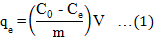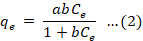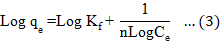Optimizing Granular Activated Carbon (Coal and Peat-Base) for Malathion Removal from Agricultural Effluent
Optimizing Granular Activated Carbon (Coal and Peat-Base) for Malathion Removal from Agricultural Effluent
Hajjar Hartini Wan Jusoh1,2, Hafizan Juahir1,2*, Azimah Ismail1, Nurfarahana Mohd Nasir3,5, Setyo Budi Kurniawan1,4 and Ahmad Jusoh5
1East Coast Environmental Research Institute, Gong Badak Campus, Universiti Sultan Zainal Abidin, Kuala Terengganu 21300, Malaysia; 2Faculty of Bioresource and Food Industry, Besut Campus, Universiti Sultan Zainal Abidin, Kuala Terengganu 22022, Malaysia; 3Faculty of Engineering, Universiti Putra Malaysia, 43400 UPM Serdang, Selangor Darul Ehsan, Malaysia; 4Department of Chemical and Process Engineering, Faculty of Engineering and Built Environment, Universiti Kebangsaan Malaysia, 43600 UKM Bangi, Selangor, Malaysia; 5Faculty of Ocean Engineering, Universiti Malaysia Terengganu, 21300 Kuala Terengganu, Terengganu.
Abstract | The usage of malathion pesticide and a subsequent release into the environment was a major concern worldwide due to detrimental effect and their toxicity to ecosystem. Concerned about the impact of malathion pesticide, this study investigated the effectiveness of granular activated carbon (GAC) in removing malathion from agricultural runoff. The study focused on how particle size and dosage of GAC impact the removal efficiency. Therefore, this study revealed that particle size and dosage of granular activated carbon significantly affect the removal efficiency of malathion from agricultural runoff. As the initial malathion concentration (7 μg L-1) was lowered to approximately 1.14 and 1.5 μg L-1 for CBAC and 2.87 μg L-1 for PBAC at respective diameters of 0.063 and 1.0 mm, the best circumstances for the highest removal efficiency of 90 % were observed. The features of adsorption behavior were described using the Langmuir and Freundlich adsorption isotherms models. The Freundlich and Langmuir models, with a maximum adsorption capacity of 248.1 mg g-1, suit the equilibrium results quite well. Therefore, this study highlights the potential of GAC as an effective absorbent material for the removal of malathion from agricultural runoff, with significant implications for mitigating the damaging consequences of environmental pesticide pollution.
Received | February 22, 2024; Accepted | August 06, 2024; Published | October 07, 2024
*Correspondence | Hafizan Juahir, Faculty of Bioresource and Food Industry, Besut Campus, Universiti Sultan Zainal Abidin, Kuala Terengganu 21300, Malaysia; Email: hafizanjuahir@unisza.edu.my
Citation | Jusoh, H.H.W., H. Juahir, A. Ismail, N.M. Nasir, S.B. Kurniawan and A. Jusoh. 2024. Optimizing granular activated carbon (coal and peat-base) for malathion removal from agricultural effluent. Sarhad Journal of Agriculture, 40(Special issue 1): 08-21.
DOI | https://dx.doi.org/10.17582/journal.sja/2024/40/s1.8.21
Keywords | Granular activated carbon, Malathion, Batch studies, Adsorption, Agricultural run off
Copyright: 2024 by the authors. Licensee ResearchersLinks Ltd, England, UK.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
The global increase in water utilization stems from socioeconomic progress and the expansion of the population, particularly in dry areas. This spike in demand exacerbates the problem of water scarcity, stressing the already limited water resources. Key markers for assessing water purity include temperature, TDS (total dissolved solids), pH levels, DO (dissolved oxygen), and the concentration of ammonium. These essential attributes can be affected by both natural events and human actions, influencing the condition of water resources (Ssomad et al., 2016). Water resource management has emerged as a critical concern that necessitates immediate action. By implementing structured and efficient water resource management strategies, it’s possible to ensure the production of uncontaminated, high-quality wastewater (Kamarudin et al., 2020). Wastewater can originate from various sources such as industrial operations, farms, and residential areas. Alongside residential waste, agricultural runoff is a significant contributor to water pollution (Hanapi et al., 2022). There’s a growing need for improved water and wastewater management practices in the agricultural sector to address these issues and mitigate the adverse effects of pollutants, like pesticides, on both the environment and public health.
Large quantities of pesticides, such as herbicides, insecticides, fungicides, rodenticides, and others, are introduced into the environment due to household, industrial, and agricultural activities. Various processes can affect the ecosystem once pesticides are released, whether through their application, disposal, or accidental spillage. Thus, there are significant concerns regarding the long-term effects of low doses of pesticides on non-target species, the environment, and human health (Ahmed et al., 2020). Due to their effectiveness and high cost, developing countries are unable to discontinue the use of various pesticides. Consequently, it is essential to reduce and eliminate these potentially harmful substances before releasing wastewater. Many researchers are exploring advanced methods to remove these pesticides from their primary sources, predominantly aquatic environments (Derbalah et al., 2021; Massoud et al., 2021).
Pesticide residues are often found in rivers, streams, groundwater, and even precipitation. The risk of unintended pesticide leaks into the environment is determined by various factors, such as agricultural practices, spraying techniques, and environmental conditions (including landforms, biodiversity, respiration, erosion). These residues can indicate the impact of pesticides on biological processes in nature, as illustrated in Figure 1. Pesticides applied or used on fields can contaminate surrounding land and water bodies in several ways. Wind drift and evaporation, for instance, can cause pesticides to spread from their original application sites and be reintroduced into the ground or water bodies through rainfall. Surface runoff and water percolating into drainage systems can carry pesticide residues into rivers and lakes, while leaching can introduce these substances into groundwater. Additionally, spills during the handling of pesticide products contribute to the dissemination of these chemicals into the environment.
Malathion, chemically known as S-1, 2-bis (ethoxycarbonyl) ethyl O, O-dimethyl phosphorodithioate, is a commonly used organophosphorous pesticide. It is effective in controlling a wide array of insects, including those infesting fruits and vegetables, as well as mosquitoes (Bhardwaj and Saraf, 2014). Malathion has become one of the most widely used active ingredients in pest control solutions around the globe, with countries such as China and India deploying substantial quantities for their agricultural production. The employment of this pesticide has been shown to result in the presence of organophosphate residues, including malathion, which possess hazardous and potentially carcinogenic properties (Khuman et al., 2020). The toxicity of malathion and other organophosphates is attributed to their ability to inhibit cholinesterase activity, affecting the nervous system. This interference disrupts normal neurotransmission, leading to a range of adverse effects on health (Bradford et al., 2020). The widespread use of Malathion highlights the critical need for careful monitoring and regulation of pesticides to ensure they do not pose threats to human health and the environment.
In response to the growing pollution of natural water bodies by pesticides, several strategies have been devised to combat this issue. These include removal techniques such as adsorption, photocatalysis, disinfection, and ozonation. Numerous studies utilizing various methods have been conducted to explore the effectiveness of these strategies in eliminating specific pesticides from water sources (Derbalah et al., 2021; Adrunik and Badja, 2021; Cosgrove et al., 2019). For instance, (Biswas and Goel, 2022) Research has explored the efficacy of the electrocoagulation method in removing pesticides, including malathion, imidacloprid, and chlorpyrifos, from contaminated water (Trellu et al., 2021) using electrochemical methods, Goh et al. (2022) and Nanofiltration treatment, Boelan and Purnomo (2019) employed a combination culture of bacteria, white-rot fungus, and other microorganisms. Nevertheless, due to their effectiveness in removing pollutants from aqueous solutions, there has been an increasing emphasis on adsorption techniques. These methods are widely recognized for their efficiency in purifying water by capturing and holding contaminants (Andrunik and Bajda, 2021). Various strategies have been devised to address the significant challenge of safely disposing of pesticides into the environment. However, many of these methods come with high costs, making them impractical for widespread use. Techniques like biological degradation, chemical oxidation, and photocatalysis have been recognized as effective ways to eliminate pesticides, but they can be expensive and require specialized equipment and expertise.
The utilization of activated carbon (AC) for adsorption has become a widely popular, straightforward, and cost-effective method for removing organic contaminants, including pesticides, from water environments. Activated carbon can be derived from a variety of natural sources such as coconut husk, peat, wood coal, or palm shell, and can be produced at a relatively low cost. Activated carbon adsorption is particularly successful in addressing taste, odor, and color issues often associated with organic compounds like pesticides (Zieliński et al., 2022). Therefore, improving water quality through activated carbon adsorption involves not only removing such impurities but also monitoring the concentration of pollutants in water through bench-scale experiments, such as group studies, to understand the removal process. The effectiveness of an adsorbent system can be determined using the sorption capacity parameter from batch experiments. The adsorption capacity of granular activated carbon (GAC) was evaluated using adsorption isotherms, specifically the Freundlich and Langmuir models. The Langmuir isotherm describes monolayer adsorption on a homogenous surface with a finite number of binding sites, while the Freundlich isotherm represents a heterogeneous surface with varying adsorption energies.
Materials and Methods
Preparation of adsorbate and adsorbent
Malathion, containing 95% active ingredient, was acquired from Sigma Aldrich. Solutions of malathion were prepared using distilled water from a Mili-Q water purifier (Millipore Corporation). The activated carbon (AC) used as the adsorbent, both coal and peat-based, was sourced from an overseas manufacturer. The supplied raw material was small, spherical, and varied in color from black to brownish, with standard sizes ranging between 0.8–1.5 mm. The activated carbon was then crushed and sieved into two sizes (1.0 mm and 0.063 mm), cleaned of particles and contaminants with distilled water, oven-dried for 24 hours at 110 °C, and subsequently stored in plastic containers for future use.
Physical and chemical analysis
To understand the mechanism of adsorption, the adsorbent was thoroughly characterized by examining its various physical and chemical properties. The preliminary behavior of the selected pesticide, malathion, was determined spectrophotometrically using a Cary 50 UV Spectrophotometer at 230 nm kmax. kmax referring to the wavelength at which the pesticide absorbs light most strongly during spectrophotometric analysis. Separately, the surface morphology was examined using scanning electron microscope (SEM), model JOEL JSM 6360 LA, Japan. The SEM analysis was performed at various magnifications, including 500x, 1500x, and 10000x, to capture details across different scales. The samples’ surface area and pore size distributions were assessed using a nitrogen adsorption–desorption technique on a Micro-meritics ASAP 2010 surface area analyzer. BET method is utilized to determine the surface area of an adsorbent material by exposing a sample of activated carbon to a known quantity of an adsorbate gas, typically nitrogen, at a specific low temperature (77K). The amount of gas adsorbed at various pressures is measured, and this data is used to calculate the surface area of the substance.
The absorbance data of malathion, recorded at thirty-minute intervals during the adsorption process, were converted into concentration data using the associated calibration curves. This spectrophotometric monitoring was pivotal in assessing the adsorption of malathion onto activated carbon derived from both coal and peat. Consequently, plots of the concentrations over time were generated to visually represent the process.
To evaluate the performance of the activated carbon adsorbents (coal- and peat-based) across a temperature range of 30 to 80°C, experiments were conducted using two distinct particle sizes: 1.0 mm and 0.063 mm. The adsorption kinetics were determined by vigorously shaking a 250 mL conical flask containing 1.0 g of the carbon samples and 20 mL of a malathion solution, with an initial concentration of 7 µg L-1. This setup was facilitated by a Bioscience Agitator, model 74574, which maintained the samples at room temperature (approximately 25°C) and agitated them at a steady speed of 150 rpm as recommended by Hamadi et al. (2004). To study the removal kinetics of malathion, samples of the solution were taken at 30-minute intervals, ranging from 30 to 300 minutes. The malathion content in the filtrate was then measured spectrophotometrically at the adsorbate’s maximum absorption wavelength (ϕmax) of 230 nm. For each sorbent size, three samples were used throughout the course of three hours to ensure accuracy and consistency in the results obtained.
The amount of malathion adsorbed (qe in mg g-1) was determined by the Equation 1.
Where C0 and Ce are the initial and final concentrations, respectively of the adsorbate in solution (µg L-1), V the volume of solution (L) and m is the mass of adsorbent (g).
Analytical statistics
For the purpose of determining the interactions between components, all research findings were thoroughly examined in triplicate using Origin 2022 software (Origin Lab Corp., USA). Graphical analyses were then produced. IBM SPSS ver. 28.0 was used to conduct statistical analysis. The data’s homogeneity of variances and normality were confirmed using the Levene’s and Shapiro-Wilk tests, respectively. One-Way Analysis of Variance (ANOVA) was used to assess the sorption and removal efficiency of activated carbon coal-based activated carbon (CBAC) and peat-based activated carbon (PBAC) at various contact times, and the Tukey HSD test was then performed. In this experiment, results were deemed statistically significant at p<0.05.
Results and Discussion
Physical and chemical characterization of GAC
The main focus of this section is on the physical and chemical properties of the activated carbon used in this study. The physical characteristics include the exterior pore size and shape, surface area, pore volume, and mean pore radius. Observation of the granular activated carbon (GAC) utilized revealed clear pores on their surfaces, with variations in pore sizes and shapes being evident. Figures 2A, B showcase the asymmetrical pore distributions, irregular structures, and porous surfaces characteristic of the adsorbents under examination. The exterior pore sizes for the coal-based activated carbon (CBAC) and peat-based activated carbon (PBAC) adsorbents ranged from 2.80 to 4.49 µm and 1.36 to 2.13 µm, respectively. The categorization of the pore sizes of both GACs falls under macropores, which are larger than 50 nm. This categorization implies that these GACs are highly suitable for liquid phase adsorption, facilitating the effective removal of contaminants from solutions. According to the study by Xie et al. (2020), these macropores provide optimal conditions for adsorption, as the adsorbing material can interact with multiple surfaces simultaneously. The size of the pores greatly influences the porosity and the total surface area of the adsorbent. Furthermore, the distribution and number of pores play crucial roles in determining the efficiency with which pollutants are absorbed. The most effective adsorption occurs when the pores are just large enough to allow the pollutants to enter. This precise matching between pore size and pollutant dimension ensures that the adsorbent can capture and retain contaminants efficiently, highlighting the importance of pore structure in the overall adsorption process.
Figure 3A, B illustrate the exterior structures of the adsorbents, coal-based activated carbon (CBAC) and peat-based activated carbon (PBAC), respectively.
CBAC with the pore size ranging from 11 µm to 30 µm is characterized under macropores. These large pores, visible at higher magnification, contribute to the initial transport of adsorbate molecules into the internal structure of the carbon. Whereas PBAC exhibits a more uneven surface structure. The analysis suggests that CBAC possesses a greater integrity of the exterior surface structure in comparison to PBAC. In this study, both adsorbents showed promising performances in the adsorption of malathion; however, CBAC was notably more effective, attributed to its more intact exterior surface structure compared to the PBAC adsorbent. This finding underscores the significant impact that the physical characteristics of adsorbents can have on their efficiency in removing contaminants from solutions.
Table 1: BET surface area of CBAC an PBAC.
|
GAC |
Surface area (m2 g-1) |
Pore volume (cm3 g-1) |
Mean pore radius (Å) |
|
CBAC |
894 |
295 |
18.9 |
|
PBAC |
566 |
187 |
21.6 |
The surface areas of coal-based activated carbon (CBAC) and peat-based activated carbon (PBAC) were measured using the Micromeritics ASAP 2010 instrument. The values for exterior area, pore volume, and pore radius for both types of activated carbons were organized into Table 1. It was found that the specific surface area of CBAC is approximately 20% higher compared to PBAC. This indicates that the additional 20% surface area of CBAC can be attributed to the external surface area of the activated carbon itself. The surface area values for the two granular activated carbons (GACs) used in this study ranged between 500 and 1500 m2g-1, which falls within the typical range for Brunauer–Emmett–Teller (BET; N2, 77K) activated carbon. This finding highlights the variation in physical properties between different types of activated carbon and suggests how these differences can impact their effectiveness in adsorption applications (Zulkefli et al., 2022).
In the context of this study, considering that the mean pore radius of the two types of granular activated carbons (GACs) used is greater than 17 Å, the malathion compounds are expected to adsorb within the range of 17 to 21 Å. This suggests that the size of the malathion molecules fits well within the pore structure of the activated carbons, allowing for efficient adsorption to occur based on the physical characteristics of the adsorbents (Krstic et al., 2008).
Adsorption occurs on the internal surface area of activated carbon. If the molecular size of the adsorbate is larger than the size of the macropores, it cannot penetrate through the structure of the activated carbon. Therefore, considering Malathion has a molecular weight of 330.36 Da and a chemical formula of C10H19O6PS2, it possesses a relatively small molecular size. This facilitates the adsorption process between the adsorbent (activated carbon) and the adsorbate (Malathion). The small size of Malathion molecules allows them to enter the macropores and even smaller pores of the activated carbon, where they can be effectively trapped and removed from the solution (Kadam et al., 2022).
According to the results, the BET surface area of coal-based activated carbon (CBAC) is larger compared to that of peat-based activated carbon (PBAC), measuring 894 m2g-1 and m2g-1, respectively. This difference in surface area can be attributed to the predominant presence of macropore-sized pores in CBAC. These macropores facilitate the transport of the adsorbate to the micropores, where the actual adsorption occurs (Shimizu and Matubayasi, 2021), Therefore, the higher porosity of CBAC compared to PBAC likely contributes to its greater adsorption capacity and a higher percentage of malathion removal. This underscores the importance of macropore presence in enhancing the effectiveness of activated carbon as an adsorbent by allowing better accessibility and transport within the adsorbent structure.
Chemical characterization of all adsorbents used in this research was conducted through analyses of their pH levels, moisture content, and ash content. The findings of these studies are compiled in Table 2, which outlines the physico-chemical properties of the adsorbents.
Table 2: Principal features of GAC.
|
Parameters |
Value |
|
|
CBAC |
PBAC |
|
|
Ash content (on dry basis) (%) |
3.02 ± 0.01 |
8.71+ 0.02 |
|
Moisture content (%) |
2.71 ± 0.00 |
4.71+ 0.08 |
|
pH |
4.36 ± 0.03 |
5.17+ 0.01 |
|
Particle size |
8–20 mesh |
8–20 mesh |
The adsorbent’s pH level can influence its capability to remove contaminants, making it a vital aspect of the adsorption process’s overall efficiency (Iftekhar et al., 2018). Adsorption processes are favored under more acidic conditions, as indicated by the acidic pH levels of the two activated carbons used, suggesting that an acidic environment could interact with the substances being removed, potentially affecting the surface properties of the adsorbents. This aligns with findings by (Iftekhar et al., 2018), which showed that adsorbents with various functional groups can effectively adsorb lanthanum (La) at a slightly acidic pH of 4, as well as at a more acidic pH of 3, due to the electrostatic repulsion between the adsorbent’s surface functional groups and H+ ions. Notably, CBAC exhibits a lower pH compared to PBAC, pointing towards a potentially higher adsorption efficiency. The more acidic pH of CBAC is attributed to the acid washing it undergoes during its activation process, which impacts its adsorption characteristics.
The moisture content values highlight that CBAC, as an impregnated carbon type, possesses a lower moisture content compared to PBAC. This characteristic is crucial since the contaminant removal mechanisms in impregnated carbons predominantly involve chemical reactions occurring within the reagent solutions held in their pores, with lower moisture levels correlating to improved efficiency. The presence of water can hinder the functionality of activated carbon in several ways, one significant issue being the blockage of the pores essential for adsorption. Given that activated carbon operates through attracting and holding pollutant molecules using chemical or physical forces, its porous structure offers an extensive surface area for adsorption activities. When water enters the pores of activated carbon, it can either displace the pollutant molecules or obstruct their entry, diminishing the adsorption efficiency. Furthermore, water exposure can lead to the deterioration or breakdown of activated carbon over time. Despite the inherent stability of activated carbon, continuous exposure to water and varying environmental conditions can lead to its degradation, undermining its ability to efficiently capture contaminants. Thus, CBAC could demonstrate enhanced adsorption effectiveness at lower moisture levels. This observation aligns with findings by Gumus and Okpeku (2015) which indicated that a reduced moisture content ranging from 1 to 1.75% increases the adsorption rate of pollutants.
The ash content of the activated carbons used in this study ranged from 3 to 9%, which is considered low. This low ash content is advantageous for adsorption processes as it can influence the porosity and, ultimately, the efficiency of the adsorbent. According to Sánchez (2011) the acceptable range for ash content in activated carbon is realistically set between 2 to 5%. This standard ensures that the activated carbon retains a high level of purity and efficacy for adsorption applications. Khalili et al. (2000) present the evidence supporting the claim that low ash content adsorbents exhibit higher activity lies in the comparative ash values of CBAC and PBAC. Specifically, with a 3.02% ash content, CBAC demonstrates characteristics of a highly efficient adsorbent. This lower ash content contributes to a more effective adsorption process, likely due to higher purity or less interference from non-carbon elements that make up the ash. On the other hand, PBAC showed a significantly higher ash content at 8.71%. This considerable difference suggests that CBAC, with its lower ash content, would have superior properties for adsorption, affecting its efficacy in removing malathion residues from agricultural wastewater more favorably than PBAC. The lower ash content in CBAC could result in a higher surface area available for adsorption and fewer impurities that could block the adsorption sites, enhancing its ability to clean agricultural wastewater of malathion effectively. This observation aligns with the findings of (Acharya et al., 2009) who assert that effective adsorbents typically have an ash content ranging between 3% and 4.45%. This range is indicative of optimal adsorption capacity and efficiency, underscoring the importance of low ash content in enhancing the performance of adsorbents.
Adsorption isotherms play a vital role in fully leveraging the potential of an adsorbent as they detail the interaction between the adsorbate and the adsorbent. This information is essential for understanding and optimizing the adsorption process (Obaid, 2020). They are fundamental in determining the maximum adsorption capacity of an adsorbent, as they illustrate the extent to which an adsorbent can adsorb substances. Furthermore, they facilitate the evaluation of how suitable an adsorbent is for specific applications (Prasath et al., 2014). As a result, various theoretical models, such as the Freundlich and Langmuir models, are utilized to elucidate the experimental data concerning adsorption. The graphical representation of the adsorption isotherm data for malathion, seen in Figure 4A, B, demonstrates these models in action.
Equation 2 contains the following formulas for a straight line for Langmuir.
Where Ce is the concentration of malathion ions at equilibrium (mg L-1), the amount of malathion adsorbed at equilibrium (mg g-1), and the Langmuir constants a (mg g-1) and b (1 mg-1) correspond to the adsorption energy and capacity, respectively.
The straight-line expressions for Freundlich given by Equation 3 are as follows:
Where Kf and n are the Freundlich constants, which indicate how favorably malathion adsorption occurs onto both activated carbons, taking into account all parameters affecting adsorption capacity.
The empirical constant value of 1/n is linked to the effectiveness of the adsorption energy of activated carbon, whereas its adsorption capacity is indicated by Kf. Table 3 presents a comparative analysis of the Langmuir isotherm between CBAC and PBAC, revealing that CBAC possesses a higher maximum adsorption capacity (qmax) compared to PBAC. This observation is in agreement with the BET surface area and pore volume metrics for CBAC provided in Table 1, which indicate superior values relative to PBAC. The fitting of the experimental data to the Freundlich and Langmuir isotherms is depicted in Figure 4A, B. Given the correlation coefficient (R2) of the Langmuir isotherm model is nearer to one compared to the Freundlich model, the experimental data are concluded to better align with the Langmuir adsorption isotherm, as detailed in Table 3. The assessment incorporating a one-way ANOVA and the subsequent post hoc multiple comparison test (Tukey’s HSD) concludes that within the horizontal axis, groups denoted by the same letter do not exhibit significant statistical differences at a specified significance level (p ≤ 0.05), while those annotated with different letters do present significant statistical discrepancies. Such findings suggest the occurrence of monolayer sorption on a surface comprising a finite number of adsorption sites (Hafizuddin et al., 2021).
Typically, an increased adsorption capacity results from having larger pore volumes and greater surface areas. The difference in surface areas between CBAC, with a value of 894 m2g-1, and CSAC, with a value of 849 m2g-1, led to CBAC having a higher adsorption capacity for malathion, measured at 248.1 and 123.5 mgg-1 for particles of 0.063 mm and 1.0 mm
|
Types of isotherms |
Parameters |
CBAC |
PBAC |
||
|
0.063 mm |
1.0 mm |
0.063mm |
1.0 mm |
||
|
Langmuir |
qmax |
248.1±0.3a |
123.5±1.12b |
113.9±0.97c |
100.6±0.63d |
|
KL |
0.05±0.005a |
0.18±0.03b |
0.39±0.009c |
0.58±0.01d |
|
|
RL |
0.0001±0.003a |
8.4±0.11b |
16.7±0.18c |
24.0±0.73d |
|
|
R2 |
0.977±0.012a |
0.937±0.12a |
0.902±0.003b |
0.917±0.007b |
|
|
Freundlich |
Kf |
11.9 ±0.76a |
17.8 ±0.54b |
34.1+0.22c |
36.3±0.22d |
|
1/n |
0.86 ±0.01a |
0.74 ±0.15b |
0.46±0.1c |
0.39±0.03d |
|
|
R2 |
0.943±0.01a |
0.913±0.01a |
0.725±0.03b |
0.719 ±0.01b |
|
Letters a, b, c, d refers to grouping. (means ± standard deviation.
in diameter, respectively, compared to PBAC’s 113.9 and 100.6 mgg-1. The superior performance of CBAC can be attributed to its larger pore volume and surface area, which are 295 cm3g-1 and 894 m2g-1, respectively. This result also underlines how particle size influences the efficiency of adsorption, with smaller particles demonstrating faster malathion removal. Smaller particle sizes offer a relatively larger exterior contact surface, improving the effectiveness of pesticide removal, more so than what is observed with PBAC. Additionally, CBAC is characterized by a wider range of pore diameters, indicative of a diverse array of surface functional groups that likely contribute to a stronger chemical interaction with the adsorbate (malathion), enhancing adsorption efficiency. Furthermore, the dimensionless factor, RL, being between 0 and 1, signifies favorable adsorption conditions for malathion and CBAC with a particle size of 0.063 mm, leading to increased malathion removal and a higher adsorption capacity value.
The values of the Langmuir constant, which represents the adsorption energy per milligram of adsorbent, denoted as KL, were determined for CBAC and PBAC across two different particle sizes, 0.063 and 1.0 mm. The recorded KL values were 0.05 and 0.18 Lmg-1 for CBAC and 0.39 and 0.58 Lmg-1 for PBAC, respectively. This exhibited a trend where PBAC consistently showed higher KL values for both particle sizes, indicating stronger adsorption energy. This variation is attributed to the differences in the source materials of each adsorbent, which in turn influences the energy dynamics of the adsorption process (Jeppu and Clement, 2012). Despite CBAC having a higher KL value than PBAC, this does not necessarily enhance its adsorption performance. A higher adsorption energy, as indicated by the KL value, does not guarantee an increase in the adsorption capacity.
Adsorption energy refers to the process where molecule interaction with another substance’s surface happens purely through physical means, without any chemical change such as bond formation or breakage. Instead, this interaction is driven by van der Waals forces. Given that CBAC boasts a larger surface area and pore volume compared to PBAC, it inherently has a higher capacity for adsorbing materials. During adsorption, malathion molecules attach to the surface of activated carbon. However, despite PBAC displaying higher adsorption energy, its rate of adsorption falls behind that of CBAC due to the limited number of available sites or space for the adsorption to take place. Consequently, while high adsorption energy might seem advantageous, it can actually impede adsorption performance if the number of adsorptive sites is insufficient.
The adsorption capacities for CBAC are noted to be significantly lower, at 11.9 and 17.8 mgg-1, in comparison to PBAC, which exhibits capacities of 34.1 and 36.3 mgg-1 across particle sizes of 0.063 and 1.0 mm respectively, as demonstrated through variations in the Freundlich isotherm data. However, these values are still notably higher than those recorded for CSAC and PSAC, which ranged from 0.9 to 1.5 mgg-1 for the same adsorbate, malathion (Jusoh et al., 2011). This discrepancy may be attributed to the principles of the Freundlich isotherm, which suggests that adsorption occurs with the formation of a multimolecular layer on the adsorbent’s surface, indicating a different adsorption mechanism at play (Vijayakumar et al., 2011). The diminished adsorption capacity observed can be attributed to the lack of heterogeneity in the adsorption sites on the CBAC surface when interacting with malathion (the adsorbate), as well as CBAC not operating at full efficiency throughout the adsorption process. This suggests that the adsorbate does not uniformly distribute over the adsorbent’s surface, leading to an underutilization of CBAC’s adsorptive potential.
Furthermore, in contrast to PBAC and the behavior described by the Freundlich isotherm, CBAC was subjected to high pressures during its activation process. While this method effectively delineates the relation between adsorption capacities and lower pressure levels, it struggles to accurately predict adsorption values under high pressure conditions (Musah et al., 2022). Consequently, despite CBAC exhibiting a higher empirical constant 1/n, indicative of stronger adsorption energy compared to PBAC, it deviates from the expected Freundlich isotherm model and demonstrates a diminished adsorption capacity, as detailed in Table 3.
Adsorption kinetics of malathion
Effect of contact time: Figure 5 presents the removal of a 7 µgL-1 malathion solution over time, specifically at intervals of 30, 60, 90, 120, 150, 180, 210, 240, 270, and 300 minutes, employing both CBAC and PBAC across the studied particle sizes of 0.063 mm and 1.0 mm. With a consistent carbon dose of 1.5 g, determined as the optimum based on experiments evaluating the impact of carbon dosage, the data revealed that for both types of adsorbents and particle dimensions, a swift adsorption occurred within the initial 60 minutes, reaching a state of equilibrium approximately after 150 minutes. The process of malathion adsorption exhibited an initially high rate, accelerated further post 60 minutes, and achieved about 90% of adsorption across all tested sizes. This observation on adsorption performance aligns with findings from several other studies that offer explanations for similar outcomes. Notably, for the particle sizes of 0.063 and 1.0 mm respectively, the initial concentration of malathion at 7 µgL-1 was reduced to about 1.14 and 1.5 µgL-1 for CBAC, and to 2.87 µgL-1 for PBAC, bringing the final concentrations of malathion well below the safety threshold of 3.0 µgL-1 for malathion in water, as per the Registration Eligibility Decision (RED) (Newhart, 2006).
Based on the investigation into the influence of particle size, it was observed that the smaller 0.063 mm particles showcased a higher adsorption capacity compared to the larger 1.0 mm particles. Across all varieties of Granular Activated Carbons (GACs) examined in this study, the reduced particle size of 0.063 mm demonstrated superior percentage removal compared to the 1.0 mm size, attributed to the increased external surface area provided by the smaller particles. This enhancement in malathion removal efficiency due to the smaller particle size is consistent across all adsorbents tested, stemming from the reduced contact time used in this research. Therefore, to mitigate the adverse effects of particle size on adsorption efficacy, an extension of contact time is recommended. Removal efficiencies of PBAC and CBAC differed significantly at varying contact times, as shown by statistical analysis (p > 0.05). Application of post hoc analyses such as Tukey’s HSD test and the Bonferroni test revealed comparable removal efficiencies at the 150 and 180-minute marks (p > 0.05), achieving over 90% removal. The adsorption process reached equilibrium after 90 and 150 minutes for CBAC and PBAC, respectively, with no further changes in equilibrium concentration observed, leading to the selection of 180 minutes as the optimal contact duration for all experimental runs.
The length of time that activated carbon is in contact with a water pollutant plays a critical role in the adsorption process. This contact duration directly impacts how effectively the activated carbon can adsorb and remove pollutants from water, influencing the overall efficiency of the purification process (Wan Jusoh et al., 2023; Arslan and Pehlivan, 2008; Hamadi et al., 2004). Longer periods of contact between the activated carbon and water contaminants facilitate the extraction of greater amounts of pollutants. The concept of equilibrium is crucial in defining the optimal duration for the adsorption process. At the point of equilibrium, there is no further change in the concentration of the solute in the solution or the surface of the adsorbent. This means the adsorption process has reached a stage where the rate of adsorbate binding to the adsorbent equals the rate of adsorbate release back into the solution, indicating the maximum adsorption capacity under the given conditions.
Effect of carbon dosage: Figure 6 illustrates the impact of varying dosages of CBAC and PBAC adsorbents on the adsorption of malathion, explored within the range of 0.5 to 3.0 g. An initial augmentation in the dosage of activated carbon leads to a marked enhancement in malathion uptake, establishing that even a modest increase in adsorbent quantity significantly boosts the removal efficiency. Beyond certain dosages, any further increase in dose results in a gradual and consistent rise in the efficacy of malathion removal, indicating that the relationship between adsorbent dosage and adsorption efficiency exhibits a positive correlation up to a certain point, beyond which the efficiency gains begin to stabilize. These results are consistent with the findings of (Amuda and Ibrahim, 2006), who reported that the removal efficiency of activated carbons tends to rise with increasing dose. The relationship between the amount of malathion adsorbed and the dosage of the adsorbent is depicted in Figure 6. It was found that elevating the dosage of activated carbons from 0.5, 1.0, 1.5, 2.0, to 3.0 g led to an increase in the quantity of malathion (contaminants) absorbed from the solution, highlighting the adsorbents’ enhanced capacity. This trend was observed consistently across all types of Granular Activated Carbon (GAC) employed in this study. Specifically, the removal efficiency of malathion improved from 45 to 88% for CBAC and from 40 to 75% for PBAC when the dosage of the adsorbent was increased from 0.5 to 1.5 g. Beyond this dosage point, the increase in adsorption efficiency was marginal. The removal efficiency at different adsorbent dosages showed significant variation for both PBAC 0.063 mm and 1.0 mm (F= 359.852, p<0.05 and F= 183.655, p<0.05, respectively). Nevertheless, the Post Hoc Tukey’s HSD test and Duncan test indicated no significant difference in removal efficiency between the 1.5 g and 3.0 g dosages (p>0.05). Hence, these findings are in line with those reported by (Boon, 1998), supporting the conclusion that a 1.5 g dosage of activated carbon is the optimum amount for all the selected GACs in this investigation.
In accordance with the Granular Activated Carbon (GAC) particle sizes examined in this study, it was observed that for both types of GACS used, the finer particle size of 0.063 mm achieved a higher percentage of contaminant removal, reaching up to 88%. This observation mirrors patterns highlighted in earlier sections, where adsorption capacity was found to increase as particle size decreased. Consequently, this indicates that the particle size significantly affects the efficiency of the adsorption process. The rationale behind this is that the adsorption mechanism necessitates an abundance of binding sites, which are more readily available due to the increased external surface area provided by the smaller particles.
As the dosage of adsorbents was increased, it was observed that the sorption density for each type of Granular Activated Carbon (GAC) utilized decreased until further additions of the adsorbent no longer resulted in any increase in overall efficiency for adsorbate removal or led to a reduction in its sorption density. When comparing an equal amount of activated carbon, it was revealed that the removal efficiency of malathion by CBAC was notably higher than that by PBAC. This is illustrated by the fact that, at an identical dosage of activated carbon (approximately 3.0 g), CBAC was capable of removing up to 90% of the adsorbate, whereas PBAC achieved only about an 80% removal rate. These results are consistent with observations made in previous sections that indicated CBAC, with its larger pore sizes and greater BET surface areas compared to PBAC, exhibits superior characteristics for the adsorption process.
CBAC was found to have a specific surface area nearly 20% larger than that of PBAC. This additional 20% in the surface area of CBAC is attributed to the exterior surface area of the activated carbon itself. This feature contributes to CBAC’s enhanced adsorption capacity and a higher percentage of malathion removal when compared to PBAC. The improved efficiency is likely due to CBAC’s more porous structure, which offers an increased number of accessible sorption surfaces (Bansode et al., 2004). The expansion in external surface area is crucial for the uptick in adsorption, providing more sites for the malathion to be adsorbed (Senthilkumaar et al., 2010). This explains the superior performance of CBAC in malathion elimination, emphasizing the importance of surface area and porosity in the effectiveness of adsorption processes.
The availability of more adsorptive sites for the removal process is directly tied to an increase in the exterior surface area (Arslan and Pehlivan, 2008). Consequently, the sorption density is determined by the BET surface area and pore volume of the specific Granular Activated Carbon (GAC) used. Following the assessment of the physical characteristics of the GACs employed in this study, CBAC is positioned at the top rank due to its superior properties, followed by PBAC. This ranking reflects the efficiency of each adsorbent in terms of available surface area and pore structure, which are critical factors for adsorption performance.
Conclusions and Recommendations
In summary, because of its larger BET surface area and pore volume, coal-based activated carbon performs better in industrial applications than peat-based activated carbon. It is also an affordable choice that is widely accessible in big quantities. Activated carbon based on coal (CBAC) exhibited the maximum ability to adsorb malathion; its macropore size increased from 2.80 to 4.49 µm, and it had a specific surface area of 894 m2g-1. Strong correlations were seen between the adsorption process and the experimental data, which was in accordance with the Langmuir adsorption isotherm model. Under the current circumstances, all four GAC types demonstrated encouraging results in eliminating malathion pollutants, with a maximum removal effectiveness of up to 90%. Therefore, eliminating malathion from aqueous solutions is a successful process when activated carbon is used as an adsorbent. A critical step is transferring lab-scale research into large-scale industrial applications. To guarantee the viability and effectiveness of employing activated carbon for malathion removal in actual agricultural effluent treatment facilities, more study and field testing are necessary.
Acknowledgements
This project was financially funded by the Ministry of Higher Education of Malaysia under Project Vot. No. 59112.
Novelty Statement
The exploration of synergetic effects of coal and peat base granular activated carbon, this research plans new territory in the realm of adsorption technology, advancing the field towards more effective and environmentally friendly strategies for pesticide removal in agricultural settings.
Author’s Contribution
Hajjar Hartini Wan Jusoh: Draft the original manuscript, formatting.
Hafizan Juahir: Supervise and comments on the critical part of manuscript.
Nurfarahana Mohd Nasir: Formatting, review and editing on the critical manuscript writing.
Setyo Budi Kurniawan: Formatting, comments on the critical manuscript writing.
Ahmad Jusoh: Funding and comments on the critical manuscript writing.
Azimah Ismail: Comments on the critical manuscript writing.
Conflict of interest
The authors have declared no conflict of interest.
References
Acharya, J., J.N. Sahu, B.K. Sahoo, C.R. Mohanty and B.C. Meikap. 2009. Removal of chromium (VI) from wastewater by activated carbon developed from Tamarind wood activated with zinc chloride. Chem. Eng. J., 150: 25–39. https://doi.org/10.1016/j.cej.2008.11.035
Ahmed, M.S., A.H. Massoud, A.S. Derbalah, A. Al-Brakati, M.A. Al-Abdawani, H.A. Eltahir, T. Yanai and E.K. Elmahallawy. 2020. Biochemical and histopathological alterations in different tissues of rats due to repeated oral dose toxicity of cymoxanil. Animals, 10: 1–14. https://doi.org/10.3390/ani10122205
Amuda, O.S. and A.O. Ibrahim. 2006. Industrial wastewater treatment using natural material as adsorbent. Afr. J. Biotechnol., 5: 1483–1487.
Andrunik, M. and T. Bajda. 2021. Removal of pesticides from waters by adsorption: Comparison between synthetic zeolites and mesoporous silica materials. A review. Materials. https://doi.org/10.3390/ma14133532
Arslan, G. and E. Pehlivan. 2008. Uptake of Cr3+ from aqueous solution by lignite-based humic acids. Bioresour. Technol., 99: 7597–7605. https://doi.org/10.1016/j.biortech.2008.02.007
Bansode, R.R., Losso, J.N., Marshall, W.E., Rao, R.M. and Portier, R.J., 2004. Pecan shell-based granular activated carbon for treatment of chemical oxygen demand (COD) in municipal wastewater. Bioresour. Technol., 94: 129–135. https://doi.org/10.1016/j.biortech.2003.12.009
Bhardwaj, J.K. and P. Saraf. 2014. Malathion-induced granulosa cell apoptosis in caprine antral follicles: An ultrastructural and flow cytometric analysis. Microsc. Microanal., 20: 1861–1868. https://doi.org/10.1017/S1431927614013452
Biswas, B. and S. Goel. 2022. Electrocoagulation and electrooxidation technologies for pesticide removal from water or wastewater: A review. Chemosphere 302: 134709. https://doi.org/10.1016/j.chemosphere.2022.134709
Boelan, E.G. and A.S. Purnomo. 2019. Biodegradation of 1,1,1-trichloro-2,2-bis (4-chlorophenyl) ethane (DDT) by mixed cultures of white-rot fungus ganoderma lingzhi and bacterium pseudomonas aeruginosa. Hayati, 26: 90–95. https://doi.org/10.4308/hjb.26.2.90
Boon, S., 1998. Removal of natural organic matter from Sg. Sireh using local manufactured activated carbon.
Bradford, B.R., E. Whidden, E.D. Gervasio, P.M. Checchi and K.M. Raley-Susman. 2020. Neonicotinoid-containing insecticide disruption of growth, locomotion, and fertility in Caenorhabditis elegans. PLoS One, 15. https://doi.org/10.1371/journal.pone.0238637
Cosgrove, S., B. Jefferson and P. Jarvis. 2019. Pesticide removal from drinking water sources by adsorption: A review. Environ. Technol. Rev., 8(1): 1-24. https://doi.org/10.1080/21622515.2019.1593514
Derbalah, A., A. Massoud and A. Ahmed. 2021. Recent advances in the removal of pesticides from water sources: A review of the efficacy of various methods. J. Environ. Manage., 284: 112116.
Derbalah, A., A. Massoud, I. El-Mehasseb, M.S. Allah, M.S. Ahmed, A. Al-Brakati and E.K. Elmahallawy. 2021. Microbial detoxification of dimethoate and methomyl residues in aqueous media. Water (Switzerland) 13. https://doi.org/10.3390/w13081117
Goh, P.S., N.A. Ahmad, T.W. Wong, L.T. Yogarathinam and A.F. Ismail. 2022. Membrane technology for pesticide removal from aquatic environment: Status quo and way forward. Chemosphere, 307: 136018. https://doi.org/10.1016/j.chemosphere.2022.136018
Gumus, R.H. and I. Okpeku. 2015. Production of activated carbon and characterization from snail shell waste (andlt; iandgt; Helixandlt;/iandgt; andlt; iandgt; pomatiaandlt; /iandgt;). Adv. Chem. Eng. Sci., 5: 51–61. https://doi.org/10.4236/aces.2015.51006
Hafizuddin, M.S., C.L. Lee, K.L. Chin, P.S. H’ng, P.S. Khoo and U. Rashid. 2021. Fabrication of highly microporous structure activated carbon via surface modification with sodium hydroxide. Polymers (Basel) 13. https://doi.org/10.3390/polym13223954
Hamadi, N.K., S. Swaminathan and X.D. Chen. 2004. Adsorption of Paraquat dichloride from aqueous solution by activated carbon derived from used tires. J. Hazard. Mater., 112: 133–141. https://doi.org/10.1016/j.jhazmat.2004.04.011
Hanapi, N.H.M., H. Monajemi, A. Ismail, A. Suhaili, A. Endut and H. Juahir. 2022. Biodegradation of textile dye wastewater with the application of response surface methodology (RSM): A factorial design approach. Trends Sci., 19. https://doi.org/10.48048/tis.2022.4168
Iftekhar, S., D.L. Ramasamy, V. Srivastava, M.B. Asif and M. Sillanpää. 2018. Understanding the factors affecting the adsorption of Lanthanum using different adsorbents: A critical review. Chemosphere, 204: 413–430. https://doi.org/10.1016/j.chemosphere.2018.04.053
Jeppu, G.P. and T.P. Clement. 2012. A modified langmuir-freundlich isotherm model for simulating pH-dependent adsorption effects. J. Contam. Hydrol., 129–130: 46–53. https://doi.org/10.1016/j.jconhyd.2011.12.001
Jusoh, A., W.J.H. Hartini, N. Ali and A. Endut. 2011. Study on the removal of pesticide in agricultural run off by granular activated carbon. Bioresour. Technol., 102: 5312–5318. https://doi.org/10.1016/j.biortech.2010.12.074
Kadam, U.S., K.H. Trinh, V. Kumar, K.W. Lee, Y. Cho, M.H.T. Can, H. Lee, Y. Kim, S. Kim, J. Kang, J.Y. Kim, W.S. Chung and J.C. Hong. 2022. Identification and structural analysis of novel malathion-specific DNA aptameric sensors designed for food testing. Biomaterials, 287: 121617. https://doi.org/10.1016/j.biomaterials.2022.121617
Kamarudin, M.K.A., N.A. Wahab, N.A.A. Jalil and S.M.H.M. Sunardi. 2020. Water quality issues in water resources management at Kenyir Lake, Malaysia. J. Teknol., 82: 1–11. https://doi.org/10.11113/jt.v82.14173
Khalili, N.R., M. Campbell, G. Sandi and J. Golaś. 2000. Production of micro- and mesoporous activated carbon from paper mill sludge: I. Effect of zinc chloride activation. Carbon N. Y., 38: 1905–1915. https://doi.org/10.1016/S0008-6223(00)00043-9
Khuman, S.N., P.G. Vinod, G. Bharat, Y.M. Kumar and P. Chakraborty. 2020. Spatial distribution and compositional profiles of organochlorine pesticides in the surface soil from the agricultural, coastal and backwater transects along the south-west coast of India. Chemosphere, 254. https://doi.org/10.1016/j.chemosphere.2020.126699
Krstic, D.Z., M. Colovic, M. Bavcon Kralj, M. Franko, K. Krinulovic, P. Trebse and V. Vasic. 2008. Inhibition of AChE by malathion and some structurally similar compounds. J. Enzyme Inhib. Med. Chem., 23: 562–573. https://doi.org/10.1080/14756360701632031
Massoud, A., A. Derbalah, I. El-Mehasseb, M.S. Allah, M.S. Ahmed, A. Albrakati and E.K. Elmahallawy. 2021. Photocatalytic detoxification of some insecticides in aqueous media using tio2 nanocatalyst. Int. J. Environ. Res. Publ. Hlth., 18. https://doi.org/10.3390/ijerph18179278
Musah, M., Y. Azeh, J. Mathew, M. Umar, Z. Abdulhamid and A. Muhammad. 2022. Adsorption kinetics and isotherm models: A review. Caliphate J. Sci. Technol., 4: 20–26. https://doi.org/10.4314/cajost.v4i1.3
Newhart, K., 2006. Environmental fate of malathion.
Obaid, S.A., 2020. Langmuir, freundlich and tamkin adsorption isotherms and kinetics for the removal aartichoke tournefortii straw from agricultural waste. J. Phys. Conf. Ser., 1664: 012011. https://doi.org/10.1088/1742-6596/1664/1/012011
Prasath, R.R., P. Muthirulan and N. Kannan. 2014. Agricultural wastes as a low cost adsorbents for the removal of Acid Blue 92 dye: A Comparative study with Commercial activated carbon. IOSR J. Agric. Vet. Sci., 7: 19–32. https://doi.org/10.9790/2380-07231932
Sánchez, A.J., 2011. Characterization of activated carbon produced from coffee residues by chemical and physical activation (Master’s thesis). KTH Chemical Science and Engineering, Stockholm, Sweden.
Senthilkumaar, S., S.K. Krishna, P. Kalaamani, C.V. Subburamaan and N.G. Subramaniam. 2010. Adsorption of organ ophosphorous pesticide from aqueous solution using “waste” jute fiber carbon. Modern Appl. Sci., https://doi.org/10.5539/mas.v4n6p67
Shimizu, S. and Matubayasi, N., 2021. Adsorbate-adsorbate interactions on microporous materials. Microporous and Mesoporous Mater., 323: 111254. https://doi.org/10.1016/j.micromeso.2021.111254
Ssomad, M.A.H.A., N.A. Husin, M. Hudzari, H. Razali, H.N. Azuan, R.M. Hudzari, M.N.A. Noordin and E. Azizah. 2016. Water quality monitoring at Bukit Nenasi highland irrigation in Kuala Terengganu, in: 3rd National Conference on Knowledge Transfer.
Trellu, C., H.O. Vargas, E. Mousset, N. Oturan and M.A. Oturan. 2021. Electrochemical technologies for the treatment of pesticides. Curr. Opin. Electrochem., 26: 100677. https://doi.org/10.1016/j.coelec.2020.100677
Tudi, M., Ruan, H.D., Wang, L., Lyu, J., Sadler, R., Connell, D., Chu, C. and Phung, D.T., 2021. Agriculture development, pesticide application and its impact on the environment. Int. J. Environ. Res. Publ. Health, https://doi.org/10.3390/ijerph18031112
Vijayakumar, G., R. Tamilarasan and M. Dharmendirakumar. 2011. Adsorption, kinetic, equilibrium and thermodynamic studies on the removal of basic dye rhodamine-B from aqueous solution by the use of natural adsorbent perlite. J. Mater. Environ. Sci., 3: 157–170.
Wan Jusoh, H.H., H. Juahir, M.N. Nasir, A. Jusoh and N.A. Mahiddin. 2023. Granular activated carbon optimization for enhanced environmental disaster resilience and malathion removal from agricultural effluent. E3S Web Conf., 437: 03006. https://doi.org/10.1051/e3sconf/202343703006
Xie, B., J. Qin, S. Wang, X. Li, H. Sun and W. Chen. 2020. Adsorption of phenol on commercial activated carbons: Modelling and interpretation. Int. J. Environ. Res. Publ. Hlth., 17. https://doi.org/10.3390/ijerph17030789
Zieliński, B., P. Miądlicki and J. Przepiórski. 2022. Development of activated carbon for removal of pesticides from water: Case study. Sci. Rep., 12(1): 20869. https://doi.org/10.1038/s41598-022-25247-6
Zulkefli, N.N., A.M.I. Noor-Azam, M.S. Masdar, N.A. Baharuddin, W.N.R. Wan Isahak and M.N. Sofian. 2022. Performance and characterization of bi-metal compound on activated carbon for hydrogen sulfide removal in biogas. Molecules, 27. https://doi.org/10.3390/molecules27249024
To share on other social networks, click on any share button. What are these?