmad, Department of Entomology, Faculty of Agricultural Sciences, University of the Punjab, Lahore 54590, Pakistan; Email: shahbaz.iags@pu.edu.pk
Citation | Ahmad, S., M. Iqbal, A. Javaid, M.B. Chattha, M. Ashfaq, T. Hussain and S. Ashraf. 2024. Plant-derived oils enhance the effectiveness of entomopathogenic fungi in controlling melon fruit fly maggots. Pakistan Journal of Weed Science Research, 30(4): 162-177.
DOI | https://dx.doi.org/10.17582/journal.PJWSR/2024/30.4.162.177
Keywords | Coriandrum sativum, Ricinus communis, Eruca sativa, Bactrocera cucurbitae, EPF, Virulence
Copyright: 2024 by the authors. Licensee ResearchersLinks Ltd, England, UK.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Introduction
Fruit fly maggots feed inside the fruits and vegetables as well as damage their quality. They also damage flowers and stems of plants (Kumar and Rathor, 2020; Mahendiran et al., 2022). The damage is mostly done by the female that lay eggs in the soft tissue of the fruits and flowers (Mani, 2022). Due to this, market value of the products is decreased and the losses vary between 30–100% depending upon the species. Fruits damage due to the melon fruit fly has been reported in the range of 41–89% (Saeed et al., 2022; Khuhro et al., 2024).
For biological control of insects, parasitoids, predators and entomopathogens (fungi, viruses, bacteria and nematodes) have been used that are effective for the betterment of the environment as compared to the use of synthetic insecticides (Thilagam et al., 2023; Joshi et al., 2024). Entomopathogenic fungi, bacteria, virus and nematodes were reported for the protection of plants against different pests (Deka et al., 2021; Ahmad et al., 2022; Grabka et al., 2022; Singh et al., 2024). Integrated pest management is proved to be more effective for reducing the pollution as well as density of the pests from the environment (Fahad et al., 2021). It is done by biological control particularly by the help of entomopathogenic fungi (Mantzoukas and Eliopoulos, 2020; Wang et al., 2024). The entomopathogenic fungi such as Isaria fumosorosea, Beauveria bassiana and Lecanicillium lecanii have been proved very effective in biological control of insects (Alwaneen et al., 2024; Quesada-Moraga et al., 2024). These occur naturally and to preserve these, proper understanding about the compatibility and interaction with other insecticides would be known that may inhibit the pathogen reproduction and growth (Bamisile et al., 2021; Mishra et al., 2024; Singh et al., 2024).
Oils and wetting agents have been extensively investigated and adopted as means of enhancing the delivery, persistence, and efficacy of myco-insecticides (Ghorui et al., 2024). Oil based formulations of Beauveria bassiana proved more efficient carrier of B. bassiana conidia than 0.01% aqueous Tween 80 for the weevil Pantorhytes plutus (Prakash et al., 2015; Kordrostami et al., 2024). Naturally occurring botanical insecticides such as neem oil and other plant essential oils, have long been used in Asia for protection against stored-grain insect pests. More recently, researchers in the Western Hemisphere have begun to assess their use as alternatives to fumigants and other chemical insecticides (Deguine et al., 2021; Zharkov et al., 2023; Albornoz et al., 2024).
The use of entomopathogenic fungi in combination with the plant essential oils proved to be more effective in controlling different types of pest with cost effective techniques. The investigation of the combined action of EPF and EOs or vegetable extracts can aid in pest management by improving control effectiveness and reducing the use of chemical pesticides (Bacova et al., 2020; Natal et al., 2021; Kordrostami et al., 2024; Rehan et al., 2024). EPF and essential oils compatibility could make it easier to choose the right items for IPM programs (Sorokan et al., 2023; Ochieng et al., 2024; Vermelho et al., 2024). According to Chaudhary et al. (2024) and Marisel et al. (2024), these combination applications can increase control effectiveness by lowering applied amounts, avoiding environmental contamination risks, and preventing the development of insect resistance. Because many synthetic pesticides are incompatible with EPFs, it is crucial to search for some natural antagonists to use in conjunction with them (Napoli et al., 2020; Valerio et al., 2021; Kordrostami et al., 2024). So, the aim of the present study was the assessment of sensitivity of entomopathogenic fungi to different oils such as coriander oil, taramira oil and castor oil against B. cucurbitae maggots.
Materials and Methods
Collection and rearing of fruit flies
Fruit flies used in this experimental trail were collected from the bitter and cucumber fields that were present in University of Punjab Lahore on weekly basis. In order to gather the flies, the fruits were first taken from the infected field and placed on the side of the plate with one side adjusted using mesh wire. As the development of the maggots was completed, they were collected in different trays.
After the period of five days pupa were collected, washed with distilled water and were placed in the plastic plates for the emergence of the flies. During this, diet such as banana, eggs and yeasts etc. was provided in rearing cages. Emerged adults were maintained in plastic cages (30 x 30 x 30 cm), approximately 180–200 flies per cage, under laboratory conditions (24±1.5°C, RH 40±5%, L:D 14:10). During the photophase, light was provided by typical neon tubes (Philips18W/865), at light intensity of approximately 1000 lux near the cages (measured with Elvos Luxmeter LM1010). Food provision was in the form of a liquid diet consisting of sugar, yeast hydrolysate enzymatic (Chembiotin) and water (ratio 4:1:5). Flies were allowed to oviposit in bitter gourd. Water was provided with a soaked cotton wick extruding from a small water container. Maggots obtained from bitter gourd for 2–3 generations in our laboratory were used in our experiments.
Procurement of entomopathogenic fungi
Nine strains of entomopathogenic fungi namely Metarhizium pinghaense (MBC709), Isaria javanica (MBC524), I. fumosorosea (MBC 053), I. farinose (MBC 389), I. cateniammulata (MBC289) Lecanicillium attenuatum (MBC807), Metarhizium anisopliae (F52), Beauveria bassiana (MBC 076), and B. brongniartii (MBC397) were used in the present research and imported from the National Center for Agriculture Utilization Research US Department of Agriculture, USA. These strains were cultured on the ¼ SADY media and incubated at 28 ºC in dark conditions for 10-14 days. Determination of conidial concentration was done microscopically, by using Bright-Line hemocytometer (Abdelmoteleb and González-Mendoza, 2020).
Oils and preparation of their concentrations
Three oils (Coriander, Taramira and Castor oil) were purchased from reliable source and seven concentrations viz. 5, 10, 15, 20, 25, 30 and 35% of each oil were prepared in Tween-20.
Experiment I: Impact of oils on growth of EPF
Before autoclaving, each concentration of the oil was mixed with growth media, poured in Petri dishes and placed inside the laminar air flow for solidification. Lazy-L-Spreader was used to inoculate fungal strains by spreading 100 µL of conidial suspension in the Petri dishes containing growth media and placed under dark condition at 28°C. Germinating spores were counted in three groups by using the given formula:
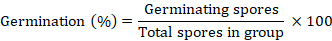
Experiment II: Impact of oils on virulence of EPF
Oil concentrations were mixed with five concentrations of every EPF strain (1×105, 1×106, 1×107 and 1×108, 1×109 spores mL-1) in all possible combinations. A suspension of oil concentration within conidial suspension of EPF was prepared by mixing 100 µL of oil and then it was spread on media plates. The larvae were fed on the diet containing all possible combinations of oil treatments. Completely randomized design was applied and 25 larvae were exposed to each treatment. Dead larvae were collected to calculate mortality after 3, 5, 7, 14 and 21 days of treatment (Dembilio et al., 2010). The mortality data were changed into percentage corrected mortality by utilizing Henderson-Tilton’s formula (Henderson and Tilton, 1955).
Data analysis
To determine LC50 and LC90 values, the data of mortality were subjected to probit analysis. The products were analyzed on the basis of their LC50 and LC90 values. The statistical analysis was conducted using the Minitab software package (Minitab, 2017). The mortality data were also analyzed by ANOVA and their mean were separated by Tukey HSD test (Dembilio et al., 2010).
Results and Discussion
Effect of oils on germination of entomopathogenic fungi
Findings showed that oils had significant effects on germination of all entomopathogenic fungal strains (P value = 0.0000 <0.05) (Table 1). Maximum germination (87%) of M. pinghaense was observed on 5% concentration as compared to other concentrations 10%, 15%, 20%, 25% and 30% have germination of M. pinghaense (80%), (72%), (65%), (58%), (51%) and (45%), respectively (Table 2). Germination due to different oils viz. coriander, taramira and castor oil was 59%, 65% and 72%, respectively (Table 3). Interaction effect between concentrations and oils revealed that maximum germination (93%) due to castor oil was at 5% concentration and minimum germination (80%) was observed at same concentration due to coriander oil. Similarly, percent germination (85% at castor oil) and minimum germination (72% at coriander) was observed on 10% concentration. While maximum percent germination (77% of castor oil) was observed on (15%) concentration and minimum germination (65% of coriander) was observed. Maximum percent germination (20%) concentration was (72%) of castor oil and minimum germination (59%) of coriander was observed. Maximum percent germination on 35% concentration was (52%) of castor oil and minimum germination (42%) of coriander was observed (Table 4).
Table 1: Analysis of variance for the effect of fungal strain, concentration and incubation period on mortality of melon fruit fly maggots.
|
SOV |
DF |
SS |
MS |
F |
P |
|
Concentrations |
5 |
194389 |
38877.8 |
99923.0 |
0.0000 |
|
Days |
4 |
106911 |
26727.8 |
68695.2 |
0.0000 |
|
Fungus |
8 |
20925 |
2615.6 |
6722.55 |
0.0000 |
|
Concentrations × Days |
20 |
15974 |
798.7 |
2052.86 |
0.0000 |
|
Concentrations × Fungus |
40 |
6480 |
162.0 |
416.39 |
0.0000 |
|
Days × Fungus |
32 |
2796 |
87.4 |
224.54 |
0.0000 |
|
Concentrations × Days × Fungus |
160 |
2626 |
16.4 |
42.18 |
0.0000 |
|
Error |
540 |
210 |
0.4 |
||
|
Total |
809 |
350312 |
Table 2: Effect of different concentrations of oils on percent germination of spores of entomopathogenic fungi.
|
Conc. |
Germination (%) (± S.E.) |
||||||||
|
Metar-hizium pinghaense |
Isaria javanica |
Isaria fumoso-rosea |
Lecani-cillium attenu-atum |
Metar-hizium anisopliae |
Isaria farinose |
Beau-veria bassi-ana |
Isaria cateniam-mulata |
Beauveria brongniartii |
|
|
5% |
87 ± 1.96A |
90 ± 0.87A |
84 ± 0.83A |
87 ± 0.85A |
94 ± 0.89A |
97 ± 0.98A |
80 ± 0.95A |
92 ± 0.82A |
81 ± 0.58A |
|
10% |
80 ± 2.06B |
83 ± 0.75B |
77 ± 0.74B |
80 ± 0.77B |
87 ± 0.82B |
90 ± 0.84B |
73 ± 0.83B |
85 ± 0.81B |
74 ± 0.51B |
|
15% |
72 ± 1.91C |
76 ± 0.67C |
70 ± 0.66C |
72 ± 0.62C |
80 ± 0.74C |
83 ± 0.73C |
66 ± 0.71C |
78 ± 0.72C |
66 ± 0.44C |
|
20% |
65 ± 1.91D |
67 ± 0.57D |
62 ± 0.61D |
64 ± 0.51D |
72 ± 0.65D |
75 ± 0.61D |
58 ± 0.62D |
69 ± 0.66D |
59 ± 0.42D |
|
25% |
58 ± 1.93E |
61 ± 0.54E |
55 ± 0.54E |
58 ± 0.46E |
65 ± 0.57E |
68 ± 0.54E |
51 ± 0.54E |
63 ± 0.61E |
52 ± 0.38E |
|
30% |
51 ± 1.69F |
54 ± 0.41F |
49 ± 0.41F |
51 ± 0.36F |
58 ± 0.52F |
62 ± 0.51F |
45 ± 0.51F |
56 ± 0.54F |
46 ± 0.35F |
|
35% |
45 ± 1.71G |
48 ± 0.29G |
43 ± 0.37G |
45 ± 0.31G |
52 ± 0.43G |
56 ± 0.43G |
39 ± 0.37G |
50 ± 0.41G |
39 ± 0.21G |
Values with different letters show significant difference (P≤0.05).
Table 3: Effect of different oils on percent germination of spores of entomopathogenic fungi.
|
Oils |
Germination (%) ± S.E. |
||||||||
|
Metar-hizium ping-haense |
Isaria javanica |
Isaria fumoso-rosea |
Lecani-cillium attenu-atum |
Metar-hizium aniso-pliae |
Isaria farinose |
Beauveria bassiana |
Isaria cateniam-mulata |
Beauveria brongniartii |
|
|
Coriander |
59 ± 2.85C |
62 ± 0.42C |
57 ± 0.64C |
58 ± 0.67C |
63 ± 0.72C |
69 ± 0.65C |
53 ± 0.32C |
64 ± 0.62C |
54 ± 0.63C |
|
Taramira |
65 ± 3.56B |
68 ± 0.55B |
63 ± 0.59B |
64 ± 0.73B |
74 ± 0.47B |
76 ± 0.69B |
59 ± 0.49B |
70 ± 0.68B |
59 ± 0.55B |
|
Castor oil |
72 ± 3.05A |
75 ± 0.83A |
69 ± 0.51A |
74 ± 0.82A |
80 ± 0.41A |
82 ± 0.71A |
65 ± 0.61A |
77 ± 0.71A |
66 ± 0.41A |
Values with different letters show significant difference (P≤0.05)
The germination of I. javanica was significantly impacted by oils (Table 1). The highest percentage of I. javanica germination occurred at 5% concentration. At concentrations of 5%, 10%, 15%, 20%, 25%, 30% and 35%, the corresponding germination was 83%, 76%, 67%, 61%, 54%, and 48% (Table 2). However, germination resulting from various oils, such as castor oil, taramira oil, and coriander oil, was 62%, 68%, and 75%, respectively (Table 3). There was a minimum germination rate of 82.01% of coriander and a maximum germination of 96% of castor oil at 5% concentration, according to the interaction impact between concentrations and oils. At 35% concentration, the highest (55% of castor oil) and the lowest germination (44% of coriander) were noted (Table 4).
In comparison to other concentrations, the highest percentage of I. fumosorosea germination (84%) was observed at 5% concentration. The corresponding germination at 10%, 15%, 20%, 25%, and 30% concentrations were 77%, 70%, 62%, 55%, and 43%, respectively (Table 2). However, the germination due to various oils (coriander, castor oil, and taramira oil) were 57%, 63%, and 69%, respectively (Table 3). Minimum germination (77% of coriander) and maximum germination (90% of castor oil) were found at 5% concentrations. The highest germination (49% of castor oil) and the lowest germination (39% of coriander) were noted at 35% concentration (Table 4).
Table 4: Interactive effect of concentrations and oils on percent germination of spores of entomopathogenic fungi.
|
Conc. (%) |
Germination (%) ± S.E. |
||||||||
|
Oils |
|||||||||
|
Cori-ander |
Tara-mira |
Castor oil |
Cori-ander |
Tara-mira |
Castor oil |
Cori-ander |
Tara-mira |
Castor oil |
|
|
Metarhizium pinghaense |
Isaria javanica |
Isaria fumosorosea |
|||||||
|
5 |
80 ± 0.86DE |
88 ± 0.88AB |
93 ± 0.95A |
82. ± 1.19CD |
91 ± 1.24AB |
96 ± 1.43A |
77 ± 1.28E |
85 ± 1.36B |
90 ± 1.49A |
|
10 |
72 ± 0.58GH |
82 ± 0.81CD |
85 ± 0.91BC |
74 ± 1.14EF |
85 ± 1.21BC |
88 ± 1.25B |
69 ± 1.22G |
80 ± 1.28D |
82 ± 1.35C |
|
15 |
65. ± 0.62HI |
75 ± 0.43FG |
77. ± 0.73EF |
69 ± 1.11FG |
78 ± 1.14DE |
80 ± 1.14CDE |
62 ± 1.17H |
72 ± 1.21F |
74 ± 1.26F |
|
20 |
59 ± 0.61JK |
63 ± 0.41IJ |
72 ± 0.61GH |
61 ± 1.07HI |
66 ± 1.11GH |
75 ± 1.17EF |
56 ± 1.13I |
60 ± 1.18H |
69 ± 1.18G |
|
25 |
52 ± 0.54KH |
57 ± 0.56JK |
65 ± 0.58HI |
55 ± 1.05KLM |
60 ± 1.04IJK |
68 ± 1.12G |
49 ± 1.11J |
54 ± 1.13I |
62 ± 1.13H |
|
30 |
47 ± 0.58MN |
50 ± 0.48HM |
58 ± 0.51JK |
49 ± 1.01MN |
53 ± 1.01LM |
61 ± 1.02HIJ |
49 ± 1.05J |
54 ± 1.13I |
55 ± 1.06I |
|
35 |
42 ± 0.41O |
42 ± 0.32O |
52 ± 0.45KH |
44 ± 1.03N |
45 ± 1.01N |
55 ± 1.02JKL |
39 ± 1.01L |
39 ± 1.05L |
49 ± 1.04J |
|
Lecanicillium attenuatum |
Metarhizium anisopliae |
Isaria farinose |
|||||||
|
5 |
79 ± 1.46C |
87 ± 1.65B |
95 ± 1.76A |
83 ± 1.76D |
97 ± 1.81AB |
99 ± 1.83A |
90±1.78CD |
93 ± 1.83AB |
97 ± 1.82A |
|
10 |
71 ± 1.37DE |
81 ± 1.54C |
87 ± 1.69B |
75 ± 1.69EF |
91 ± 1.74BC |
94. ± 1.77B |
82 ± 1.75EF |
93 ± 1.81BC |
96 ± 1.79B |
|
15 |
64 ± 1.27FG |
74 ± 1.48D |
79 ± 1.63C |
69 ± 1.53FGH |
84 ± 1.72D |
86 ± 1.65CD |
76 ± 1.65FG |
86 ± 1.76DE |
88±1.73CDE |
|
20 |
58 ± 1.21HIJ |
62± 1.41FGH |
74 ± 1.57D |
62 ± 1.45IJ |
72 ± 1.56FG |
81 ± 1.54DE |
69 ± 1.60HI |
74 ± 1.71GH |
82 ± 1.71EF |
|
25 |
51 ± 1.18KL |
56 ± 1.37IJ |
67 ± 1.42EF |
55 ± 1.38KL |
66 ± 1.51HI |
74 ± 1.46F |
62±1.57KLM |
67 ± 1.66IJK |
75 ±1.65G |
|
30 |
46h±1.12MN |
49 ± 1.32LM |
60±1.41GHI |
49 ± 1.32LM |
59 ± 1.43JK |
67 ± 1.34GHI |
56±1.52MN |
60 ± 1.64LM |
68±1.61HIJ |
|
35 |
41 ± 1.06N |
41 ± 1.16N |
54 ± 1.35JK |
45± 1.26M |
51 ± 1.32LM |
61 ± 1.26IJK |
52 ± 1.44N |
53 ± 1.51N |
63±1.56JKL |
|
Beauveria bassiana |
Isaria cateniammulata |
Beauveria brongniartii |
|||||||
|
5 |
73 ± 1.46CD |
82 ± 1.52AB |
86 ± 1.56A |
84 ± 1.53CD |
93 ± 1.61AB |
98 ± 1.65A |
73.93±1.36DE |
82 ± 1.43AB |
87 ± 1.52A |
|
10 |
65 ± 1.44EF |
76 ± 1.47BC |
79 ± 1.49B |
77 ± 1.49EF |
87 ± 1.57BC |
90 ± 1.62B |
66 ± 1.34G |
76 ± 1.41CD |
79 ± 1.47BC |
|
15 |
59 ± 1.36FG |
69 ± 1.42DE |
71±1.43CDE |
71 ± 1.43FG |
80 ± 1.54DE |
82 ± 1.55CDE |
59 ± 1.21H |
69 ± 1.34FG |
71 ± 1.43EF |
|
20 |
52 ± 1.31HI |
57 ± 1.39GH |
65 ± 1.32EF |
63 ± 1.28HI |
68 ± 1.51GH |
77 ± 1.51EF |
53 ± 1.15IJ |
57 ± 1.32HI |
66 ± 1.38G |
|
25 |
45.23±1.25KLM |
50 ± 1.32IJK |
58 ± 1.26G |
57± 1.21KLM |
62 ± 1.39IJK |
70 ± 1.48G |
46 ± 1.12L |
51 ± 1.25JK |
59±1.35H |
|
30 |
39 ± 1.17MN |
44 ± 1.26LM |
51 ± 1.21HIJ |
1 ± 1.19MN |
55 ± 1.34LM |
63 ± 1.45HIJ |
41 ± 1.06MN |
44 ± 1.21LM |
52 ± 1.31J |
|
35 |
35 ± 1.06N |
36 ± 1.15N |
46 ± 1.17JKL |
47 ± 1.15N |
47 ± 1.31N |
57 ± 1.41JKL |
36 ± 1.01N |
36 ± 1.16N |
46 ± 1.27KL |
Values with different letters show significant difference (P≤0.05).
The germination of L. attenuatum was significantly affected by oils (P value= 0.0000<0.05) (Table 1). It was highest (87%) at 5% concentration. The germination was 72%, 64%, 58%, 51%, and 45% at 10%, 15%, 20%, 25%, 30%, and 35%, respectively (Table 2). In different oil (coriander, taramira, and castor oil) treatments, germination was of 58%, 64%, and 74%, respectively (Table 3). The maximum percentage of germination (95% of castor oil) was recorded on 5% concentration, while the minimal germination (79% of coriander) was demonstrated by the interaction impact between concentrations and oils. The lowest percentage of germination (41% of coriander) and the highest percentage of germination (54% of castor oil) were noted at 35% concentration (Table 4).
The highest percentage of M. anisopliae germination (95%) was observed at a concentration of 5%. In contrast, M. pinghaense germination was observed at concentrations of 10%, 15%, 20%, 25%, 30%, and 35% was 87%, 79%, 74%, 67%, 60%, and 54%, respectively) (Table 2). In treatments of coriander, taramira, and castor oil, germination was 63%, 74%, and 80%, respectively (Table 3). There was a minimum germination (83%) of coriander and a maximum germination of 99% in castor oil on 5% concentration, according to the interaction impact between concentrations and oils. At 35% concentration, the highest percentage of germination (61% of castor oil) and the lowest percentage of germination (45% of coriander) were noted (Table 4).
I. farinose germination was highest at 5% concentration (97%), whereas at 10%, 15%, 20%, 25%, 30%, and 35%, I. farinose germination was 90, 83%, 75%, 68%, 62%, and 56%, respectively (Table 2). However, the germination affected by the various oils (castor oil, taramira, and coriander oil) were 82%, 76%, and 70%, respectively (Table 3). The interaction between concentrations and oils showed that the lowest germination (90% of coriander) and the highest germination (97% of castor oil) were both seen at 5% concentration. The highest percentage of germination (63% of castor oil) and the lowest percentage of germination (52% of coriander) were noted at 35% concentration (Table 4).
The highest percentage of B. bassiana germination occurred at 5% concentration (80%). The corresponding germination at 10%, 15%, 20%, 25%, 30%, and 35% of B. bassiana was 73%, 66%, 58%, 51%, 45%, and 39%, respectively (Table 2). Coriander, taramira, and castor oil caused germination of 53%, 59%, and 65%, respectively (Table 3). The maximum percentage of germination (86% of castor oil) and the minimum germination (73% of coriander) were shown to be caused by the interaction impact between concentrations and oils. At a 35% concentration, the highest percentage of germination (46% of castor oil) and the lowest percentage of germination (35% of coriander) were noted (Table 4).
T1 (control) had the highest percentage of I. cateniammulata germination (80%), followed by T2, T3, T4, T5, T6, and T7 (10%, 15%, 20%, 25%, 30%, and 35%), which had I. cateniammulata germination of 85%, 78%, 69%, 63%, 56%, and 50%, respectively (Table 2). Coriander, taramira, and castor oil treatments showed 64%, 70%, and 77% germination, respectively (Table 3). The maximum percentage of germination (98% of castor oil) and minimal germination (84% of coriander) were found to occur when concentrations and oils interacted. At 35% concentration, the highest percentage of germination (57% of castor oil) and the lowest percentage of germination (47% of coriander) were noted (Table 4).
B. brongniartii at 5% concentration showed maximum germination (81%) followed by T2, T3, T4, T5, T6 and T7 where germination of B. brongniartii was 74%, 66%, 59%, 52%, 46% and 39%, respectively (Table 2). Germination due to different oils (coriander, taramira, and castor oil) was 54%, 59% and 66%, respectively (Table 3). Interaction effect between concentrations and oils revealed that maximum germination (87% of castor oil) was observed at 5% concentration and minimum germination was 74% due to coriander oil. Maximum percent germination (46.06% of castor oil) was observed at 35% concentration and minimum (36%) due to coriander (Table 4).
Lethal concentrations (LC50 & LC90) of microbial insecticides after 3 days of exposure to B. cucurbitae maggots
EPF strain MBC 053 has demonstrated the least LC50 value (8.56 × 107 conidia mL-1) and proves highly effective against maggots of B. cucurbitae. Unlikely, EPF strain MBC 397 exhibited the highest LC50 value (2.83 × 1011 conidia mL-1) and proved least effective against maggots of B. cucurbitae. EPF strains MBC 709, MBC 524, MBC 807, F 52, MBC 389, MBC 076 and MBC 289 exhibited 7.61 × 109, 1.45 × 1010, 1.02 × 1010, 6.84 × 109, 1.85 × 109, 4.51 × 109 and 5.96 × 109 LC50, respectively. The order of pathogenicity of tested EPF strains were MBC053 > MBC389 > MBC076 > MBC289 > MBC709 > F52 > MBC807 > MBC524 > MBC397. The pathogenicity of all EPF strains against B. cucurbitae maggots varied significantly as fiducial limits of EPF tested strains did not overlap with each other (Table 5).
Table 5: Lethal concentration values of different entomopathogenic fungi after 3 days of exposure to Bactrocera cucurbitae maggots.
|
Fungal strain |
LC50 |
FL limit |
LC90 |
FL limit |
Slope±S.E. |
x2 |
DF |
P |
|
M. pinghaense (MBC709) |
7.61 × 109 |
1.70 × 109 - 1.18 × 1011 |
2.63 × 1012 |
1.55×1011 - 7.03×1014 |
0.21 ± 0.04 |
1.62 |
3 |
0.65 |
|
I. javanica (MBC524) |
1.45 × 1010 |
3.18 × 109- 2.53 × 1011 |
1.43 × 1012 |
1.08 × 1011 - 2.52 × 1014 |
0.26 ± 0.04 |
7.60 |
3 |
0.05 |
|
I. fumosorosea (MBC 053) |
8.56 × 107 |
1.66 × 109-1.95 × 1011 |
6.10 × 1012 |
2.47 × 1011 - 4.48 × 1015 |
0.18 ± 0.03 |
2.63 |
3 |
0.45 |
|
L. attenuatum (MBC807) |
1.02 × 1010 |
2.46 × 109- 1.38 × 1011 |
1.25 × 1012 |
1.02 × 1011 - 1.62 × 1014 |
0.24 ± 0.04 |
8.37 |
3 |
0.03 |
|
M. anisopliae (F52) |
6.84 × 109 |
1.29 × 109- 1.71 × 1011 |
8.91 × 1012 |
2.94 × 1011 - 1.16 × 1016 |
0.16 ± 0.03 |
0.11 |
3 |
0.99 |
|
I. farinose (MBC 389) |
1.85 × 109 |
6.42 × 108- 9.72 × 109 |
4.27 × 1011 |
5.27 × 1010 - 1.68 × 1013 |
0.22 ±0.03 |
7.53 |
3 |
0.05 |
|
B. bassiana (MBC 076) |
4.51 × 109 |
6.65 × 108-2.99 × 1011 |
9.28 × 1013 |
8.94 × 1011 - 8.10 × 1018 |
0.12 ± 0.02 |
0.53 |
3 |
0.91 |
|
I. cateniammulata (MBC289) |
5.96 × 109 |
1.06 × 107 - 1.80 × 1011 |
1.68 × 1013 |
4.13 × 1011 - 5.25 × 1016 |
0.15 ± 0.02 |
3.73 |
3 |
0.29 |
|
B. brongniartii (MBC397) |
2.83 × 1011 |
3.73 × 109 - 2.406 × 1021 |
3.22 × 1018 |
6.62 × 1013 - 7.03 × 1044 |
0.07 ± 0.02 |
2.79 |
3 |
0.42 |
Table 6: Lethal concentration values of different entomopathogenic fungi after 5 days of exposure to Bactrocera cucurbitae maggots.
|
Insecticides |
LC50 |
FL limit |
LC90 |
FL limit |
Slope±S.E |
X2 |
DF |
P |
|
M. pinghaense (MBC709) |
8.13×109 |
1.06 × 109 to 7.92 × 1011 |
1.08×1014 |
1.01 × 1012 to 1.08 × 1019 |
0.12±0.03 |
0.31 |
3 |
0.96 |
|
I. javanica (MBC524) |
2.45×1010 |
3.23 × 109 to 1.79 × 1012 |
2.33×1013 |
5.11 × 1011 to1.25 × 1017 |
0.17 ±0.03 |
2.05 |
3 |
0.56 |
|
I. fumosorosea (MBC 053) |
3.46×109 |
8.74 × 108 to 3.98 × 1010 |
2.25×1012 |
1.35 × 1011 to 5.43 × 1014 |
0.18 ± 0.03 |
4.15 |
3 |
0.24 |
|
L. attenuatum (MBC807) |
1.72×1010 |
2.53 × 109 to 8.97 × 1011 |
1.77×1013 |
4.41 × 1011 to 5.97 × 1016 |
0.42 ± 0.03 |
2.80 |
3 |
0.42 |
|
M. anisopliae (F52) |
1.07×109 |
2.31 × 108 to 2.08 × 1010 |
2.29×1013 |
3.95 × 1011 to 2.55 × 1017 |
0.12 ±0.02 |
0.39 |
3 |
0.94 |
|
I. farinose (MBC 389) |
4.32×108 |
1.57 × 108 to 1.93 × 109 |
5.26×1011 |
5.03 × 1010 to 3.66 × 1013 |
0.16 ± 0.02 |
0.34 |
3 |
0.95 |
|
B. bassiana (MBC 076) |
5.40×108 |
1.16 × 108 to 1.03 × 1010 |
4.38×1013 |
4.94 × 1011 to 2.58 × 1018 |
0.10 ± 0.02 |
1.07 |
3 |
0.78 |
|
I. cateniammulata (MBC289) |
1.00×109 |
2.34 × 108 to 1.46 × 1010 |
1.29×1013 |
3.02 × 1011 to 4.85 × 1016 |
0.12 ±0.02 |
2.92 |
3 |
0.40 |
|
B. brongniartii (MBC397) |
1.25×109 |
1.68 × 108 to 2.07 × 1011 |
1.26×1015 |
3.24 × 1012 to 1.32 × 1024 |
0.08 ±0.02 |
0.41 |
3 |
0.93 |
Table 7: Lethal concentration (LC) values of different entomopathogenic fungi after 7 days of exposure to Bactrocera cucurbitae maggots.
|
Fungal strain |
LC50 |
FL limit |
LC90 |
FL limit |
Slope±S.E |
X2 |
DF |
P |
|
M. pinghaense (MBC709) |
5.06×108 |
1.32 × 108 to 5.19 × 109 |
8.37×1012 |
2.23 × 1011 to 2.15 × 1016 |
0.12 ± 0.02 |
2.12 |
3 |
0.55 |
|
I. javanica (MBC524) |
1.88×1010 |
1.36 × 109 to 2.83 × 1013 |
2.21×1015 |
3.92 × 1012 to 5.14 × 1023 |
0.10 ± 0.02 |
0.47 |
3 |
0.92 |
|
I. fumosorosea (MBC 053) |
1.26×109 |
1.51 × 108 to 4.38 × 1011 |
3.82×1015 |
3.60 × 1012 to 8.87 × 1025 |
0.08 ± 0.02 |
1.53 |
3 |
0.67 |
|
L. attenuatum (MBC807) |
8.51×1010 |
2.56 × 109 to 4.63 × 1016 |
9.25×1016 |
1.83 × 1013 to 8.74 × 1031 |
0.08 ± 0.02 |
2.72 |
3 |
0.43 |
|
M. anisopliae (F52) |
1.76×108 |
4.20 × 107 to 2.06 × 109 |
3.31×1013 |
3.62 × 1011 to 2.78 × 1018 |
0.09 ± 0.02 |
0.26 |
3 |
0.96 |
|
I. farinose (MBC 389) |
7.38×107 |
3.02 × 107 to 2.11 × 108 |
1.64×1011 |
2.06 × 1010 to 6.16 × 1012 |
0.15 ±0.02 |
1.93 |
3 |
0.58 |
|
B. bassiana (MBC 076) |
1.25×108 |
2.37 × 107 to 2.70 × 109 |
3.17×1014 |
9.27 × 1011 to 1.93 × 1022 |
0.08 ± 0.02 |
0.61 |
3 |
0.89 |
|
I. cateniammulata (MBC289) |
1.83×108 |
4.28 × 107 to 2.26 × 109 |
4.07×1013 |
4.09 × 1011 to 4.63 × 1018 |
0.09 ±0.02 |
1.96 |
3 |
0.57 |
|
B. brongniartii (MBC397) |
1.05×109 |
1.34 × 106 to 6.05 × 107 |
5.01×1013 |
2.85 × 1011 to 2.16 × 1020 |
0.07 ±0.02 |
0.27 |
3 |
0.96 |
Lethal concentrations (LC50 & LC90) of microbial insecticides after 5 days of exposure to B. cucurbitae maggots
EPF strain MBC 389 has demonstrated the least LC50 value (4.32 × 108 conidia mL-1) and proves highly effective against maggots of B. cucurbitae. Unlikely, EPF strain MBC 524 exhibited the highest LC50 value (2.45 × 1010 conidia mL-1) and proved least effective against maggots of B. cucurbitae. EPF strains MBC 709, MBC 053, MBC 807, F 52, MBC 076, MBC 289 and MBC 397 exhibited 8.13 × 109, 3.46 × 109, 1.72 × 1010, 1.07 ×109, 5.40 × 108, 1.00 × 109 and 1.25 × 109 LC50 respectively. The order of pathogenicity of tested EPF strains were MBC389 > MBC076 > MBC289 > F52 > MBC397 > MBC053 > MBC709 > MBC807 > MBC524. The pathogenicity of all EPF strains against Bactrocera cucurbitae maggots varied significantly fiducial limits of EPF tested strains did not overlap with each other (Table 6).
Lethal concentrations (LC50 & LC90) of microbial insecticides after 7 days of exposure to B/ cucurbitae maggots
EPF strain MBC 389 has demonstrated the least LC50 value (7.38 × 107 conidia mL-1) and proves highly effective against maggots of B. cucurbitae. Unlikely, EPF strain MBC 807 exhibited the highest LC50 value (8.51 × 1010 conidia mL-1) and proved least effective against maggots of B. cucurbitae. EPF strains MBC 709, MBC 053, MBC 524, F 52, MBC 076, MBC 289 and MBC 397 exhibited 5.06 × 108, 1.26 × 109, 1.88 × 1010, 1.76 × 108, 1.25 × 108, 1.83 × 108 and 1.05 × 109 LC50, respectively. The order of pathogenicity of tested EPF strains were MBC 389 > MBC 076 > MBC289 > F52 > MBC709 > MBC 397 > MBC053 > MBC524 > MBC807. The pathogenicity of all EPF strains against B. cucurbitae maggots varied significantly as fiducial limits of EPF tested strains did not overlap with each other (Table 7).
Table 8: Lethal concentration (LC) values of different microbial insecticides after 14 days of exposure to Bactrocera cucurbitae maggots.
|
Fungal strain |
LC50 |
FL limit |
LC90 |
FL limit |
Slope±S.E |
X2 |
DF |
P |
|
M. pinghaense (MBC709) |
6.63×107 |
2.35 × 107 to 2.35 × 108 |
6.01×1011 |
4.27 × 1010 to 9.59 × 1013 |
0.13±0.0 |
1.57 |
3 |
0.66 |
|
I. javanica (MBC524) |
1.79×108 |
3.63 × 107 to 3.63 × 109 |
1.62 ×1014 |
7.36 × 1011 to 7.97 × 1020 |
0.08±0.02 |
0.10 |
3 |
0.99 |
|
I. fumosorosea (MBC 053) |
7.85×106 |
2.25 × 106 to 2.18 × 107 |
1.34×1011 |
1.34 × 1010 to 9.68 × 1012 |
0.12±0.02 |
3.05 |
3 |
0.38 |
|
L. attenuatum (MBC807) |
4.60×109 |
5.18 × 108 to 1.15 × 1012 |
7.89×1014 |
2.28 × 1012 to 1.54 × 1022 |
0.09±0.02 |
2.20 |
3 |
0.53 |
|
M. anisopliae (F52) |
1.48×107 |
4.12 × 106 to 4.83 × 107 |
6.42×1011 |
3.51 × 1010 to 2.50 × 1014 |
0.11±0.02 |
0.41 |
3 |
0.93 |
|
I. farinose (MBC 389) |
1.40×107 |
4.46 × 106 to 3.99 × 107 |
2.04×1011 |
1.83 × 1010 to 1.97 × 1013 |
0.12±0.02 |
1.35 |
3 |
0.71 |
|
B. bassiana (MBC 076) |
2.02×106 |
2.55 × 105 to 7.39 × 106 |
4.81×1011 |
2.21 × 1010 to 4.31 × 1014 |
0.09±0.01 |
0.36 |
3 |
0.94 |
|
I. cateniammulata (MBC289) |
5.49×106 |
1.06 × 106 to 1.87 × 107 |
7.38×1011 |
3.23 × 1010 to 6.64 × 1014 |
0.10±0.01 |
0.82 |
3 |
0.84 |
|
B. brongniartii (MBC397) |
1.86×106 |
1.59 × 105 to 7.90 × 106 |
1.98×1012 |
4.65 × 1010 to 2.11 × 1016 |
0.08±0.01 |
0.30 |
3 |
0.95 |
Table 9: Lethal concentration (LC) values of different entomopathogenic fungi after 21 days of exposure to Bactrocera cucurbitae maggots.
|
Insecticides |
LC50 |
FL limit |
LC90 |
FL limit |
Slope±S.E |
X2 |
DF |
P |
|
M. pinghaense (MBC709) |
5.16×106 |
1.64 × 106 to 1.2 × 107 |
2.69×1010 |
4.64 × 109 to 5.35 × 1011 |
0.14±0.02 |
2.08 |
3 |
0.55 |
|
I. javanica (MBC524) |
1.98×106 |
2.88 × 105 to 6.81 × 106 |
2.43×1011 |
1.50 × 1010 to 8.39 × 1013 |
0.10±0.01 |
0.04 |
3 |
0.99 |
|
I. fumosorosea (MBC 053) |
7.41×105 |
1.12 x 105 to 2.43 × 106 |
2.23×1010 |
3.20 × 109 to 7.67 × 1011 |
0.11±0.01 |
1.76 |
3 |
0.62 |
|
L. attenuatum (MBC807) |
1.86×107 |
6.88 × 106 to 4.79 × 107 |
9.36×1010 |
1.22 × 1010 to 3.32 × 1012 |
0.14±0.02 |
1.78 |
3 |
0.61 |
|
M. anisopliae (F52) |
1.12×106 |
1.44 × 105 to 3.94 × 106 |
1.08×1011 |
8.77 × 109 to 1.74 × 1013 |
0.10±0.01 |
0.23 |
3 |
0.97 |
|
I. farinose (MBC 389) |
4.46×105 |
5.04 ×104 to 1.64 × 106 |
2.32×1010 |
3.05 × 109 to 1.05 × 1012 |
0.11±0.01 |
1.88 |
3 |
0.59 |
|
B. bassiana (MBC 076) |
1.57×105 |
1.39 × 104 to 6.44 × 105 |
6.02×109 |
1.09 × 109 to 1.27 × 1011 |
0.11±0.01 |
0.50 |
3 |
0.91 |
|
I. cateniammulata (MBC289) |
3.11×105 |
4.43 × 104 to 1.05 × 106 |
4.67×109 |
9.99 × 108 to 6.42 × 1010 |
0.12±0.01 |
0.21 |
3 |
0.97 |
|
B. brongniartii (MBC397) |
4.49×103 |
4.23 × 101 to 4.48 × 104 |
1.12×109 |
2.25 × 108 to 2.31 × 1010 |
0.09±0.01 |
0.14 |
3 |
0.98 |
Lethal concentration (LC50 & LC90) values of different microbial insecticides after 14 days of exposure to B. cucurbitae maggots
EPF strain MBC 397 has demonstrated the least LC50 value (1.86 × 106 conidia mL-1) and proves highly effective against maggots of B. cucurbitae. Unlikely, EPF strain MBC 807 exhibited the highest LC50 value (4.60 × 109 conidia mL-1) and proved least effective against maggots of B. cucurbitae. EPF strains MBC 709, MBC 053, MBC 524, F 52, MBC 076, MBC 289 and MBC 389 exhibited 6.63 × 107, 7.85 × 106, 1.79 × 108, 1.48 × 107, 2.02 × 106, 5.49 × 106 and 1.40 × 107 LC50, respectively. The order of pathogenicity of tested EPF strains were MBC 397 >MBC076 > MBC289 > MBC053 > MBC389 > F52 > MBC709 > MBC524 > MBC807. The pathogenicity of all EPF strains against B. cucurbitae maggots varied significantly. Fiducial limits of EPF tested strains did not overlap with each other (Table 8).
Lethal concentrations (LC50 & LC90) of microbial insecticides after 21 days of exposure to B. cucurbitae maggots
EPF strain MBC 397 has demonstrated the least LC50 value (4.49 × 103 conidia mL-1) and proves highly effective against maggots of B. cucurbitae. Unlikely, EPF strain MBC 807 exhibited the highest LC50 value (1.86 × 107 conidia mL-1) and proved least effective against maggots of B. cucurbitae. EPF strains MBC 709, MBC 053, MBC 524, F 52, MBC 076, MBC 289 and MBC 389 exhibited 5.16 × 106, 7.41 × 105, 1.98 × 106, 1.12 × 106, 1.57 ×105, 3.11 × 105 and 4.46 × 105 LC50, respectively. The order of pathogenicity of tested EPF strains were MBC397 > MBC076 >MBC289 > MBC389 > MBC053 > F52 > MBC524 > MBC709 > MBC807. The pathogenicity of all EPF strains against B. cucurbitae maggots varied significantly as fiducial limits of EPF tested strains did not overlap with each other (Table 9).
Percent mortality of B. cucurbitae maggots due to entomopathogenic fungi
Analysis of variance showed that the total percentage mortality against B. cucurbitae has significant results after the application of all treatments. Mean comparison for total treatments against B. cucurbitae showed highest mortality rate (52.58%) was recorded when highest concentration (1 × 109 conidia mL-1) of entomopathogenic fungi was used and minimum mortality was recorded in control treatment (5.82%) followed by other concentrations (1 × 105, 1 × 106, 1 × 107 and 1 × 108 conidia mL-1) was 21.79%, 29.79%, 38.20% and 45.56% respectively against B. cucurbitae (Table 10).
Table 10: Effect of different concentrations of entomopathogenic fungi on mortality of melon fruit fly maggots.
|
Concentrations (conidia mL-1) |
Mortality (%) |
|
1 × 105 |
21.79 ± 0.43E |
|
1 × 106 |
29.79 ± 0.54D |
|
1 × 107 |
38.20 ± 0.61C |
|
1 × 108 |
45.56 ± 0.62B |
|
1 × 109 |
52.58 ± 0.78A |
|
Control |
5.82 ± 0.21F |
Values with different letters show significant difference (P≤0.05).
Mean comparison for total treatments against B. cucurbitae showed highest mortality rate (50.06%) after 21 days of application of treatments and minimum mortality was recorded (17.45%) after 3 days of application, while 23.41% mortality was recorded after 5 days, 31.61% mortality after 7 days and after 14 days of application of treatments mortality was 38.95% for B. cucurbitae (Table 11).
Table 11: Percent mortality of melon fruit fly maggots exposed to different entomopathogenic fungal strains for different days.
|
Days |
Mortality (%) |
|
3 day |
17.45 ± 0.35E |
|
5 day |
23.41 ± 0.45D |
|
7 day |
31.61 ± 0.53C |
|
14 day |
38.95 ± 0.74B |
|
21 day |
50.06 ± 0.86A |
Values with different letters show significant difference (P≤0.05).
Mean comparison for total treatments against B. cucurbitae showed highest mortality rate (40.18%) was recorded when MBC397 strain of entomopathogenic fungi was used and minimum mortality 22.66% was recorded in MBC807 strain followed by other strains (MBC076, MBC289, MBC389, F52, MBC053 MBC709 and MBC524) was recorded 37.16%, 34.91%, 33.95%, 33.57%, 31.81%, 30.30% and 26.09%, respectively against B. cucurbitae (Table 12).
Table 12: Percent mortality of melon fruit fly maggots exposed to different entomopathogenic fungal strains.
|
Fungus |
Mortality (%) ± S.E. |
|
MBC397 |
40.18 ± 0.75A |
|
MBC076 |
37.16 ± 0.56B |
|
MBC289 |
34.91 ± 0.51C |
|
MBC389 |
33.95 ± 0.47D |
|
F52 |
33.57 ± 0.45E |
|
MBC053 |
31.81 ± 0.41F |
|
MBC709 |
30.30 ± 0.33G |
|
MBC524 |
26.09 ± 0.26H |
|
MBC807 |
22.66 ± 0.16I |
Values with different letters show significant difference (P≤0.05).
B. cucurbitae which were treated with 1 × 105 conidia mL-1, showed 6.63% mortality after 3 days of application of treatments while 1 × 106, 1 × 107, 1 × 108, and 1 × 109 conidia mL-1 showed mortality 11.75%, 21.99%, 28.03% and 33.61%, respectively, for B. cucurbitae after 3 days of application of treatments. Minimum mortality (2.67%) was recorded in control where no treatment was applied. B. cucurbitae which were treated with 1 × 105 conidia mL-1, showed 12.67% mortality after 5 days of application of treatments while 1 × 106, 1 × 107, 1 × 108, and 1 × 109 conidia mL-1 showed mortality 20.14%, 27.59%, 34.09% and 41.52%, respectively, for B. cucurbitae after 5 days of application of treatments. Minimum mortality (4.45%) was recorded in control where no treatment was applied. B. cucurbitae which were treated with 1 × 105 conidia mL-1, showed 22.13% mortality after 7 days of application of treatments while 1 × 106, 1 × 107, 1 × 108, and 1 × 109 conidia mL-1 showed mortality 30.57%, 36.63%, 44.07% and 50.17%, respectively, for B. cucurbitae after 7 days of application of treatments. Minimum mortality (6.07%) was recorded in control where no treatment was applied. B. cucurbitae which were treated with 1 × 105 conidia mL-1, showed 28.68% mortality after 14 days of application of treatments while 1 × 106, 1 × 107, 1 × 108, and 1 × 109 conidia mL-1 showed mortality 36.94%, 46.11%, 53.32% and 61.10%, respectively, for Bactrocera cucurbitae after 14 days of application of treatments. Minimum mortality (7.60%) was recorded in control where no treatment was applied. B. cucurbitae which were treated with 1 × 105 conidia mL-1, showed 38.88% mortality after 21 days of application of treatments while 1 × 106, 1 × 107, 1 × 108, and 1 × 109 conidia mL-1 showed mortality 49.56%, 58.72%, 68.33% and 76.55%, respectively, for B. cucurbitae after 3 days of application of treatments. Minimum mortality (8.34%) was recorded in control where no treatment was applied (Table 13).
B. cucurbitae which were treated with MBC709 strain showed 16.15% mortality after 3 days of application of treatments while strains, MBC524, MBC053, MBC807, F52, MBC389, MBC076, MBC289 and MBC397 showed mortality 11.73%, 15.72%, 11.67%, 18.53%, 18.52%, 22.03%, 19.24% and 23.43% respectively for B. cucurbitae after 3 days of application of treatments. B. cucurbitae which were treated with MBC709 strain showed 22.73% mortality after 5 days of application of treatments while strains, MBC524, MBC053, MBC807, F52, MBC389, MBC076, MBC289 and MBC397 showed mortality 12.78%, 20.22%, 15.37%, 27.24%, 25.67%, 29.29%, 26.68% and 30.71% respectively for B. cucurbitae after 5 days of application of treatments. B. cucurbitae which were treated with MBC709 strain showed 31.12% mortality after 7 days of application of treatments while strains, MBC524, MBC053, MBC807, F52, MBC389, MBC076, MBC289 and MBC397 showed mortality 23.52%, 30.83%, 22.50%, 33.78%, 33.66%, 35.22%, 32.83% and 41.01% respectively for B. cucurbitae after 7 days of application of treatments. B. cucurbitae which were treated with MBC709 strain showed 36.26% mortality after 14 days of application of treatments while strains, MBC524, MBC053, MBC807, F52, MBC389, MBC076, MBC289 and MBC397 showed mortality 35.65%, 42.45%, 25.37%, 39.73%, 39.74%, 44.11%, 42.15% and 45.16% respectively for B. cucurbitae after 14 days of application of treatments. B. cucurbitae which were treated with MBC709 strain showed 45.29% mortality after 21 days of application of treatments while strains, MBC524, MBC053, MBC807, F52, MBC389, MBC076, MBC289 and MBC397 showed mortality 46.76%, 49.83%, 38.44%, 48.60%, 52.20%, 55.16%, 53.66% and 60.61%, respectively, for B. cucurbitae after 21 days of application of treatments (Table 14).
Table 13: Percent mortality of melon fruit fly maggots exposed to different concentrations of entomopathogenic fungi for different time intervals.
|
Conc. (conidia mL-1) |
Mortality (%) ± S.E. |
||||
|
3 day |
5 day |
7 day |
14 day |
21 day |
|
|
1 × 105 |
6.63± 0.35R |
12.67±0.31O |
22.13± 0.45N |
28.68± 0.42M |
38.88±1.21I |
|
1 × 106 |
11.75± 0.68OP |
20.14± 0.51N |
30.57± 0.65L |
36.94± 1.02J |
49.56± 1.12F |
|
1 × 107 |
21.99± 0.98N |
27.59± 0.67M |
36.63± 0.87J |
46.11± 1.07G |
58.72± 1.21D |
|
1 × 108 |
28.03± 1.01M |
34.09± 0.76K |
44.07± 1.21I |
53.32± 1.15E |
68.33± 1.46B |
|
1 × 109 |
33.61± 1.14K |
41.52± 1.05H |
50.17± 1.06FG |
61.10± 1.41C |
76.55±1.53A |
|
Control |
2.67± 0.12T |
4.45± 0.04S |
6.07±0.07R |
7.60± 0.13Q |
8.34± 0.15P |
Values with different letters show significant difference (P≤0.05).
Table 14: Percent mortality of melon fruit fly maggots exposed to different entomopathogenic fungi for different time intervals.
|
Fungal strains |
Percent mortality ± S.E. |
||||
|
3 days |
5 days |
7 days |
14 days |
21 days |
|
|
MBC709 |
16.15 ± 1.04T |
22.73 ± 1.14P |
31.12 ± 1.16J |
36.26 ± 1.17H |
45.29 ± 1.32E |
|
MBC524 |
11.73 ± 1.04V |
12.78 ± 1.05S |
23.52 ± 1.18M |
35.65 ± 1.21H |
46.76 ± 1.38D |
|
MBC053 |
15.72 ± 1.06T |
20.22 ± 1.12Q |
30.83 ± 1.21K |
42.45 ± 1.32F |
49.83 ± 1.67D |
|
MBC807 |
11.67 ± 1.12V |
15.37 ± 1.15T |
22.50 ± 1.25P |
25.37 ± 1.16M |
38.44 ± 1.53G |
|
F52 |
18.53 ± 1.26R |
27.24 ± 1.04M |
33.78 ± 1.28I |
39.73 ±1.18G |
48.60 ± 1.54D |
|
MBC389 |
18.52 ± 1.16R |
25.67 ± 1.06N |
33.66 ± 1.32I |
39.74 ± 1.22G |
52.20 ± 1.47C |
|
MBC076 |
22.03 ± 1.15O |
29.29 ± 1.08L |
35.22 ± 1.35H |
44.11 ± 1.27EF |
55.16 ±1.61B |
|
MBC289 |
19.24 ± 1.23Q |
26.68 ± 1.11LM |
32.83 ± 1.36H |
42.15 ± 1.35F |
53.66 ± 1.74C |
|
MBC397 |
23.43 ± 1.35O |
30.71 ± 1.14K |
41.01 ±1.42F |
45.16 ± 1.43E |
60.61 ± 1.87A |
Values with different letters show significant difference (P≤0.05).
Table 15: Percent mortality of melon fruit fly maggots exposed to different concentrations of the entomopathogenic fungi.
|
EPF strains |
Percent mortality ± S.E. |
|||||
|
1 × 105 |
1 × 106 |
1 × 107 |
1 × 108 |
1 × 109 |
Control |
|
|
MBC709 |
19.28 ± 1.01V |
23.45 ± 1.17T |
35.11 ± 1.24O |
45.11 ± 1.54I |
51.78 ± 1.34EF |
7.12 ± 1.04Z |
|
MBC524 |
17.68 ± 1.05X |
22.68 ± 1.12T |
30.18 ± 1.31Q |
36.01 ± 1.66M |
43.51 ± 1.42I |
6.47 ± 1.02Z |
|
MBC053 |
19.73 ± 1.03V |
30.78 ± 1.19Q |
37.63 ± 1.28M |
45.27 ± 1.52I |
52.01 ± 1.43D |
5.42 ± 1.06a |
|
MBC807 |
10.47 ± 1.05Y |
20.69 ± 1.51U |
25.80 ± 1.43S |
34.27 ± 1.43P |
39.38 ± 1.75K |
5.41 ± 0.76a |
|
F52 |
23.99 ± 1.06T |
30.76 ± 1.12Q |
39.28 ± 1.16L |
46.92 ± 1.54H |
54.53 ± 1.53D |
5.99 ± 0.62a |
|
MBC389 |
21.49 ± 1.07U |
28.34 ± 1.27R |
39.28 ± 1.25L |
51.13 ± 1.46EF |
57.94 ± 1.43BC |
5.56 ± 0.57a |
|
MBC076 |
26.59 ± 1.11RS |
36.77 ± 1.15M |
45.24 ± 1.28I |
50.35 ± 1.62F |
58.03 ± 1.96B |
5.99 ± 0.41a |
|
MBC289 |
23.24 ± 1.17T |
33.87 ± 1.22P |
42.73 ± 1.22J |
48.06 ± 1.59G |
56.09 ± 1.87C |
5.47 ± 0.32a |
|
MBC397 |
33.73 ± 1.14P |
40.77 ± 1.31K |
48.61 ± 1.44G |
52.97 ± 1.64D |
60.01 ± 1.98A |
5.00 ± 0.25a |
B. cucurbitae which were treated with different strains (MBC709, MBC524, MBC053, MBC807, F52, MBC389, MBC076, MBC289 and MBC397) at 1 x 105 conidia mL-1 showed mortality 19.28%, 17.68%, 19.73%, 10.47%, 23.99%, 21.49%, 26.59%, 23.24% and 33.73%, respectively. B. cucurbitae which were treated with different strains (MBC709, MBC524, MBC053, MBC807, F52, MBC389, MBC076, MBC289 and MBC397) at 1 × 106 conidia mL-1 showed mortality 23.45%, 22.68%, 30.78%, 20.69%, 30.76%, 28.34%, 36.77%, 33.87% and 40.77%, respectively. B. cucurbitae which were treated with different strains (MBC709, MBC524, MBC053, MBC807, F52, MBC389, MBC076, MBC289 and MBC397) at 1 × 107 conidia mL-1 showed mortality 35.11%, 30.18%, 37.63%, 25.80%, 39.28%, 39.28%, 45.24%, 42.73% and 48.61%, respectively. B. cucurbitae which were treated with different strains (MBC709, MBC524, MBC053, MBC807, F52, MBC389, MBC076, MBC289 and MBC397) at 1 × 108 conidia mL-1 showed mortality 45.11%, 36.01%, 45.27%, 34.27%, 46.92%, 51.13%, 50.35%, 48.06% and 52.97%, respectively. B. cucurbitae which were treated with different strains (MBC709, MBC524, MBC053, MBC807, F52, MBC389, MBC076, MBC289 and MBC397) at 1 × 109 conidia mL-1showed mortality 51.78%, 43.51%, 52.01%, 39.38%, 54.53%, 57.94%, 58.03%, 56.09% and 60.01%, respectively. B. cucurbitae showed mortality rates of 7.12%, 6.47%, 5.42%, 5.41%, 5.99%, 5.56%, 5.99%, 5.47% and 5.00% in control treatment of different strains, MBC709, MBC524, MBC053, MBC807, F52, MBC389, MBC076, MBC289 and MBC397, respectively (Table 15).
Virulence of different entomopathogenic fungi was evaluated against maggots of B. cucurbitae. Susceptibility to fruit flies by different pathogens has been confirmed by various scientist (Peña et al., 2015; Cheng et al., 2017). Results of present experiment revealed that mortality range 10 to 95%, results supported by (Bedini et al., 2018; Chergui et al., 2020) who found that 7 to 100% mortality was observed against Ceratitis capitata when treated with B. bassiana. In present study nine strains were evaluated against maggots of melon fruit fly. Among of them B. brongniartii (MBC397) proved to be most effective for the control melon fruit fly maggots.
Higher concentration (1×109) and maximum exposure interval (21) days, Metarhizium pinghaense (MBC709) (51.78%), Isaria javanica (MBC524), (43.51%) Isaria fumosorosea (MBC 053) (52.01%), Lecanicillium attenuatum (MBC807) (39.38%), Metarhizium anisopliae (F52) (54.53%), Isaria farinose (MBC 389), (57.94%) Beauveria bassiana (MBC 076), (58.03%), Beauveria bassiana (MBC 076), Isaria cateniammulata (MBC289) (56.09%) and Beauveria brongniartii (MBC397) (60.01%) mortality against maggots of melon fruit fly. These results also reported by Yang et al. (2015) topical application method encouraged significantly greater mortality than feeding alone. C. capitata adults demonstrated mortality level from 20 to 98.7% in the assessment of 16 strains of B. bassiana, Qazzaz et al. (2015). In the identical way, (Ugwu and Nwaokolo, 2020) stated mortality range between 82 and 100% against B. bassiana and M. anisopliae for the control of Anastrepha ludens adults. Similarly, Nwaokolo et al. (2023) reported that larvae of Mediterranean fruit fly showed that M. anisopliae discovered higher mortality rate than B. bassiana reaching 73.80% at 1×109 concentration of volume 20ml/100g result showed difference because Khlaywi et al. (2014) used distilled water for solution making and in our research Tween 20 were used as solvent for prepration of suspension. Iqbal et al. (2021) reported that Cucurbits fruit fly larvae showed (75%) mortality the highest was at concentration of 1×109 spores/ml of B. bassiana isolates was measured depending on the adult’s emergence rates. The adult’s emergence rate was decreased with increasing of concentration.
When these EPFs were applied on maggots through diet bioassy all tested entomopathogenic microbial insecticides, LC50 for maggots of B. cucurbitae decreased with increase in exposure interval. At maximum exposure intervals (21 days), B. bassiana demonstrated least LC50 against B. cucurbitae maggots (1.57 × 105 conidia mL-1) followed by I. farinose which explained LC50 value of 4.46 x 105 conidia mL-1against maggots of B. cucurbitae, at maximum exposure interval (21 days). Likewise, Isaria farinose explained LC50 value of 1.0 × 107 conidia mL-1against larvae of B. cucurbitae, at maximum exposure interval (21 days); while, Isaria cateniannulata demonstrated highest LC50 against B. cucurbitae larvae (1.9 × 107 conidia mL-1). At highest concentration (1 × 109 conidia mL-1), LT50 values for larvae of B. cucurbitae in sand bioassay were 9.04 days, 11.78 days, 12.78 days and 13.04 days for B. bassiana, Metarhizium pinghaense, Isaria farinose and Isaria cateniannulata, respectively. Gul et al. (2015) reported that B. zonata showed that LC50 value 2×108 conidia/mL for B. bassiana for 5 days result difference due to different application method. Shahid et al. (2024) reported that LC50 values, Bb100 show more sensitive against male the LC50 value was 8.3 x 103 spores mL-1. and then Bb 17 (2.7 x 105 spores mL-1) and BE47 (5 × 106 spores mlL-1). At concentration of 1×109 spores mL-1show varied lethal time, which articulates on virulence, showed superior of Bb100 with LT50 2.5 days, then the isolate of BE47 (5.5 days) and Bb17 (9 days). Here is little difference in result due to solvent Xia et al. (2023) were used Tween 80 rather than tween 20. Dimbi et al. (2003) and Singh et al. (2024) also reported that C. rosa var. fasciventris and C. capitata showed that the LT90 value ranged 3–4 days in both insects.
Acknowledgement
The researcher would like to express gratitude to the University of the Punjab Lahore Campus’s Department of Entomology as well as everyone else who helped with this research.
Novelty Statement
In order to effectively manage melon fruit flies and eliminate the need for insecticides for pest control, the current work is a pioneer in comprehending the synergistic effect of oils in improving the virulence effect of entomopathogenic fungus.
Author’s Contribution
Mubashar Iqbal and Shahbaz Ahmad: Developed the research plan, analyzed the data, and authored the manuscript.
Arshad Javaid and Muhammad Bilal Chattha: Critically analyzed the data and provided guidance on the research plan and statistical analysis.
Muhammad Ashfaq, Tajamal Hussain and Sumra Ashraf: Reviewed the manuscript drafts and prepared the tables.
Conflict of interest
The authors have declared no conflict of interest.
References
Abdelmoteleb, A. and D. González-Mendoza. 2020. A novel Streptomyces rhizobacteria from desert soil with diverse anti-fungal properties. Rhizosphere, 16: 100-143. https://doi.org/10.1016/j.rhisph.2020.100243
Ahmad, S., A. Sarwar, A. Shoaib, A. Javaid, M.S. Hanif and Q. Ali. 2022. Sustainable management of guava fruit fly, Bactrocera zonata (Tephritidae: Diptera) by entomopathogenic fungi. Fresenius Environ. Bull., 31(6): 5522-5527.
Albornoz, M.V., C.C. Santander and A. Alfaro-Tapia. 2024. A review of the arrival, effects, and management of Bagrada hilaris in South America: The case of Chile. Horticulture, 10(10): 1072-1084. https://doi.org/10.3390/horticulturae10101072
Alwaneen, W.S., M. Tahir, P.B. Avery, W. Wakil, N.G. Kavallieratos, N. Eleftheriadou, M.C. Boukouvala, K.G. Rasool, M. Husain and A.S. Aldawood. 2024. Initial evaluation of the entomopathogenic fungi Beauveria bassiana and Metarhizium robertsii, and the entomopathogenic nematode Heterorhabditis bacteriophora, individually and in combination against the noxious Helicoverpa armigera (Lepidoptera: Noctuidae). Agronomy, 14(7): 1395-1405. https://doi.org/10.3390/agronomy14071395
Bacova, K., K. Zitterl-Eglseer, L. Chrastinova, A. Laukova, M. Madarova, S. Gancarcikova, D. Sopkova, Z. Andrejcakova and I. Placha. 2020. Effect of thymol addition and withdrawal on some blood parameters, antioxidative defense system and fatty acid profile in rabbit muscle. Animals, 10(8): 1248-1255. https://doi.org/10.3390/ani10081248
Bamisile, B.S., K.S. Akutse, J.A. Siddiqui and Y. Xu. 2021. Model application of Entomopathogenic fungi as alternatives to chemical pesticides: Prospects, challenges, and insights for next-generation sustainable agriculture. Front. Plant Sci., 12: 741804-741811. https://doi.org/10.3389/fpls.2021.741804
Bedini, S., S. Sarrocco, R. Baroncelli, G. Vannacci and B. Conti. 2018. Pathogenic potential of Beauveria pseudobassiana as bioinsecticide in protein baits for the control of the medfly Ceratitis capitata. Bull. Insectol., 71(1): 31-38.
Chaudhary, R., A. Nawaz, Z. Khattak, M.A. Butt, M. Fouillaud, L. Dufossé, M. Munir, Inam ul Haq and H. Mukhtar. 2024. Microbial bio-control agents: A comprehensive analysis on sustainable pest management in agriculture. J. Agric. Food Res., 76(2): 101-111. https://doi.org/10.1016/j.jafr.2024.101421
Cheng, D., Z. Guo, M. Riegler, Z. Xi, G. Liang and Y. Xu. 2017. Gut symbiont enhances insecticide resistance in a significant pest, the oriental fruit fly Bactrocera dorsalis (Hendel). Microbiology, 5(1): 1-12. https://doi.org/10.1186/s40168-017-0236-z
Chergui, S., K. Boudjemaa, A. Benzehra and I. Karaca. 2020. Pathogenicity of indigenous Beauveria bassiana (Balsamo) against Ceratitis capitata Wiedemann (Diptera: Tephritidae) under laboratory conditions. Egypt. J. Biol. Pest Contr., 30(1): 128-137. https://doi.org/10.1186/s41938-020-00331-z
Deguine, J.P., J.N. Aubertot, R.J. Flor, F. Lescourret, K.A. Wyckhuys and A. Ratnadass. 2021. Integrated pest management: Good intentions, hard realities. A review. Agron. Sust. Dev., 41(3): 38-47. https://doi.org/10.1007/s13593-021-00689-w
Deka, B., C. Baruah and A. Babu. 2021. Entomopathogenic microorganisms: Their role in insect pest management. Egypt. J. Biol. Pest Contr., 31(1): 1-8. https://doi.org/10.1186/s41938-021-00466-7
Dembilio, O., E. Quesada-Moraga, C. Santiago-Alvarez and J.A. Jacas, 2010. Potential of an indigenous strain of the entomopathogenic fungus Beauveria bassiana as a biological control agent against the red palm weevil, Rhynchophorus ferrugineus. J. Invertebr. Pathol., 104: 214–221. https://doi.org/10.1016/j.jip.2010.04.006
Dimbi, S., N.K. Maniania, S.A. Lux, S. Ekesi and J.K. Mueke. 2003. Pathogenicity of Metarhizium anisopliae (Metsch.) Sorokin and Beauveria bassiana (Balsamo) Vuillemin, to three adult fruit fly species: Ceratitis capitata (Weidemann), C. rosa var. fasciventris Karsch and C. cosyra (Walker) (Diptera: Tephritidae). Mycopathologia, 156: 375-382.
Fahad, S., S. Saud, A. Akhter, A.A. Bajwa, S. Hassan, M. Battaglia, M. Adnan, F. Wahid, R. Datta, E. Babur and S. Danish. 2021. Bio-based integrated pest management in rice: An agro-ecosystems friendly approach for agricultural sustainability. J. Saudi Soc. Agric. Sci., 20(2): 94-102. https://doi.org/10.1016/j.jssas.2020.12.004
Ghorui, M., S. Chowdhury and S. Burla. 2024. The science behind entomopathogenic fungi: Mechanisms and applications. In: Entomopathogenic fungi: Prospects and Challenges. Singapore: Springer Nature Singapore, pp. 3-35. https://doi.org/10.1007/978-981-97-5991-0_1
Grabka, R., T.W. d’Entremont, S.J. Adams, A.K. Walker, J.B. Tanney, P.A. Abbasi and S. Ali. 2022. Fungal endophytes and their role in agricultural plant protection against pests and pathogens. Plants, 11(3): 384-392. https://doi.org/10.3390/plants11030384
Gul, H.T., S. Freed, M. Akmal and M.N. Malik. 2015. Vulnerability of different life stages of Bactrocera zonata (Tephritidae: Diptera) against entomogenous fungi. Pak. J. Zool., 47(2): 307-317.
Henderson, C.F. and E.W. Tilton. 1955. Tests with acaricides against the brow wheat mite, J. Econ. Entomol., 48: 157-161. https://doi.org/10.1093/jee/48.2.157
Iqbal, M., M.D. Gogi, B. Atta, M.J. Nisar, M.J. Arif and N. Javed. 2021. Assessment of pathogenicity of Beauveria bassiana, Metarhizium anisopliae, Verticillium lecanii and Bacillus thuringiensis var. kurstaki against Bactrocera cucurbitae Coquillett (Diptera: Tephritidae) via diet-bioassay technique under controlled conditions. Int. J. Trop. Insect Sci., 41: 1129-1145. https://doi.org/10.1007/s42690-020-00298-2
Joshi, M.D., A.K. Srivastava, M. Ashaq, S. Jaggi, P. Gupta, W. Hasan and S. Gupta. 2024. Biocontrol agents and plant protection. Uttar Pradesh J. Zool., 45(16): 109-131. https://doi.org/10.56557/upjoz/2024/v45i164292
Kachur, K. and Z. Suntres. 2020. The antibacterial properties of phenolic isomers, carvacrol and thymol. Crit. Rev. Food Sci. Nutr., 60(18): 3042-3053. https://doi.org/10.1080/10408398.2019.1675585
Khlaywi, S.A., M.W. Khudhair, H.F. Alrubeai, A.K. Shbar and S.A. Hadi. 2014. Efficacy of Beauveria bassiana and Metarhizium anisopliae to control mediterranean fruit fly, Ceratitis capitata. Int. J. Entomol. Res., 02(03): 169–173. http://www.escijournals.net/IJER
Khuhro, N.H., M. Abubakar, Q.B. Pirzado, Q.A. Soomro, M.I. Kubar, M.U. Asif and R.M. Memon. 2024. Life history parameters of melon fruit fly, Bactrocera cucurbitae (Coquillett) (Diptera: Tephritidae) on four economically important vegetables. Agric. Sci. J., 6(1): 49-57. https://doi.org/10.56520/asj.v6i1.318
Kordrostami, M., F. Sanjarian, S. Shahbazi and A.A. Ghasemi-Soloklui. 2024. Exploring low-dose gamma radiation effects on monoterpene biosynthesis in Thymus vulgaris: insights into plant defense mechanisms. Environ. Sci. Poll. Res., 7(1): 1-21. https://doi.org/10.1007/s11356-024-33269-y
Kumar, R. and V.S. Rathor. 2020. Nature and types of damage by insect pests. J. Entomol. Res., 44(4): 639-646. https://doi.org/10.5958/0974-4576.2020.00106.1
Mahendiran, G., S. Lal and O.C. Sharma 2022. Pests and their management on temperate fruits: Apple, pear, peach, apricot, cherry, persimmon, walnut, olive, kiwifruit and strawberry. Trend. Hortic. Entomol., 6(1): 891-941. https://doi.org/10.1007/978-981-19-0343-4_36
Mani, M., 2022. Pests and their management in minor fruits: (Avocado, Breadfruit, Carambola, Durian, Langsat, Longan, Mangosteen, Passion Fruit, Rambutan, Rose Apple, Santol, Star Apple, Velvet Apple, and Water Nut). Trends Hort. Entomol., pp. 863-890. https://doi.org/10.1007/978-981-19-0343-4_35
Mantzoukas, S. and P.A. Eliopoulos. 2020. Endophytic entomopathogenic fungi: A valuable biological control tool against plant pests. Appl. Sci., 10(1): 360-368. https://doi.org/10.3390/app10010360
Marisel, O.G., R.R. Yoania, R.R. Santiago, Z.L.H. Alberto and C.D.I. Fernando. 2024. Bioprospecting a mountain-derived phosphorus-solubilizing bacterium: Bacillus thuringiensis B3 as a plant-growth promoter in lettuce and tomato horticultural crops. Sci. Hortic., 337(12): 113568. https://doi.org/10.1016/j.scienta.2024.113568
Minitab, L.L.C., 2017. Getting started with Minitab 18; Minitab Inc.: State College, PA, USA, pp. 73.
Mishra, S., A. Srivastava, A. Singh, G.C. Pandey and G. Srivastava. 2024. An overview of symbiotic and pathogenic interactions at the fungi-plant interface under environmental constraints. Front. Fungal Biol., 5(13): 63-76. https://doi.org/10.3389/ffunb.2024.1363460
Napoli, E., L. Siracusa and G. Ruberto. 2020. New tricks for old guys: Recent developments in the chemistry, biochemistry, applications and exploitation of selected species from the Lamiaceae Family. Chem. Biol., 17(3): 19-34. https://doi.org/10.1002/cbdv.201900677
Natal, C.M., M.J.G. Fernandes, N.F. Pinto, R.B. Pereira, T.F. Vieira, A.R.O. Rodrigues, D.M. Pereira, S.F. Sousa, A.G. Fortes, E.M. Castanheira and M.S.T. Gonçalves. 2021. New carvacrol and thymol derivatives as potential insecticides: Synthesis, biological activity, computational studies and nanoencapsulation. RSC Adv., 11(54): 34024-34035. https://doi.org/10.1039/D1RA05616F
Nwaokolo, V.M., S.G. Jonathan, J.A. Ugwu and E.I. Eziashi. 2023. Potential of Beauveria bassiana and Metarhizium anisopliae as biological management agents of Phytolyma fusca (Hemiptera, Psylloidea). Nigeria Agric. J., 54(1): 336-341.
Ochieng, T.A., K.S. Akutse, I.J. Ajene, D.C. Kilalo, M. Muiru and F.M. Khamis. 2024. Interactions between Bacillus thuringiensis and selected plant extracts for sustainable management of Phthorimaea absoluta. Sci. Rep., 14(1): 92-99. https://doi.org/10.1038/s41598-024-60140-4
Peña, J.M., M.A. Carrillo and E.A. Hallem. 2015. Variation in the susceptibility of Drosophila to different entomopathogenic nematodes. Infect. Immun., 83(3): 1130-1138. https://doi.org/10.1128/IAI.02740-14
Prakash, G.V.S.B., U.R. Sankar, and V. Padmaja. 2015. Development and testing of mycopesticide formulations of Metarhizium anisopliae (Metschnikoff) for shelf life and field application against Spodoptera litura (Feb) larvae. Int. J. Biol., 4(9): 4284-4289.
Prinsloo, G. and N. Nogemane. 2018. The effects of season and water availability on chemical composition, secondary metabolites and biological activity in plants. Phyt. Rev., 17(4): 889-902. https://doi.org/10.1007/s11101-018-9567-z
Qazzaz, F.O., M.I. Al-Masri and R.M. Barakat. 2015. Effectiveness of Beauveria bassiana native isolates in the biological control of the Mediterranean fruit fly (Ceratitis capitata). Adv. Entomol., 3(2): 44-55. https://doi.org/10.4236/ae.2015.32006
Quesada-Moraga, E., N. Gonzalez-Mas, M. Yousef-Yousef, I. Garrido-Jurado and Fernández- M. Bravo. 2024. Key role of environmental competence in successful use of entomopathogenic fungi in microbial pest control. J. Pest Sci., 97(1): 1-15. https://doi.org/10.1007/s10340-023-01622-8
Rathod, N.B., P. Kulawik, F. Ozogul, J.M. Regenstein and Y. Ozogul. 2021. Biological activity of plant-based carvacrol and thymol and their impact on human health and food quality. Trend Food Sci. Technol., 116: 733-748. https://doi.org/10.1016/j.tifs.2021.08.023
Rehan, M.M., A.A. Abouzaid, H.G. Abo-Al-Ela, M.S. Abdou and N.R. Elsaidy. 2024. Utilization of origanum oil as a health promoter in Nile tilapia (Oreochromis niloticus) challenged with Pseudomonas aeruginosa. Aquaculture, 584: 740-753. https://doi.org/10.1016/j.aquaculture.2024.740683
Saeed, M., T. Ahmad, M. Alam, L.A. Al-Shuraym, N. Ahmed, M.A. Alshehri, H. Ullah and S.M. Sayed. 2022. Preference and performance of peach fruit fly (Bactrocera Zonata) and Melon fruit fly (Bactrocera Cucurbitae) under laboratory conditions. Saudi J. Biol, Sci., 29(4): 2402-2408. https://doi.org/10.1016/j.sjbs.2021.12.001
Shahid, M., M.D. Gogi, A. Akhtar, S. Saeed, S. Kanwal, S. Fazal, R.A. Hassan, A. Anees and A. Nadeem. 2024. Impact of almond oil, amla oil and coconut oil on the growth and virulence of entomopathogenic strains against Bactrocera Zonata maggots: Effect of oils on entomopathogenic strains against maggots. J. Zool., 7(1): 36-43. https://doi.org/10.54393/mjz.v5i02.112
Singh, K.A., I. Nangkar, A. Landge, M. Rana and S. Srivastava. 2024. Entomopathogens and their role in insect pest management. J. Biol. Con., 38(1): 321-331. https://doi.org/10.18311/jbc/2024/35752
Sorokan, A., V. Gabdrakhmanova, Z. Kuramshina, R. Khairullin and I. Maksimov. 2023. Plant-associated Bacillus thuringiensis and Bacillus cereus: inside agents for biocontrol and genetic recombination in phytomicrobiome. Plants, 12(23): 40-47. https://doi.org/10.3390/plants12234037
Thilagam, P., C.H. Sharanappa, S. Roy, L. Deb, S. Padhan, S. Srividhya and P. Awadhiya. 2023. A review on advances in biocontrol techniques for managing insect pests in sustainable agriculture. Int. J. Environ. Clim. Change, 13(11): 2114-2125. https://doi.org/10.9734/ijecc/2023/v13i113373
Ugwu, J.A. and V.M. Nwaokolo. 2020. Biocidal activity of selected botanicals and Beauveria bassiana on oriental fruit fly, Bactrocera dorsalis (Diptera; Tephritidae). J. Res. For. Wildl. Environ., 12(1): 53-61.
Valerio, F., G.N. Mezzapesa, A. Ghannouchi, D. Mondelli, A.F. Logrieco and E.V., Perrino. 2021. Characterization and antimicrobial properties of essential oils from four wild taxa of Lamiaceae family growing in Apulia. Agronomy, 11(7): 1431-1439. https://doi.org/10.3390/agronomy11071431
Vermelho, A.B., J.V. Moreira, I.T. Akamine, V.S. Cardoso and F.R. Mansoldo. 2024. Agricultural pest management: The role of microorganisms in biopesticides and soil bioremediation. Plants, 13(19): 2762-2769. https://doi.org/10.3390/plants13192762
Wang, L., N.O. Keyhani, Y. Xia and J. Xie. 2024. The potential and limitations of entomopathogenic fungi as biocontrol agents for insect pest management. Entomol. Generalis, 44(4): 341-356. https://doi.org/10.1127/entomologia/2024/2498
Xia, Y., S. Yu, Q. Yang, J. Shang, Y. He, F. Song, Q. Li and C. Jiang. 2023. Sublethal effects of Beauveria bassiana strain BEdy1 on the development and reproduction of the white-backed plant hopper, Sogatella furcifera (Horváth) (Hemiptera: Delphacidae). J. Fungi, 9(1): 123-134. https://doi.org/10.3390/jof9010123
Yang, Y., Y. Zhang, M. Wang, S.S. Li, X.Y. Ma and Z.H. Xu. 2015. Bioefficacy of entomopathogenic Aspergillus strains against the melon fly, Bactrocera cucurbitae (Diptera: Tephritidae). App. Entomol. Zool., 50: 443-449. https://doi.org/10.1007/s13355-015-0351-x
Zharkov, D., T. Nizamutdinov, D. Dubovikoff, E. Abakumov and A. Pospelova. 2023. Navigating agricultural expansion in harsh conditions in Russia: Balancing development with insect protection in the era of pesticides. Insects, 14(6): 557-564. https://doi.org/10.3390/insects14060557
To share on other social networks, click on any share button. What are these?





