Olive Cultivars and Harvest Index Determines Quality of Olive Oil
Research Article
Olive Cultivars and Harvest Index Determines Quality of Olive Oil
Riaz Alam1*, Muhammad Sajid2, Imtiaz Hussain3, Gulzar Ullah1, Hussain Shah3, Muhammad Arshad Farooq3 and Rashid Muhammad4
1PARC-PFRS ARI, Tarnab Peshawar, Khyber Pakhtunkhwa, Pakistan; 2Department of Horticulture, The University of Agriculture, Peshawar, Khyber Pakhtunkhwa, Pakistan; 3Pakistan Agricultural Research Council (PARC), Islamabad, Pakistan; 4Ecotoxicology Research Program, National Agricultural Research Centre, Islamabad, Pakistan.
Abstract | The oil extracted from Manzanilla, Picual, Ottobratica, Pendolino, and Frontoio olive cultivars were assessed for quality attributes at various stages of harvesting, i.e., Lemon green, Semi-ripe and Ripe. The extracted oil from Manzanilla fruits has lower Peroxide Value (POV) (3.21 meq kg-1) with high chlorophyll (4.58 mg L-1) as well as Phenol (616.67 mg kg-1) contents. The extracted oil from Frontoio fruits exhibited a lower percentage of Free Fatty Acids (FFA) (0.43%) and a higher carotene content (2.70 mg L-1). These quality values fell within the established standards for extra virgin olive oil category by International Olive Council (IOC, 2003) except for the FFA levels in Ottobratica fruit ripe stage oil that met the criteria for virgin olive oil category. The semi-ripe stage fruits oil recorded maximum total phenols (530 mg kg-1) along with lower FFA percentage (0.25%) as well as POV (3.04 meq kg-1). In contrast, the extracted oil from ripe stage fruits had a lower phenol (361.67 mg kg-1) as well as elevated FFA (1.50%) and POV (4.43 meq kg-1), Furthermore, there was a significant reduction in carotenoids and chlorophyll content from 3.17 to 1.49 mg L-1 and 4.99 to 2.41 mg L-1, respectively in the extracted oil as fruits progressed from lemon green stage to ripe stage of harvesting. Olive cultivars Frontoio, Manzanilla and Picual are recommended for the production of extra virgin oil, while semi-ripe stage of harvesting is the optimum stage for quality oil extraction in olives in the local environmental conditions of Sangbhatti, Mardan-Pakistan.
Received | August 03, 2023; Accepted | December 09, 2023; Published | February 01, 2024
*Correspondence | Riaz Alam, PARC-PFRS ARI, Tarnab Peshawar, Khyber Pakhtunkhwa, Pakistan; Email: riazalamhort@gmail.com, riazalam@parc.gov.pk
Citation | Alam, R., M. Sajid, I. Hussain, G. Ullah, H. Shah, M.A. Farooq and R. Muhammad. 2024. Olive cultivars and harvest index determines quality of olive oil. Sarhad Journal of Agriculture, 40(1): 100-108.
DOI | https://dx.doi.org/10.17582/journal.sja/2024/40.1.100.108
Keywords | Olive cultivars, Harvesting stages, Free fatty acids, Peroxide value, Phenols, Oil quality
Copyright: 2024 by the authors. Licensee ResearchersLinks Ltd, England, UK.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Introduction
Olive is an important commodity in many parts of the world because of its economic payback (Fernández and Moreno, 2000) and potential health benefits (Gutiérrez et al., 1999; Larsen et al., 1999; Ranalli et al., 1999; Visioli and Galli, 1998). The response of olive cultivars to environmental conditions and specific traits is influenced by their genetic inheritance (Lavee and Wodner, 2004; Tubeileh et al., 2008), however the genotype greatly influence particular traits compared to the climate (Lanteri et al., 2002). Olive oil production is determined by the genotype and environment (Mailer, 2005; Ceci and Carelli, 2010), and the interaction between these two factors greatly influence the ultimate quality of olive oil (Torres et al., 2009; Mannina et al., 2001).
Olive fruit and its oil composition to some extent is determined by the variety of the oil as well as the extent of fruit ripeness and is an important factor for the stability of virgin olive oil which changes as the olive ripens. The acidity, peroxide index, oxidative stability, pigments, phenolic compounds and fatty acid composition is decreased while the oil content increased during ripening (Gutiérrez et al., 1999). To produce high-quality olive oil, its essential to harvest the fruits when they reach the right stage of maturity without damaging their skins, and then processed within a window of 12 to 24 hours after harvesting, fruits are needed to be graded by quality and processed separately (Hermoso Fernández et al., 1998).
The duration of oil accumulation period and rate of oil synthesis directly influence the oil concentration in fleshy mesocarp portion of olive fruit (Trentacoste et al., 2012) which in turn establish the harvesting index. The capacity of oil synthesis and fruit development has been identified in different genotypes (Hammami et al., 2011; Lavee and Wodner, 2004; Trentacoste et al., 2010). The variation in skin and flesh colours due to pigmentation relate to fruit ripening and altering in its oil’s chemical composition (Beltrán et al., 2004). Oils extracted from olive fruits that have undergone a washing process are usually low in quality with reduction in bitterness and flavour (Hermoso Fernández et al., 1998; Ranalli et al., 1999) also the extra moisture can decrease oil extraction efficiency due to formation of water and oil emulsions.
Olive is considered as a sub-tropical plant that can thrive well in the mountainous areas of Balochistan and Khyber Pakhtunkhwa provinces of Pakistan (Baloch, 1994). An estimated number of 80 million wild olives (Olea cuspidata) are cultivated in Balochistan, newly merged areas of Khyber Pakhtunkhwa Province (formerly Federally Administered Tribal areas) and Potohar region of Punjab, demonstrating successful olive adaptation in Pakistan (Bonji and Palliotti, 1994).
Although different olive cultivars such as Frontoio, Pendolino and Manzanilla are being cultivated in Pakistan (Campus, 2021), there is limited scientific information available about thse introduced olive cultivars in the country. To date, limited research work has been done on olive cultivars and harvesting stages in Pakistan. Assessment of promising olive cultivars and determination of appropriate harvest index for quality oil production will lead toward augmentation of olive cultivation in the country and will assist self-reliance in olive oil production that are of high edible value.
Materials and Methods
The experimental research was carried out at Olive Model Farm Sangbhatti Mardan, Khyber Pakhtunkwa Pakistan and involved five olive cultivars namely Picual, Frontoio, Ottobratica, Manzanilla and Pendolino. The trees were spaced at 6 x 6 meters and the soil in this farm is classified as silt loam (0.67 % organic matter and a pH of 7.00). The region experiences an average rainfall of approximately 600 mm annually, which occurs primarily during July and August with dry season spanning May and June. The lowest temperature varied from 2 °C in Dec-Jan and 25 °C in June-July whereas the highest temperature ranged from 18 to 38 °C.
For this study, three olive trees having similar size were assigned to each treatment and were observed on daily basis for taking relevant data. Soil ploughing four times annually via a cultivator along with routine cultural practices were carried out during the experimental period. Additionally, every tree was provided annually with well-rotten Farm Yard Manure at a rate of 15 kg during winter season while inorganic fertilizers specifically Di-ammonium Phosphate (DAP) at the rate of 1.5 kg and Urea at the rate of 3 kg (administered in three equal split doses).
For the purpose of olive oil extraction, a total of three plants from each cultivar were harvested and biochemical analysis of oil extracted at each ripening stage was carried out in the laboratories for qualitative traits.
Fruits from all the studied cultivars were hand-picked at each stage of harvesting (lemon green, semi ripe and ripe) and safely transported in ventilated plastic buckets to the olive oil extraction mill at ARI Tarnab, Peshawar. The oil samples extracted from each cultivar at each harvesting stage were stored in dark brown glass bottles at room temperature (15-25 °C). All the biochemical analyses repeated thrice for each sample and were carried out at National Agricultural Research Centre, Islamabad and Biochemistry Laboratory, Agricultural Research Institute Tarnab, Peshawar. The analyses were completed within one month after processing of the oils.
Free fatty acid (FFA) percentage
The acid value or free fatty acid, given as percent of oleic acid was determined by directly titration of the oil in an alcoholic medium against standard potassium hydroxide solution. Weighed twenty eight gram oil sample in a conical flask added 50 ml neutralized ethanol along with 2 ml of phenolphthalein indicator then heated the mixture on hot plate at 40 oC. Titrated the solution with standard KOH, shacked vigorously until the pink colour, stable for at least thirty seconds was obtained. The results were presented as percentage oleic acid; the expression is given according to American Oil Chemists Society, Official Method Ca 5a-40.
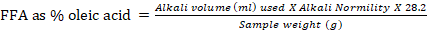
Where 28.2 is the molecular weight of oleic acid (282) divided by 10.
Peroxide value “POV” (meq kg-1)
A 5 g sample was placed into 250 ml Erlenmeyer flask and 30 ml of solvent mixture (3:2, acetic acid: chloroform) was added. The mixture was then swirled to achieve dissolution and 0.5 ml of saturated solution of KI was introduced and shaken well for 60 seconds. To stop the reaction, 30 ml distilled water was added and titration with 0.1N sodium thiosulfate continued until the yellow iodine colour vanished. Then 2.0 ml starch solution was added with titration continued along with shaking to release all iodine from solvent layer. Thiosulfate solution was further added drop by drop until the blue colour disappeared. A blank experiment was performed wherein the blank titration required no more than 0.1 ml of 0.1N sodium thiosulfate solution.

Total phenol (mg kg-1)
Total phenol from each oil sample of each treatment was determined spectrophotometrically according to the Folin Ciocalteu method. Ten gram of olive oil was dissolved in 50 ml hexane was further extracted with a 20 ml of 60% methanol. The mixture was vigorously shaken for 120 seconds and the resulting extract was then evaporated to dryness using a vacuum rotary evaporator at 40 °C. The residue was subsequently reconstituted in 1 ml of methanol.
Total phenol concentration was determined in the methanolic extract with Folin-Ciocalteu reagent. A 0.1ml sample was diluted with water to a final volume of 5 ml in a 10 ml volumetric flask. To this 0.5 ml Folin Ciocalteu reagent was added and left for 180 seconds before adding 1 ml of saturated Na2Co3 solution. The content was determined after an hour at a wavelength of 725 nm against a reagent blank. For the calibration curve, caffeic acid was used as a standard, ranging 100-1000 µg/100 ml assay solution.
Chlorophyll value (mg L-1)
Chlorophyll was quantified for the oil samples extracted at each treatment with a spectrophotometer based on the Beer Lambert Law and the extinction coefficient for chlorophyll following the Arnon equation for quantification of the total chlorophyll content in an 80% acetone extract.
Total chlorophyll (µg ml-1) = 20.2 (A645) + 8.02 (A663)
Where A663 is the solution absorbance at 663 nm and A645 is the absorption at 645.
Carotenoids (mg L-1)
Carotenoids were quantified for the oil samples extracted at each treatment with a spectrophotometer based on the Beer Lambert Law and the extinction coefficient for carotenoids following the Lichtenthaler and Welburn (1983) equation for carotenoids content in 80 % acetone extracts:
Carotenoids (µg ml-1) = (1000A470 - 3.27(chl a)) - 104(chl b) / 227
Statistical procedure
The experiment was conducted following the Randomized Complete Block Design (RCBD). The Statistix 8.1 software was used to analyse the recorded data and LSD (Least Significant Difference) test at P ≤ 0.05 was applied for mean comparison (Steel and Torrie, 1980).
Results and Discussion
Free fatty acid (%)
Significant differences (P ≤ 0.05) were found for free fatty acid (FFA) in the oils extracted from fruits of olive cultivars and for the oil extracted from the fruits harvested at different stages and also for their interaction. The more FFA percentage (1.02%) was detected in oil of Ottobratica fruits that was significantly different from the other cultivars extracted oil; followed by the FFA (0.80%) determined in the Pendolino’s fruits extracted oil however less percentage of FFA (0.43%) was noted in Frontoio’s fruits extracted oil. Considering harvest stages, extracted oil from fruits showed a decreasing and then increasing trend for the FFA percentage. For lemon green stage of harvest, the recorded FFA in the extracted oil was 0.36% while for the semi-ripe stage of harvest, the extracted oil from fruits resulted in decreased percentage that is 0.25%. At ripe stage of harvest, FFA enhanced to 1.50% in the oil extracted from fruits (Table 1). Regarding the interaction between cultivars and harvesting stages, FFA (%) was more in oil extracted from Ottobratica fruits at ripe stage whereas a lower FFA percentage was observed in oil extracted from Frontoio fruits at semi ripe stage (Figure 1). The FFA expressed as acidity of olive oil increased during fruit maturation and is probably due to the activity of endogenous lipases which increased with the development of maturity (Kiritsakis and Markakis, 1984; Kirtisakis and Tsipeli, 1992). In the present trial, acidity decreased in the extracted oil in the fruits that were harvested at lemon green stage to semi ripe and then increased, it might be due to the lipases got more activation from semi-ripe to ripe stage of maturation. However, acidity of oil was lower for all the studied cultivars at lemon green and semi ripe stages, even it was less at ripe stage for Frontoio; than the maximum limit (≤ 0.8%) set for extra virgin grade oil, by the International Olive Council. The oil acidity of remaining cultivars, extracted at ripe stage qualified the standard of virgin category (≤ 2.0), except Ottobratica (2.23%), which fulfil for ordinary virgin grade (≤ 3.3).
Table 1: FFA (%), POV (meq kg-1), total phenol (mg kg-1), chlorophyll (mg L-1) and carotenoids (mg L-1) contents of oil of olive cultivars as affected by harvesting stages.
|
Olive cultivars (Cv) |
Parameters |
||||
|
FFA (%) |
POV (meq kg-1) |
Total phenol (mg kg-1) |
Chlorophyll (mg L-1) |
Carotenoids (mg L-1) |
|
|
Frontoio |
0.43d |
3.44d |
451.67c |
4.22b |
2.70a |
|
Manzanilla |
0.48c |
3.21e |
616.67a |
4.58a |
2.51b |
|
Ottobratica |
1.02a |
4.09a |
330.00e |
3.10e |
2.11c |
|
Pendolino |
0.80b |
3.80b |
370.60d |
3.33d |
2.03d |
|
Picual |
0.79b |
3.57c |
473.33b |
3.18c |
2.17d |
|
LSD (α = 0.05) |
0.0304 |
0.0515 |
1.0522 |
0.0743 |
0.0641 |
|
Harvesting stages (S) |
|||||
|
Lemon green |
0.36b |
3.40b |
453.67b |
4.99a |
3.17a |
|
Semi-ripe |
0.25c |
3.04c |
530.00a |
4.07b |
2.26b |
|
Ripe |
1.50a |
4.43a |
361.67c |
2.41c |
1.49c |
|
LSD (α = 0.05) |
0.0236 |
0.0399 |
0.8150 |
0.0575 |
0.0497 |
|
Interaction between olive cultivars and harvesting stages (Cv × S) |
|||||
|
Significance levels |
* (Figure 1) |
*(Figure 2) |
*(Figure 3) |
*(Figure 4) |
* (Figure 5) |
Peroxide value (meq kg-1)
The statistical analysis of the data revealed that cultivars and harvesting stages as well as their interaction had a significant impact on the Peroxide value (POV) at P ≤ 0.05. The fruits of Ottobratica produced the oil with high Peroxide value (4.09 meq kg-1), followed by the POV (3.80 meq kg-1) for the oil of Pendolino, while low peroxide value (3.21 meq kg-1) was determined in the oil of Manzanilla. The harvesting stages greatly influenced the oil quality in terms of peroxide value as it turned down in the extracted oil when fruits were harvested at lemon green stage toward semi ripe which then turned up in the oil at ripe stage of harvesting. The maximum peroxide value (4.43 meq kg-1) was observed in the extracted oil in the fruits that were picked at ripe stage, followed by POV (3.40 meq kg-1) in the extracted oil of fruits picked at lemon green stage, while minimum peroxide value (3.04 meq kg-1) resulted in the extracted oil of fruits picked at a stage of semi-ripening (Table 1). The interactive effect between cultivars and harvesting stages showed that high peroxide value was determined in the oil extracted from Ottobratica fruits at ripe stage, whereas oil from Manzanilla fruits at semi-ripe stage resulted in low value (Figure 2). The oxidation is initiated in the fruit and increases slowly during fruit maturation, resultantly peroxide value proceed at a same trend in oil (Kiritsakis and Markakis, 1984). The increase might be related to lipoxygenases which exist in the fruit (Mohamed-Mousa et al., 1996) and also correlated with phenol content (Osman et al., 1994) similar changes occurs during ripening in olive varieties with marked decrease in POV (Gutiérrez et al., 1999), the behaviour can be explained by decrease in the activity of the enzyme lipoxygenase in the studied cultivars. The peroxide values obtained in the present study are much lower than the 20 meq kg-1 limit, set by the International Olive Council, for the extra virgin grade of olive oil.
Total phenol (mg kg-1)
Statistically analysed data showed that the cultivars and harvesting stages as well as interaction of both had affected the total phenol contents of oils significantly, extracted from fruits of olive cultivars. The maximum value of total phenol (616.67 mg kg-1) was observed in the extracted oil from the cultivar Manzanilla’s fruits, followed by the phenol contents (473.33 mg kg-1) noted in extracted oil samples of Picual’s fruits, while less amount (330.00 mg kg-1) was recorded in oil of Ottobratica. The harvesting stages influenced the accumulation of total phenol in olive oil and enhanced from lemon green stage toward semi ripe and then turned down from semi-ripe to ripe stage of harvesting. More phenols (530.00 mg kg-1) were found in extracted oil from the semi-ripe stage fruits, followed by 453.67 mg kg-1 phenol recorded at lemon green while the oil from ripe stage fruits revealed lower amount (361.67 mg kg-1) (Table 1). The interaction between cultivars and harvesting stages showed that maximum phenol content was noted in the oil of Manzanilla fruits picked at semi ripe stage, while oil of Ottobratica fruits harvested in their ripe stage showed lower amount (Figure 3). There is no official limit for polyphenol content, findings of the present study provided sufficient variability for olive cultivars to polyphenol content in their respective oils, similar results were obtained by (Vossen, 2005) in olive cultivars which produced oil with variation in polyphenol content ranges from the very high to very low levels. It is of key significance to keep a check of any fluctuations in phenolic compounds concentration in olive oil as these substances directly influence oil’s resistance to oxidation and organoleptic characteristics. The phenolic content in the olive fruit flesh varies depending on the degree of pigmentation which shows a medium level at the green stage increasing to high at the semi black and ultimately decreasing to low levels at the black stage (Fantozzi, 1978). The polyphenol content reaches its optimum level in the semi black stage in case of olives and hence results in better quality olive oils. This pattern of polyphenol content varying from low to high as olives maturity stage change from the green to semi-black, followed by a decrease at the black stage, is also in accordance with the study of Mahhou et al. (2012). The phenol compounds present in the virgin olive oils are one of the bases of the nutritional importance of oil (Beltrán et al., 2005) and the phenol content within the range of 200-450 ppm was recorded in the oil of olive cultivar Hojiblanca (Uceda et al., 1992).
Chlorophyll value (mg L-1)
Olive cultivars and harvesting stages as well as their interaction recorded significant difference regarding the chlorophyll contents of olive oil. The more chlorophyll value (4.58 mg L-1) was found in the oil of cultivar Manzanilla fruits, followed by chlorophyll value (4.22 mg L-1) recorded in oil of Frontoio, while less chlorophyll content (3.10 mg L-1) was noted in the Ottobratica fruits oil. The linear reduction was observed in the chlorophyll value observed in the extracted oil of fruits at different stages. Extracted oil from lemon green stage fruits showed higher chlorophyll content (4.99 mg L-1), followed by 4.07 mg L-1 in oil of semi ripe stage fruits, while lower chlorophyll value (2.41 mg L-1) was noted in oil in ripe stage fruits (Table 1). Regarding interaction data of cultivars and harvesting stages, chlorophyll content was more in lemon green stage fruits oil of cultivar Manzanilla, while minimum value of chlorophyll was noted in oil of Pendolino, at ripe stage of harvesting (Figure 4). Chlorophyll is recognized as one of the primary pigments of olive oil possesses antioxidant properties (Dabbou et al., 2011) and can have a significant influence on olive oil regarding its quality and oxidation stability. The quantity of these valuable pigments exhibit considerable variation contingent upon factors such as fruit cultivar, the stage of ripeness and the method used for oil extraction (Aguilera et al., 2005; Desouky et al., 2009; Rotondi et al., 2004). The olive cultivars show different amount of chlorophyll in oil and the contents decrease throughout ripening stage whereas the chlorophyll disappear marginally higher than that of carotenoids (Zaringhalami et al., 2015). The content of chlorophyll pigments and carotenoids decreased markedly during ripening in olive varieties (Gutiérrez et al., 1999). In a study, reported that Chlorophyll content of olive decreased as the ripening index increased Furthermore, the climatic conditions and varieties influenced the chlorophyll content (Škevin et al., 2003). Results of the present study are in conformity with the findings of the above cited authors.
Carotenoids value (mg L-1)
There were significant variations at P ≤ 0.05 among olive cultivars, harvesting stages and their interaction regarding carotenoids value determined in olive oil. The maximum carotenoids value (2.70 mg L-1) was recorded in extracted oil of Frontoio fruits, followed by carotenoids value (2.51 mg L-1) noted in the oil of Manzanilla, while minimum value (2.03 mg L-1) was observed in the oil extracted of Pendolino’s fruits. Regarding different harvesting stages, a gradual decline in carotenoids value was noted in extracted oil from fruits. More carotenoids (3.17 mg L-1) were recorded in the extracted oil of fruits that were picked at lemon green stage, followed by carotenoids contents (2.26 mg L-1) noted in the extracted oil of semi ripe stage fruits, while less quantity of carotenoids (1.49 mg L-1) was observed in the extracted oil at ripe stage (Table 1). In the interaction high value of carotenoids was determined in the oil extracted from Frontoio fruits, picked at lemon green stage, while lower amount was determined in oil of Pendolino ripe stage fruits (Figure 5). The olive cultivars produce different amount of pigments in oil, also the carotenoids contents decrease throughout ripening process (Zaringhalami et al., 2015). The total pigment contents (chlorophylls + carotenoids) decreased in olive with ripening and oil obtained from less ripe fruits had more pigmentation, indicated the highest values for the chlorophyll and carotenoids (Gandul-Rojas et al., 2000). The same trend was observed in the present trial.
Conclusions and Recommendations
On the basis of average data collected, the cultivar Frontoio showed promising among the studied cultivars under the local conditions of Sangbhatti, Mardan-Pakistan and deserves great focus for production of extra virgin grade oil. The cultivars Manzanilla and Picual were categorized for good oil extraction having more phenols and chlorophyll contents and less peroxide value, qualify for extra virgin oil grade. The oil of Pendolino just qualified for extra virgin olive grade in terms of FFA, however, peroxide value was within the range set for extra virgin oil grade. The quality of extracted oil from the Ottobratica ripe stage fruits in terms of FFA was not satisfactory and fulfilled the requirements of virgin category. The extracted oil from semi ripe stage fruits qualified for all the quality tests, ranked as extra virgin grade.
Olive cultivars: Frontoio Manzanilla and Picual are recommended to be cultivated for production of extra virgin oil under the local agro-climatic conditions of Sangbhatti, Mardan and other similar environments. Pendolino is also recommended, however marginally qualified for extra virgin olive grade in terms of free fatty acid percentage. The fruits of Ottobratica are not recommended to be harvested at ripe stage of harvesting for quality oil extraction in terms of FFA, which fulfilled the requirements of virgin category. Olive fruits are strongly recommended to be harvested for quality oil extraction at semi ripe stage.
Novelty Statement
Olive fruits harvested at appropriate stage of maturity for extraction of quality oil production will determine the grade according to the standard of international olive council.
Author’s Contribution
Riaz Alam: Planned the experiment, conducted the experiment, data collected and analyses, writeup of the manuscript.
Muhammad Sajid: Facilitated in planning of the experiment, statistically analyses the data.
Imtiaz Hussain: Supervision, conceptualization.
Gulzar Ullah: Data collection, sample analysis.
Hussain Shah: Facilitated in data collection, coordinated for sample collection and analyses.
Muhammad Arshid Farooq: Collected review of literature to support of experiment, proof reading of the paper.
Rashid Muhammad: Analyses of oil samples.
Conflict of interest
The authors have declared no conflict of interest.
References
Aguilera, M.P., G. Beltrán, D. Ortega, A. Fernández, Jiménez A. and M. Uceda. 2005. Characterisation of virgin olive oil of Italian olive cultivars: Frantoio and Leccino, grown in Andalusia. Food Chem., 89: 387-391. https://doi.org/10.1016/j.foodchem.2004.02.046
Baloch, A., 1994. Hortic. Phases of plant growth. National Book Foundation Islamabad, pp. 633.
Beltrán, G., M.P. Aguilera, C. Del Rio, S. Sanchez and L. Martinez. 2005. Influence of fruit ripening process on the natural antioxidant content of Hojiblanca virgin olive oils. Food Chem., 89: 207-215. https://doi.org/10.1016/j.foodchem.2004.02.027
Beltrán, G., C. del Río, S. Sánchez and L. Martínez. 2004. Seasonal changes in olive fruit characteristics and oil accumulation during ripening process. J. Sci. Food Agric., 84: 1783-1790. https://doi.org/10.1002/jsfa.1887
Bonji, G. and A. Palliotti. 1994. Olive in hand book of environmental physiology of fruit crop, Anderson CRC Press Inc., Boca Raton.
Campus, A.M.K., 2021. Assessment of phenological, carpometric and yield allied attributes of olive cultivars harvested at different maturity stages. J. Pure Appl. Agric., 6: 44-53.
Ceci, L. and A. Carelli. 2010. Compositional data review of monovarietal Argentinean olive oils. Advances in fats and oil research. Transworld Res. Netw. Kerala, India, pp. 71-97.
Dabbou, S., F. Brahmi, S. Dabbou, M. Issaoui, S. Sifi and M. Hammami. 2011. Antioxidant capacity of Tunisian virgin olive oils from different olive cultivars. Afr. J. Food Sci. Technol., 2: 92-97.
Desouky, I., L.F. Haggag, M. Abd El-Migeed and E. El-Hady. 2009. Changes in some physical and chemical properties of fruit and oil in some olive oil cultivars during harvesting stage. World J. Agric. Sci., 5: 760-765.
Fantozzi, P., 1978. Dosage des composés phénoliques dans les drupes d’olives récoltés à différents stades de maturation.
Fernández, J. and F. Moreno. 2000. Water use by the olive tree. J. Crop Prod., 2: 101-162. https://doi.org/10.1300/J144v02n02_05
Gandul-Rojas, B., M.R.L. Cepero and M.I. Mínguez-Mosquera. 2000. Use of chlorophyll and carotenoid pigment composition to determine authenticity of virgin olive oil. J. Am. Oil Chem. Soc., 77: 853-858. https://doi.org/10.1007/s11746-000-0136-z
Gutiérrez, F., B. Jimenez, A. Ruiz and M. Albi. 1999. Effect of olive ripeness on the oxidative stability of virgin olive oil extracted from the varieties Picual and Hojiblanca and on the different components involved. J. Agric. Food Chem., 47: 121-127. https://doi.org/10.1021/jf980684i
Hammami, S.B., T. Manrique and H.F. Rapoport. 2011. Cultivar-based fruit size in olive depends on different tissue and cellular processes throughout growth. Sci. Hortic., 130: 445-451. https://doi.org/10.1016/j.scienta.2011.07.018
Hermoso Fernández, M., J. Gonzáles, M. Uceda, A. García-Ortiz, J. Morales, L. Frías and A. Fernández. 1998. Elaboración de aceite de oliva de calidad II—Obtención por el sistema de dos fases. Manual from the Estación de Olivicultura y Elaiotecnia. Finca “Venta del Llano” Mengíbar (Jaén), Spain. Junta de Andalucía 61.
Kiritsakis, A. and P. Markakis. 1984. Effect of olive collection regime on olive oil quality. J. Sci. Food Agric., 35: 677-678. https://doi.org/10.1002/jsfa.2740350614
Kirtisakis, A. and A. Tsipeli. 1992. Hydrolysis and oxidation of olive oil during the time that olive fruit remain on the tree. Riv. Ital. Sostanze Grasse, 69: 453-456.
Lanteri, S., C. Armanino, E. Perri and A. Palopoli. 2002. Study of oils from Calabrian olive cultivars by chemometric methods. Food Chem., 76: 501-507. https://doi.org/10.1016/S0308-8146(01)00370-3
Larsen, L.F., J. Jespersen and P. Marckmann. 1999. Are olive oil diets antithrombotic? Diets enriched with olive, rapeseed, or sunflower oil affect postprandial factor VII differently. Am. J. Clin. Nutr., 70: 976-982. https://doi.org/10.1093/ajcn/70.6.976
Lavee, S. and M. Wodner. 2004. The effect of yield, harvest time and fruit size on the oil content in fruits of irrigated olive trees (Olea europaea), cvs. Barnea and Manzanillo. Sci. Hortic., 99: 267-277. https://doi.org/10.1016/S0304-4238(03)00100-6
Mahhou A., Y. Nabil, A. Hadiddou, A. Oukabli and A. Mamouni. 2012. Performance of the arbequina, haouzia and Menara olive varieties in rainfed conditions in the Meknès region of Morocco. Olivæ, pp. 3-21.
Mailer, R., 2005. Variation in oil quality and fatty acid composition in Australian olive oil. Aust. J. Exp. Agric., 45: 115-119. https://doi.org/10.1071/EA04045
Mannina, L., G. Fontanazza, M. Patumi, G. Ansanelli and A. Segre. 2001. Italian and Argentine olive oils: A NMR and gas chromatographic study. Grasasy Aceites, 52: 380-388. https://doi.org/10.3989/gya.2001.v52.i6.348
Mohamed-Mousa, Y., D. Gerasopoulos, I. Metzidakis and A. Kiritsakis. 1996. Effect of altitude on fruit and oil quality characteristics of ‘Mastoides’ olives. J. Sci. Food Agric., 71: 345-350. https://doi.org/10.1002/(SICI)1097-0010(199607)71:3<345::AID-JSFA590>3.0.CO;2-T
Osman, M., I. Metzidakis, D. Gerasopoulos and A. Kiritsakis. 1994. Qualitative changes in olive oil of fruits collected from trees grown at two altitudes. Riv. Ital. Sostanze Grasse, 71: 187-190.
Ranalli, A., M. Ferrante, G. De Mattia and N. Costantini. 1999. Analytical evaluation of virgin olive oil of first and second extraction. J. Agric. Food Chem., 47: 417-424. https://doi.org/10.1021/jf9800256
Rotondi, A., A. Bendini, L. Cerretani, M. Mari, G. Lercker and T.G. Toschi. 2004. Effect of olive ripening degree on the oxidative stability and organoleptic properties of cv. Nostrana di Brisighella extra virgin olive oil. J. Agric. Food Chem., 52: 3649-3654. https://doi.org/10.1021/jf049845a
Škevin, D., D. Rade, D. Štrucelj, Z. Mokrovšak, S. Neđeral and D. Benčić. 2003. The influence of variety and harvest time on the bitterness and phenolic compounds of olive oil. Eur. J. Lipid Sci. Technol. 105: 536-541. https://doi.org/10.1002/ejlt.200300782
Steel, R.G.D. and J.H. Torrie. 1980. Principles and procedures of statistics, a biometrical approach McGraw-Hill Kogakusha, Ltd.
Torres, M.M., P. Pierantozzi, M.E. Cáceres, P. Labombarda, G. Fontanazza and D.M. Maestri. 2009. Genetic and chemical assessment of Arbequina olive cultivar grown in Córdoba province, Argentina. J. Sci. Food Agric., 89: 523-530. https://doi.org/10.1002/jsfa.3483
Trentacoste, E.R., C.M. Puertas and V.O. Sadras. 2010. Effect of fruit load on oil yield components and dynamics of fruit growth and oil accumulation in olive (Olea europaea L.). Eur. J. Agron., 32: 249-254. https://doi.org/10.1016/j.eja.2010.01.002
Trentacoste, E.R., C.M. Puertas and V.O. Sadras. 2012. Modelling the intraspecific variation in the dynamics of fruit growth, oil and water concentration in olive (Olea europaea L.). Eur. J. Agron., 38: 83-93. https://doi.org/10.1016/j.eja.2012.01.001
Tubeileh, A., F. Turkelboom, M. Abdeen and A. Al-Ibrahem. 2008. Fruit and oil characteristics of three main Syrian olive cultivars grown under different climatic conditions. Acta Hortic., 791: 409-414. https://doi.org/10.17660/ActaHortic.2008.791.60
Uceda, M., M. Frías and M. Ruano. 1992. Diferenciación de variedades de aceituna por la composición ácidica de su aceite, First International Symposium on Olive Growing. Córdoba. Spain. pp. 35-38.
Visioli, F. and C. Galli. 1998. The effect of minor constituents of olive oil on cardiovascular disease: New findings. Nutr. Rev., 56: 142-147. https://doi.org/10.1111/j.1753-4887.1998.tb01739.x
Vossen, P., 2005. Olive oil production. Olive production manual. Olive production manual. Sibbet, SG and Ferguson, L. (Eds.) University of California. Agriculture and natural resources. USA. Publication 3353: 157-173.
Zaringhalami, S., M. Ebrahimi, Z.P. Vanak and A. Ganjloo. 2015. Effects of cultivar and ripening stage of Iranian olive fruit on bioactive compounds and antioxidant activity of its virgin oil. Int. Food Res. J., 22.
To share on other social networks, click on any share button. What are these?








