Oil Palm Empty Fruit Leverage Concerning Delignification and Fermentation in Sheep
Research Article
Oil Palm Empty Fruit Leverage Concerning Delignification and Fermentation in Sheep
Firda Dimawarnita1*, Edwina Gabriela Tesalonika Mustamu2, Yora Faramitha1, Haryo Tejo Prakoso1, Wildan Aulia Noorsy1, Komang Gede Wiryawan2
1Indonesian Oil Palm Research Institute, Jalan Taman Kencana No. 1 Bogor, West Java, Indonesia; 2Animal Science Department of Agricultural Faculty, IPB University, Bogor, West Java, Indonesia.
Abstract | Empty Fruit Bunches (EFB) are the by-product of the palm oil plantation industry. EFB has tremendous lignin content and low palatability. We aimed to assess the impact of EFB supplementation by the delignification process and in-vitro rumenal fermentation. The treatments were raw EFB (P0) and delignified EFB (P1). We evaluated rumen pH, total volatile fatty acids (VFA), ammonia concentration (NH3), dry matter digestibility (DMD), crude fiber digestibility (CFD), organic matter digestibility (OMD), methane gas production, fulvic acid content, and bacterial population. The results indicated that the treatment did not have a significant effect on DMD, OMD, pH, NH3 concentration, methane gas, and bacterial populations. Treatment showed a significant result (P<0.05) on total VFA. The contents of fulvic acid in raw and delignified EFB, both unfermented and fermented using rumen fluid in-vitro, were around 6.12-6.46%. The use of delignified EFB can accelerate rumen fermentation and increase VFA production.
Keywords | Coefficient of digestibility, Delignification, Empty fruit bunches of palm oil, Fulvic acid, In-vitro, Volatile fatty acid
Received | July 27, 2023; Accepted | December 03, 2023; Published | January 10, 2024
*Correspondence | Firda Dimawarnita, Indonesian Oil Palm Research Institute, Jalan Taman Kencana No. 1 Bogor, West Java, Indonesia; Email: [email protected]
Citation | Dimawarnita F, Mustamu EGT, Faramitha Y, Prakoso HT, Noorsy WA, Wiryawan KG (2024). Oil palm empty fruit leverage concerning delignification and fermentation in sheep. Adv. Anim. Vet. Sci., 12(1):180-188.
DOI | https://dx.doi.org/10.17582/journal.aavs/2024/12.1.180.188
ISSN (Online) | 2307-8316
Copyright: 2024 by the authors. Licensee ResearchersLinks Ltd, England, UK.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
INTRODUCTION
A feed crisis plagues the world because of the continued demand for feed while raw material production remains stagnant or even decreases (Awasthi et al., 2020). One effective way to address this issue is using biorefinery from empty fruit bunches (EFB). Biorefineries have the potential to enable a circular bio-economy by connecting flows and cycles, thereby increasing the value of certain products. They convert biomass into a wide array of goods and fuels, including intermediate and end products such as feed, food, components (fibers, starches, and wood), chemicals, heat, oil, gas, minerals, electricity, and water (de Jong and Jungmeier, 2015; Zhou and Xu, 2019). Utilizing agricultural waste products, like EFB from the oil palm industry, offers an effective solution to this challenge. Processing 1 ton of fresh fruit bunches results in approximately 22–23% EFB, equivalent to 220–230 kg of EFB (Dimawarnita et al., 2022). EFBs are either left to decompose naturally or burned for ash production and then used as fertilizer. Over the past decade, the quality of Indonesian oil palm plantations has significantly improved (Rulli et al., 2019).
Synonym: The growth of Indonesian oil palm plantations has been significant, marked by an annual expansion of 367 thousand hectares from 2000 to 2010. It produces approximately 9.5 million tons of EFB annually, largely unused as animal feed. Feed quality is determined by digestibility value and directly affects livestock productivity (Iqbal et al., 2015). High digestibility means a more significant portion of nutrients absorbed by livestock. Yang and Rode (2001) stated that digestibility can be expressed in terms of organic matter (OM) and dry matter (DM), so that in percentage, it can be called the digestibility coefficient. EFB is a significant solid waste of approximately 6 million tonnes, but its utilization remains limited. However, there is potential to use EFB as animal feed. Research has shown that the optimal level of palm oil use in feed is around 40% (Silalahi et al., 2018). The inclusion of EFB in cattle rations typically ranges from 30-50%, and the EFB needs to be chopped into suitable sizes (2 cm long) for consumption. Sumathi et al. (2008) categorized EFB as a fiber feed of inferior quality, characterized by its high lignin content (14%) and protein, as well as its low digestibility and palatability (Wu et al., 2020). This poses a challenge since livestock generally do not prefer EFB as feed. Nevertheless, studies have investigated the potential EFB as animal feed through the delignification process.
There is potential for other ingredients to be used as livestock feed additives, such as fulvic acid (FA). FA is a fraction of humic compounds that can dissolve in acidic and alkaline solutions. Compared to other fractions of humic compounds, FA has the most negligible molecular weight, approximately 2000 Daltons. It has a high oxygen content, ranging from 45-48% (Islam, 2005). FA serves several functions, including enhancing nutrient availability and absorption in ruminant digestion, facilitating nutrient transfer, catalyzing enzymatic reactions and cellular processes, stimulating cell metabolism and synthesis, and improving water and gas absorption in cell membranes (Bai et al., 2013). The delignification process can also increase the availability of FA in animal feed ingredients, commonly referred to as humus extraction for fertilizer. Adding fulvic acid to sheep feed has been demonstrated to promote growth, enhance meat quality and also nutrient utilization efficiency in sheep (Wang et al., 2020). Thus, we aimed to assess the impact of EFB supplementation by the delignification process and in-vitro rumenal fermentation.
MATERIALS AND METHODS
Material
The primary materials utilized in the study were raw EFB and delignified EFB (obtained from Indonesian Oil Palm Research Institute). The nutrient content of EFB was analyzed at the Feed Science and Technology Laboratory, IPB University. The other materials utilized were rumen fluid (obtained from local sheep in the Cattle Nutrition Meat, Faculty of Animal Husbandry, IPB University), Mc Dougall (Merck KGaA), Vaseline (Unilever), distilled water (Indonesian Oil Palm Research Institute), boric acid indicator (Thermo Fisher Scientific), mercury chloride (HgCl2) solution (for analysis EMSURE Reag. Ph Eur,ACS, Merck KGaA), 0.2% HCl (for analysis EMSURE Reag. Ph Eur,ACS, Merck KGaA), pepsin (from porcine gastric mucosa, for biochemistry, Merck KgaA), saturated HgCl2 (for analysis EMSURE Reag. Ph Eur,ACS, Merck KGaA), saturated sodium carbonate (Na2CO3) anhydrous (for analysis EMSURE ISO, Merck KGaA), 0.05N H2SO4 (for analysis EMSURE ISO, Merck KGaA), 0.5 N HCl (for analysis EMSURE ISO, Merck KGaA), 15% Sulfuric acid (H2SO4) (for analysis EMSURE ISO, Merck KGaA), 0.5N Sodium hydroxide (NaOH) (pellet for analysis EMSURE® Merck KGaA), 0.1% phenolphthalein indicator (indicator ACS, Reag. Ph Eur, Merck KGaA), TBSF (Tris buffered saline, with Tween 20, Merck KGaA), CMC (Sodium carboxymethyl cellulose) (Merck KGaA), hemin (Merck KGaA), resazurin (Resazurin sodium salt, Merck KGaA), cysteine (for biochemistry, Merck KGaA), brain-heart infusion (BHI) medium (NutriSelect® Plus, suitable for microbiology, Merck KGaA), starch (soluble GR for analysis, Merck KgaA), and glucose (D(+)-Glucose anhydrous for biochemistry Reag., Merck KGaA).
Study procedure
Delignification of oil palm empty bunches
EFB was dried in an oven (Memmert universal oven UN55, Memmert GmbH), cooled, weighed to 50 gram, and added to NaOH solution (120 g/1 liter of distilled water). The mixture was agitated and homogenized using a hot plate stirrer (Thermo scientific, SP88854100) at 600-700 rpm/2 hours to facilitate delignification. When the temperature reaches 90oC, it can be increased to 180oC while ensuring the stirrer remains stable. After 2 hours, the lignin sample was filtered using a filter cloth or gauze, and the filtered samples were rinsed using hot water until the pH of the samples reached 7.
Rumen fluid collection
Hot water at 40-50oC was provided in a thermos. The contents of the sheep’s rumen were then extracted using a stomach tube inserted through the mouth. The collected rumen fluid was filtered and transferred into a hot water thermos. The thermos was tightly sealed and brought to the laboratory for further testing on in-vitro fermentability and digestibility tests.
In-vitro technique
The in-vitro experiment was carried out after Terry (1963). The method involves using a 100 ml fermenter tube (artificial rumen) with Mc Douglass’s solution (a substitute for sheep’s saliva or rumen fluid). Each sample weighing 0.5 g was added to the fermenter tube, 40 ml of McDougall’s solution and 10 ml of rumen fluid. CO2 was introduced inside the tube for 30 seconds and sealed with a ventilated rubber cap to create anaerobic conditions. The tube was positioned within a shaker bath and fermented for 4 hours. The rubber cap was removed to stop the fermentation, and two drops of saturated HgCl2 were introduced to exterminate the microbes. The tube was subsequently centrifuged at 3000 rpm/15 minutes, then the supernatant was collected to analyze ammonia (NH3) and volatile fatty acid (VFA).
Preparation of mcdougall’s buffer solution
McDougall Buffers were prepared (11.76 g of NaHCO3 (for analysis EMSURE ISO, Merck KGaA), 5.58 g of Na2HPO4.7H2O (for analysis EMSURE ISO, Merck KGaA), 0.684 g of KCl (for analysis EMSURE ISO, Merck KGaA), 0.564 g of NaCl (for analysis EMSURE ISO, Merck KGaA), and 0.144 g of MgSO4.7H2O (for analysis EMSURE ISO, Merck KGaA) in an Erlenmeyer. Distilled water was then added with the primary purpose of diluting the mixture and was subsequently homogenized using a magnetic stirrer. Then, the material weighed was added to the Erlenmeyer and dissolved with distilled water using a magnetic stirrer. Once dissolved, 0.48 g of CaCl2 (for analysis EMSURE ISO, Merck KGaA) was added. Distilled water was included until reaching a volume of 1200 ml, and the solution was redissolved using a magnetic stirrer. The solution was subjected to a flow of CO2 to lower the pH to 6.8.
pH measurement
The samples that underwent a 4-hour incubation were subjected to analysis using a calibrated pH meter (HI98107 Hanna) (calibrated with Buffer solutions 4 and 7). CO2 was added to the supernatant, and the resulting pH was measured. The electrode rods of the pH meter were washed thoroughly before switching to a different sample.
Analysis of digestibility coefficient of dry matter and organic matter
The OMD and DMD were quantified after Terry (1963). In-vitro samples were positioned in a 100 ml fermenter tube and incubated anaerobically in a water bath shaker for 48 hours. Two drops of HgCl2 were added and, the contents were centrifuged at 3000 rpm/10 minutes. Subsequently, the precipitate was placed back into the fermenter tube and mixed with 50 ml of pepsin and 0.2% HCl solution. The fermenter tube underwent incubation for 48 hours at 39°C within a water bath shaker. The remnants post-incubation were filtered through Whatman 41 filter paper, and the obtained residue was collected and stored in a porcelain cup and subjected to baking for 24 hours at 105oC.
To calculate the DMD, insert the cup containing the residue into the 600oC furnace (Nabertherm GmbH) for 4 hours and cool it using a desiccator for 15 minutes. Then, the cup was weighed again, and the OMD was calculated. The DMD and OMD can be calculated using the following formulas:


Crude fiber digestibility coefficient analysis
Following in-vitro incubation, the CF analysis was conducted following the AOAC (2005) method. One gram from the sample was placed in a beaker with a heater extract tool. 50 mL of 0.3N H2SO4 was heated for 30 minutes. A Pre-weighed filter paper, previously heated in an oven at 105oC for 1 hour, was used with a Buchner funnel, using either a vacuum pump (ROCKER 300) or water jet. The remnants were successively rinsed with 50 mL of hot water, 50 mL of 0.3N H2SO4, and 25 mL of acetone. The filter paper along with its contents was moved to a porcelain cup and subjected to drying in an oven (Memmert universal oven UN55, Memmert GmbH) at 105oC for 1 hour. After cooling inside a desiccator (Thermofisher scientific), the cup was weighed. The cup was returned to the furnace, cooled again, and weighed to determine the crude fiber content. The digestibility of crude fiber can be calculated using the following formula:

Ammonia (NH3) analysis
The NH3 concentration analysis was performed using the Conway microdiffusion technique (O’Malley, 1942). One ml of centrifuged rumen fluid was blended with 1 ml of saturated Na2CO3 solution. The edge of the Conway cup was evenly smeared with Vaseline, and the rumen fluid was dripped onto the left end, the Na2CO3 (for analysis EMSURE ISO, Merck KGaA) solution into the right end, and the boric acid (Thermo Fisher Scientific) solution to the center of the Conway cup. The Conway cup was sealed to create a vacuum condition and left undisturbed for 24 hours. Later, the boric acid solution exhibited a blue color change and was then subjected to titration using H2SO4 (for analysis EMSURE ISO, Merck KGaA) solution until pink color development. The NH3 concentration was determined utilizing the subsequent formula:
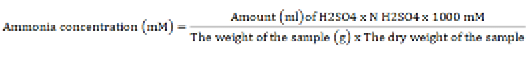
Partial VFA analysis
VFA was analyzed using the gas chromatography technique. The in-vitro samples were incubated in a 100 ml fermenter tube in a water bath shaker (Memmert, wtb50) and incubated anaerobically for 48 hours. A 1.5 ml portion of the filtrate was obtained and centrifuged at 1200 rpm/10 minutes. Subsequently, the filtrate was injected into the gas chromatography system (Shimadzu GC-2014). The curve results on the chromatogram can be utilized to calculate the VFA using the following formula.
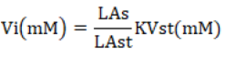
Information: Vi = Individual VFA levels (mM); LAs = Sample area and LAst chromatogram: Standard area of VFA. Individual; KVs = Standard VFA concentrations consist of acetate (40 mM), propionate (10 mM), butyrate (10 mM); Vi = Individual VFA levels (mM); LAs = Sample area and LAst chromatogram: Standard area of VFA; Individual KVs = Standard VFA concentrations consist of acetate (40 mM), propionate (10 mM), butyrate (10 mM).
Total VFA analysis
The total VFA concentration can be assessed using steam distillation (O’Malley, 1942). Set up a distillation apparatus with boiling water and allow the steam to flow into the condenser. A 5 ml portion of the supernatant sample was then taken and placed in a distillation tube, which was heated using steam. One milliliter of 15% H2SO4 solution was introduced into the distillation tube. containing the supernatant, and then the tube was closed tightly. The distillation results were collected in an Erlenmeyer flask that contains 5 ml of 0.5 N NaOH solution until the volume reached approximately 250 ml. The phenolphthalein (PP) indicator was added to an Erlenmeyer flask containing two drops of distillate, and the mix was titrated using 0.5 N HCl until a light pink coloration appeared.
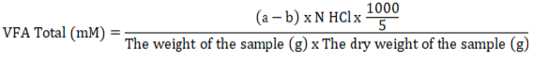
Information: a = blank titrant volume (ml); b = sample titrant volume (ml); N = normality of HCl solution
Measurement of methane gas production
The estimation of methane was calculated using the partial VFA proportion approach. Estimated methane can be calculated with the following formula (Angela and John, 2000).
CH4= 0.45 (Acetate) − 0.275 (Propionate) + 0.40 (Butyrate)
Measurement of total fulvic acid
1 g sample was weighed, and 25 ml of 0.1 N Na-pyrophosphate solution was added. The mix was shaked for 30 minutes. Allow to stand overnight, and the mixture underwent filtration using filter paper. 1 ml or 1 g of filtrate was pipetted into a 100 ml volumetric flask, and 5 ml of K2Cr2O7 solution (2 N) and 7.5 ml of H2SO4 were added. The spectrophotometer (Double beam UV-VIS, Shimadzu AA700) was used to measure the absorbance of the mixture at a wavelength of 561 nm.
Bacterial population count
The rumen bacterial population was calculated by culturing the samples in Brain Heart Infusion (BHI, 3.7 g BHI flour, 0.05 g of glucose, 1 ml of CMC 1%, 0.05 g of starch, 0.05 g of cysteine-HCl, 0.5 ml of Hemin, 0.05 ml of resazurin and aquadest till 100 ml) agar. The diluent media was made by mixing 0.3 g of Na2CO3 (for analysis EMSURE ISO, Merck KGaA) and 0.1 g of cysteine (for biochemistry, Merck KGaA) with distilled water in an Erlenmeyer tube. Then 7.5 ml of Minerals and 0.05 ml of Resazurin (Merck KGaA) were added and homogenized while flowed by CO2 gas until the color turned clear. The mixture was heated until the color changed from yellowish brown to reddish or orange, cooled, and flowed by CO2 gas to create anaerobic conditions, creating a yellowish color. Five mL of media was placed inside a Hungate tube (Thermo Fisher Scientific) containing 0.15 g of Bacto agar and sterilized using an autoclave (GEA medical LS-50 Lj) at 121°C for 15 minutes. Dilution was conducted by adding 0.05 mL of rumen fluid into 5 mL of the first diluent medium, and then the second, third, fourth, and subsequent dilutions were conducted with the same procedure. Each sample obtained from the diluent tube was taken 0.1 mL and subsequently inoculated into the agar medium. The bacteria cultivated on BHI agar medium were placed in an incubator at 39°C for 24 hours, followed by the enumeration of colony counts. The bacterial population is calculated by the following formula:
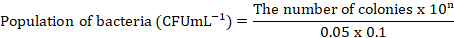
Information: n = serial dilution tube
Data analysis
The collected data underwent analysis using analysis of variance (ANOVA), with subsequent application of the Duncan Multiple Range Test in case significant differences were observed (SAS Institute, 2003). The statistical software used in this study is SPSS software (version 28, IBM).
RESULT AND DISCUSSION
The feed ingredients used in the study were raw EFB and delignified EFB with nutrient contents, as follows in Table 1.
Delignification modifies the chemical structure of lignocellulose biomass to selectively degrade lignin and break its chemical bonds, including covalent, hydrogen, and van der Walls bonds, with other chemical components. By dissolving the lignin content in the material, delignification facilitates the separation of lignin from the fiber. Delignification can be achieved through physical (thermal), chemistry, and biological means (Saritha et al., 2012). The delignification process begins with the absorption of NaOH solution by the EFB wall, leading to hydrolysis and the formation of hydroxide ions. These hydroxide ions break the ethylene and ester bonds in lignin, resulting in easier lignin biodegradation. As the NaOH solution is absorbed, the lignin and cellulose components in EFB swell, enhancing the accessibility of cellulose to cellulase enzymes and facilitating the release of lignin from EFB fibers by optimizing temperature and pH to optimize lignin breakdown. The hydroxide ions from the NaOH solution react with the phenolic groups in lignin and produce simpler organic compounds such as phenolic acids. The delignification process contributes to releasing the lignin content without causing damage to the cellulose and hemicellulose content in the material (Dimawarnita et al., 2022).
Table 1: Nutrient content of oil palm empty bunches.
|
Nutrient contents (%) |
Treatments |
|
|
P0 |
P1 |
|
|
Dry Ingredients* |
88.86 |
99.13 |
|
Ash Content* |
4.15 |
1.60 |
|
Crude protein* |
8.02 |
5.56 |
|
Crude Fat* |
0.53 |
0.20 |
|
Coarse Fiber* |
50.96 |
59.11 |
|
BETN* |
36.34 |
33.52 |
|
Lignin* |
17.25 |
7.95 |
|
Hemicellulose* |
27.27 |
14.40 |
|
ADF* |
73.58 |
77.90 |
|
Cellulose* |
56.19 |
59.42 |
|
Silica* |
0.14 |
0.44 |
|
NDF* |
99.16 |
92.29 |
|
TDN** |
46.87 |
44.29 |
Fermentability test
The in-vitro fermentability of EFB can be assessed by examining the rumen pH value and the concentration of fermented products, such as total volatile fatty acids (VFA) and ammonia (NH3). The effect of delignification on rumen fermentability is presented in Table 2. The untreated (control) and delignified EFB exhibit no significant effect on pH and ammonia concentration. However, a significant result (P<0.05) was shown on the total concentration of volatile fatty acids (VFA).
Table 2: Effect of delignification of oil palm empty fruit bunches on rumen fermentability.
|
Parameters |
Treatments |
P value |
|
|
P0 |
P1 |
||
|
pH |
6.26 ± 0.30 |
6.52 ± 0.59 |
NS |
|
NH3 (mM) |
1.94 ± 0.23 |
1.91 ± 0.37 |
NS |
|
VFA(mM) |
42.20 ± 6.54b |
73.58 ± 14.97a |
0.003 |
|
Partial VFA (%) |
|||
|
Acetate |
48.84 ± 4.25 |
49.17 ± 8.16 |
NS |
|
Propionate |
21.26 ± 3.16 |
21.49 ± 3.16 |
NS |
|
Butyrate |
12.06 ± 1.01 |
11.59 ± 2.01 |
NS |
|
Iso Butyrate |
1.87 ± 0.50 |
1.79 ± 0.39 |
NS |
|
Valerat |
1.50 ± 0.37 |
1.21 ± 0.49 |
NS |
|
Iso Valerat |
2.42 ± 0.41 |
2.45 ± 0.77 |
NS |
|
Methane gas (CH4) (mM) |
7.02 ± 0.91 |
6.22 ± 1.45 |
NS |
P0 = raw EFB, P1 = delignified EFB, NS = Not significance. Different superscripts on the same line with different letters show significant differences (P<0.05).
Rumen pH value
pH (Farobie et al., 2022) plays a fundamental role in regulating various processes within the rumen. It supports the growth of rumen microbes and influences the production of NH3 and VFA (Abderzak, 2012). The rumen pH was not significantly affected but remained within the normal range of 6.25-6.26 (McDonald et al., 2010). Rumen microorganisms function optimally at a pH of 5.5-6.5.
Ammonia concentration (NH3)
Ammonia (NH3) is the primary product of the fermentation of feed protein within the rumen via rumen microbes. Higher concentrations of NH3 indicate increased protein fermentation in the rumen. Rumen microbes utilize NH3 for their metabolism (Cobellis et al., 2016). The results indicated low NH3 levels in the rumen compared to the optimal range for rumen microbial activity (6–21 mM) (McDonald et al., 2010). The low ammonia levels in this study may be attributed to the low protein content of the feed samples used, as reflected in the nutrient content table of EFB, which shows a protein content of only 7.13%. Insufficient protein in animal feed leads to low rumen ammonia concentrations, which can hinder the growth of rumen microorganisms and subsequently affect carbohydrate breakdown (McDonald et al., 2010).
The delignification process of EFB involves the removal of a significant portion of lignin, a non-carbohydrate organic component present in empty fruit bunches. This lignin removal can also partially remove lignin-bound proteins, leading to decreased protein levels. No significant effect on P0 and P1 was observed, indicating that the delignification process did not influence or alter ammonia levels.
VFA concentration
By rumen microbes, VFAs are produced through the breakdown and fermentation of carbohydrates, including Nitrogen-Free Extract Material and crude fiber. VFAs serve as the primary energy source for ruminant animals (Hernaman, 2021). Various factors, such as feed intake, rumen fluid conditions, microbial activity within the rumen, and feeding frequency, influence the concentration of rumen VFAs. The type of feed consumed also affects the synthesis of VFA within the rumen (Bannink et al., 2008).
P1 (delignified EFB) exhibits significantly (P<0.05) higher VFA levels compared to P0 (untreated EFB). P1 described a significant effect (P<0.05) compared to P0. These findings indicated that the treated samples demonstrate normal VFA levels, whereas the untreated samples have lower VFA content than the normal limit (80-160 mM). Thus, delignified EFB is shown to produce higher energy when consumed by livestock and undergoes faster fermentation in the rumen. The availability of better nutrients and substrates can increase total VFA synthesis within the rumen (Dijkstra et al., 2012).
The levels of acetate, butyrate, propionate, isovalerate, isobutyrate, and n-valerate did not exhibit a significant effect due to the EFB delignification treatment. However, it is worth noting that acetic acid had the highest concentration compared to other partial VFA content. This is probably attributed to the elevated crude fiber content found in EFB, as acetic acid is a product of fermenting fibrous feed in the rumen. Microbes also require other nutrients to generate partial VFA, which may limit the overall production of partial VFA.
An increase followed total VFA production in certain partial VFAs such as acetate, butyrate, and propionate. This relationship can be attributed to the type and proportion of rumen microbes involved in the fermentation process and the substrate composition being degraded (Dijkstra et al., 2012). Suppose if the ratio of acetic acid exceeds the standard ratio. In that case, it may be due to the development of bacteria that produce acetic acid, which thrives with the specific feed composition provided. Many factors influence the composition of VFAs, including the composition of the rumen microbial population.
Methane gas production (CH4)
Methane gas (CH4) is produced via the anaerobic fermentation of both structural and non-structural carbohydrates by methanogens, specifically methane-producing archaea, within the rumen of ruminants. It is emitted into the atmosphere during the eructation (burping) process (Setiawan, 2021). The release of CH4 from the rumen indicates energy loss from the ruminant’s body, accounting for approximately 7%–12% of the consumed energy (Hariadi, 2007). Furthermore, CH4 produced by ruminants significantly contributes to global warming, with the ruminant population responsible for 12%–15% of CH4 pollution in the atmosphere (Moss et al., 2000).
The methane gas (CH4) production in untreated oil palm EFB did not differ significantly from the delignified EFB, with an average of 6.22%-7.02%. This finding may be attributed to the relatively low archaea population in the rumen, which may limit their ability to produce fermentation products (Setiawan, 2021). Another possible explanation for the low gas production is the decrease in rumen microbial activity due to reduced fermentable substrates (Jayanegara et al., 2009).
Dry matter digestibility coefficient and organic matter digestibility coefficient
Feed digestibility (McDonald et al., 2010) at higher levels indicates that the livestock utilizes significant nutrients from the feed for production. Digestibility can be quantified concerning both dry matter and organic matter, often represented as the digestibility coefficient, which is a percentage value indicating the degree of digestibility and the benefits of the feed for the livestock (Hartono and Sulistyowati, 2015; McDonald et al., 2010).
The in-vitro digestibility of both delignified and untreated EFB did not significantly influence the values of the digestibility coefficients for dry matter, organic matter, and crude fiber. The results suggested that the digestibility of both organic matter and dry matter within EFB, whether delignified or untreated, is very low. However, the digestibility of crude fiber appears to be higher (Baiti, 2013), stating that digestibility values in livestock typically range from 58.05-65.42% (Table 3).
Low digestibility can be caused by the feed’s physical form, which is fibers that still contain lignin and cellulose. Andre et al. (2019) stated that feed digestibility can be affected by various factors such as animal species, physical form of feed, feed composition, feeding level, temperature, and age of livestock. The OMD is dependent on the nutrient composition of the feed ingredients consumed. In the case of EFB, the nutrient content is relatively low, likely contributing to the OMD in both untreated and delignified EFB (Table 3). One possible solution is to mix EFB with other quality feed ingredients (Nur-Nazratul et al., 2021).
The digestibility of crude fiber demonstrates an inverse relationship with the digestibility of protein. The better the feed, the lower the crude fiber content. The obtained digestibility of crude fiber is relatively high, possibly due to the low nutrient content of EFB (Nur-Nazratul et al., 2021).
Table 3: Effect of delignification of EFB on dry matter digestibility coefficient, organic matter digestibility coefficient, and crude fiber digestibility coefficient.
|
Parameter |
Treatment |
P value |
|
|
P0 |
P1 |
||
|
Dry matter digestibility (%) |
15.83±1.53 |
15.31±2.96 |
NS |
|
Organic matter digestibility (%) |
15.09±2.40 |
15.86±0.82 |
NS |
|
Crude fiber digestibility (%) |
51.91±2.37 |
53.83±3.42 |
NS |
P0 = raw EFB, P1 = delignified EFB, NS = Not significance.
Rumen bacterial population
Rumen bacteria can be categorized into anaerobic, facultative anaerobic, and aerobic bacteria (Vasta et al., 2010). These bacteria can originate from feed ingredients or through direct contact with other materials containing bacteria (Lima et al., 2015). The population of rumen bacteria is presented in Table 4.
Table 4: Rumen bacterial population.
|
Parameter |
Treatment |
P value |
|
|
P0 |
P1 |
||
|
Bacterial population (log CFU mL-1) |
8.50 ± 1.15 |
8.67 ± 1.95 |
NS |
Description: P0=raw EFB, P1=delignified EFB, NS = Not significance.
The activity of rumen microorganisms can be affected by diverse factors, encompassing the dry matter content of the feed, pH levels, temperature, and buffer capacity (Wu et al., 2015). The bacterial population had no significant difference between the control and treatment groups. These fingdings suggested that the population number is slightly lower than the normal range (9- 10 log CFU ml-1), so fermentation in the rumen can occur (McDonald et al., 2010). However, a lower bacterial population in this study is still considered acceptable, as it does not have the potential to disrupt the function and health of the rumen.
Content of fulvic acid (Fulvic Acid)
Fulvic acid is an organic acid that naturally occurs during the decomposition of organic substances, often referred to as humus or humic compounds. Fulvic acid is a part of humic acid which can dissolve in alkalis and acids under neutral conditions (Islam, 2005). Fulvic acid can help the production of enzymes, hormone structure, and the need for vitamins. The fulvic acid content of EFB fermented using rumen fluid with the in-vitro method can be seen in Table 5.
Table 5: The fulvic acid content of fermented empty fruit bunches of palm oil using in-vitro rumen fluid method.
|
Parameter |
Treatment |
|||
|
P0 |
P1 |
F0 |
F1 |
|
|
Fulvic acid (%) |
6.22 |
6.19 |
6.46 |
6.12 |
Description: P0 = raw EFB, P1 = delignified EFB, F0 = Product from rumen fermentation of raw EFB, F1= product from rumen fermentation of delignified EFB
The content of fulvic acid in untreated EFB and delignified EFB that have not been fermented using rumen fluid in-vitro did not show significant changes, with the value relatively low, around 6%. This might be due to several factors, including the quality of raw materials, the delignification process, and specific chemicals used during delignification. NaOH, for example, can contribute to low levels of fulvic acid, as it may not decompose completely (Furnata and Nurlina, 2017).
Although limited information is available on other fractions of humate in rumen fermentation, fulvic acid can be utilized as a supplement in ruminant feed. Fulvic acid can contribute to animal nutrition. Previous studies have demonstrated using humate or fulvic acid as a replacement therapy for digestive disorders such as malnutrition and diarrhea. It can improve feed conversion efficiency in calves, dogs, and cats (Islam, 2005). The addition of humic compounds (39.4% humic acid and 27.8% fulvic acid) at a 10% inclusion rate in pig feed significantly increased body weight gain and feed conversion (Wang et al., 2008). On the other hand, supplementation of humic compounds in broilers did not significantly affect performance and carcass characteristics. However, there was a slight improvement in feed conversion with the inclusion of 0.1% humate (Karaoglu et al., 2016). Further research on fulvic acid in animal feed additives holds potential for exploration, as fulvic acid can be a beneficial component for livestock nutrition.
CONCLUSIONS and Recommendations
The delignification process of oil palm EFB has been demonstrated to enhance VFA production, suggesting its potential as an animal feed source. However, it is essential to note that EFB should not be considered a standalone feed source but fortified with other feed nutrients. This is because EFB alone may not provide a complete and balanced diet to meet the nutritional requirements of animals. By combining EFB with other feed ingredients that offer complementary nutrients, such as proteins, vitamins, and minerals, it is possible to create a more balanced and nutritionally adequate feed for livestock. Careful formulation and supplementation strategies can ensure that EFB contributes to the overall feed composition, enhancing its potential as an animal feed source.
ACKNOWLEDGMENTS
The author would like to thank the BPDPKS 2022 Oil Palm Research Grant and the BPDPKS ODS team, the technicians who have helped to achieve the output as promised.
NOVELTY STATEMENT
Oil palm empty fruit bunches potential as animal feed
AUTHOR’S CONTRIBUTION
Firda Dimawarnita: Conceptualization, PIC grant research, review writing as well as editing.
Edwina Gabriela T.M: Original draft, analysis, and verification of laboratory data.
Yora Faramitha: review writing, data analysis
Haryo Tejo Prakoso: translator, review writing, and editing
Wildan Aulia Noorsy: translator, review writing, and editing
Komang Gede Wiryawan: data analysis and data interpretation
Ethical approval
All experimental procedures were conducted following the institutional guidelines of Animal Ethics Committee School of Veterinary Medicine and Biomedical Sciences, IPB University (Ethical approval No. 131/KEH/SKE/X/2023).
Conflict of interests
The authors have declared no conflict of interest.
References
Abderzak LPN, Mathieu S, Diego PM, Claudette B, Cécile M (2012). Rumen microbial and fermentation characteristics are affected differently by bacterial probiotic supplementation during induced lactic and subacute acidosis in sheep. BMC Microbiol., 12(142): 1-12. https://doi.org/10.1186/1471-2180-12-142
Andre FM, Fenny R, Wolayan, Cathrien A, Rahasia, Mursye N, Regar (2019). Digestion of organic materials, crude fiber and crude fats of broil feeding from yellow pumpkin waste flour (Cucurbita moschata). Zootec, 39(2): 257-265. https://doi.org/10.35792/zot.39.2.2019.24870
Angela RMJPJ, John N (2000). Methane production by ruminants: its contribution to global warming. Ann. Zootech, 49: 231-253. https://doi.org/10.1051/animres:2000119
AOAC. 2005. Official Methods of Analysis. 18th edn. Association of Official Analytical Chemists. Arlington, VA, USA.
Awasthi MK, Sarsaiya S, Patel A, Juneja A, Singh RP, Yan B, Taherzadeh MJ (2020). Refining biomass residues for sustainable energy and bio-products: An assessment of technology, its importance, and strategic applications in circular bio-economy. Renew. Sustain. Energy Rev., 127: 109876. https://doi.org/10.1016/j.rser.2020.109876
Bai HX, Chang QF, Shi BM, Shan AS (2013). Effects of fulvic acid on growth performance and meat quality in growing-finishing pigs. Livest. Sci., 158(1-3): 118-123. https://doi.org/10.1016/j.livsci.2013.10.013
Baiti, LZLN, Pangestu E, Wahyono F, Achmadi J (2013). Effect of bagasse portion in diet on body composition of goat. Indonesian Trop. Anim. Agric., 38(3): 199-204. https://doi.org/10.14710/jitaa.38.3.199-204
Bannink A, France J, Lopez S, Gerrits WJJ, Kebreab E, Tamminga S, Dijkstra J (2008). Modelling the implications of feeding strategy on rumen fermentation and functioning of the rumen wall. Anim. Feed Sci. Technol., 143(1-4): 3-26. https://doi.org/10.1016/j.anifeedsci.2007.05.002
Cobellis G, Trabalza-Marinucci M, Marcotullio MC, Yu Z (2016). Evaluation of different essential oils in modulating methane and ammonia production, rumen fermentation, and rumen bacteria in vitro. Anim. Feed Sci. Technol., 215: 25-36. https://doi.org/10.1016/j.anifeedsci.2016.02.008
de Jong E, Jungmeier G (2015). Biorefinery concepts in comparison to petrochemical refineries. 3: 33. https://doi.org/10.1016/B978-0-444-63453-5.00001-X
Dijkstra J, Ellis JL, Kebreab E, Strathe AB, López S, France J, Bannink A (2012). Ruminal pH regulation and nutritional consequences of low pH. Anim. Feed Sci. Technol., 172(1-2): 22-33. https://doi.org/10.1016/j.anifeedsci.2011.12.005
Dimawarnita F, Perwitasari U, Marsudi S, Faramitha Y, Suharyanto S (2022). The utilization of oil palm empty fruit bunches for growth of oyster mushroom (Pleurotus ostreatus) and biodelignification process during planting cycle. Agrivita J. Agric. Sci., 44(1): 165-177. https://doi.org/10.17503/agrivita.v44i1.2311
Farobie O, Alifiah GJ, Sembada P, Firmanto S, Ibnu SB, Soma T, Wijaya H (2022). Production of local microorganism by utilizing organic matter in PT ultra peternakan bandung selatan. E3S Web of Conferences, 348: 00018. https://doi.org/10.1051/e3sconf/202234800018
Furnata MDIS, Nurlina (2017). The effect of solvent on humic acid extraction from cow manure compost. J. Kimia Khatulistiwa, 6(3): 58-65.
Gong G, Xu L, Zhang Y, Liu W, Wang M, Zhao Y, Li Y (2020). Extraction of fulvic acid from lignite and characterization of its functional groups. ACS Omega, 5(43): 27953-27961. https://doi.org/10.1021/acsomega.0c03388
Hariadi BSBT (2007). Effect of Acacia mangium willd supplementation on pennisetum purpureum on fermentation and production characteristics methane gas in vitro. Media Petern., 30(2): 106-113.
Hartono RYF, Sulistyowati E (2015). In vitro dry matter digestibility, organic matter digestibility and N-NH3 production of durian (Durio zibethinus) fermented with white rod (Pleurotus ostreatus) in different incubation time. Sains Peternakan Indonesia, 10(2): 87-94. https://doi.org/10.31186/jspi.id.10.2.87-94
Hernaman I (2021). Fermentabilitas dan Kecernaan Ransum Domba yang Mengandung Kulit Buah Pisang Muli (in vitro). J. Ilmu Ternak Univ. Padjadjaran, 20(2): 152. https://doi.org/10.24198/jit.v20i2.31416
Iqbal MA, Asif I, Nadeem A, Haroon ZK, Rana NA (2015). A study on feed stuffs role in enhancing the productivity of milch animals in Pakistan- existing scenario and future prospect. Glob. Vet., 14(1): 23-33.
Islam KMS (2005). Humic acid substances in animal agriculture. Pak. J. Nutr., 4(3): 126-134. https://doi.org/10.3923/pjn.2005.126.134
Jayanegara A, Togtokhbayar N, Makkar HPS, Becker K (2009). Tannins determined by various methods as predictors of methane production reduction potential of plants by an in vitro rumen fermentation system. Anim. Feed Sci. Technol., 150(3-4): 230-237. https://doi.org/10.1016/j.anifeedsci.2008.10.011
Karaoglu M, Aksu MI, Esenbuga N, Kaya M, Macit M, Durdag H (2016). Effect of dietary probiotic on the pH and colour characteristics of carcasses, breast fillets and drumsticks of broilers. Anim. Sci., 78(2): 253-259. https://doi.org/10.1017/S1357729800054047
Lima FS, Oikonomou G, Lima SF, Bicalho ML, Ganda EK, Filho JC, Bicalhoa RC (2015). Prepartum and postpartum rumen fluid microbiomes: Characterization and correlation with production traits in dairy cows. Appl. Environ. Microbiol., 81(4): 1327-1337. https://doi.org/10.1128/AEM.03138-14
McDonald, PRAE, Hreenhalgh JFD, Morgan CA, Sinclair LA, Wilkinson RG (2010). Animal nutrition seventh edition (Vol. 7): Pearson.
Moss, A.R., Jouany, J.P., Newbold, J. (2000). Methane production by ruminants: its contribution to global warming. In Annales de zootechnie (Vol. 49, No. 3, pp. 231-253). EDP Sciences.
Nur-Nazratul FMY, Rakib MRM, Zailan MZ, Yaakub H (2021). Enhancing in vitro ruminal digestibility of oil palm empty fruit bunch by biological pre-treatment with Ganoderma lucidum fungal culture. PLoS One, 16(9): e0258065. https://doi.org/10.1371/journal.pone.0258065
O’Malley ECAE (1942). Microdiffusion methods ammonia and urea using buffered absorbents (revised methods for ranges greater than 10μg. N. Biochem. J., 36(7-9): 655. https://doi.org/10.1042/bj0360655
Rulli MC, Casirati S, Dell’Angelo J, Davis KF, Passera C, D’Odorico P (2019). Interdependencies and telecoupling of oil palm expansion at the expense of Indonesian rainforest. Renewable Sustain. Energy Rev., 105: 499-512. https://doi.org/10.1016/j.rser.2018.12.050
Saritha M, Arora A, Lata (2012). Biological pretreatment of lignocellulosic substrates for enhanced delignification and enzymatic digestibility. Indian J. Microbiol., 52(2): 122-130. https://doi.org/10.1007/s12088-011-0199-x
SAS Institute (2003). SAS/STATR user’s guide: Statistics. Ver. 9.1, SAS Institute Inc., Cary, NC, USA.
Setiawan W (2021). The effect of mixed leaves tannin sources (Acacia mangium Willd, Switenia mahagoni, and Artocarpus heterophyllus) in pellets on in vitro methane production. Semin. Anim. Nutr. Feed Sci. (ISANFS 2021) 21: 270-274. https://doi.org/10.2991/absr.k.220401.055
Silalahi FRL, Rauf A, Hanum C, Siahaan D (2018). The characteristic and problems of beef cattle palm oil integration in Indonesia. IOP Conf. Ser. Earth Environ. Sci., 205: 012016. https://doi.org/10.1088/1755-1315/205/1/012016
Sumathi S, Chai SP, Mohamed AR (2008). Utilization of oil palm as a source of renewable energy in Malaysia. Renewable Sustain. Energy Rev., 12(9): 2404-2421. https://doi.org/10.1016/j.rser.2007.06.006
Terry JTAR (1963). A two stage technique for the in vitro digestion of forage crops. Grass Forage Sci., 18(2): 104-111. https://doi.org/10.1111/j.1365-2494.1963.tb00335.x
Thornton PK, van de Steeg J, Notenbaert A, Herrero M (2009). The impacts of climate change on livestock and livestock systems in developing countries: A review of what we know and what we need to know. Agric. Syst., 101(3): 113-127. https://doi.org/10.1016/j.agsy.2009.05.002
Vasta V, Yanez-Ruiz DR, Mele M, Serra A, Luciano G, Lanza M, Priolo A (2010). Bacterial and protozoal communities and fatty acid profile in the rumen of sheep fed a diet containing added tannins. Appl. Environ. Microbiol., 76(8): 2549-2555. https://doi.org/10.1128/AEM.02583-09
Wang Y, Wang R, Hao X, Hu Y, Guo T, Zhang J, Qi J (2020). Growth performance, nutrient digestibility, immune responses and antioxidant status of lambs supplemented with humic acids and fermented wheat bran polysaccharides. Anim. Feed Sci. Technol., 269: 114644. https://doi.org/10.1016/j.anifeedsci.2020.114644
Wang Y, Xu Z, Bach SJ, McAllister TA (2008). Effects of phlorotannins from Ascophyllum nodosum (brown seaweed) on in vitro ruminal digestion of mixed forage or barley grain. Anim. Feed Sci. Technol., 145(1-4): 375-395. https://doi.org/10.1016/j.anifeedsci.2007.03.013
Wu H, Meng Q, Yu Z (2015). Effect of pH buffering capacity and sources of dietary sulfur on rumen fermentation, sulfide production, methane production, sulfate reducing bacteria, and total Archaea in in vitro rumen cultures. Bioresour. Technol., 186: 25-33. https://doi.org/10.1016/j.biortech.2015.02.110
Wu H, Zhou Z, Yang Y, Meng Q (2020). Effect of steam explosion of oil palm frond and empty fruit bunch on nutrient composition and ruminal fermentation characteristics. Trop. Anim. Health Prod., 52(3): 1223-1228. https://doi.org/10.1007/s11250-019-02117-4
Yang, WZKAB, Rode LM (2001). Effects of grain processing, forage to concentrate ratio, and forage particle size on rumen pH and digestion by dairy cows. Dairy Sci., 84: 2203-2216. https://doi.org/10.3168/jds.S0022-0302(01)74667-X
Zhou X, Xu Y (2019). Integrative process for sugarcane bagasse biorefinery to co-produce xylooligosaccharides and gluconic acid. Bioresour. Technol., 282: 81-87. https://doi.org/10.1016/j.biortech.2019.02.129
To share on other social networks, click on any share button. What are these?





