Obtaining and Use of the Recombinant Bovine Pregnancy-Associated Glycoprotein 1
Obtaining and Use of the Recombinant Bovine Pregnancy-Associated Glycoprotein 1
Anara Ryskeldina, Indira Iskakova, Nurgul Sarina , Alexander Shevtsov , Laura Syzdykova, Alexander Shustov, Yerlan Ramankulov, Marat Kuibagarov*
Sequence of a cloned DNA fragment encoding boPAG1. Three clones were sequenced and all three clones contained the same insert. The entire sequence of the insert is shown, including primers at the ends of the PCR product. The primers PAG1_S and PAG1_AS are indicated. The arrow above the ATG codon signifies the start codon for the reading frame. The arrow above the TAA codon shows the translational stop.
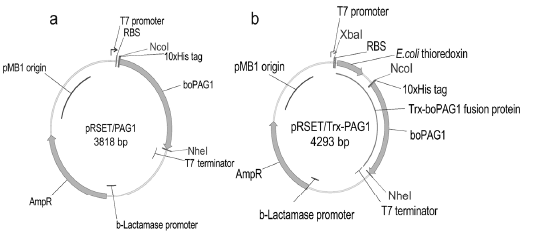
Genetic maps of plasmids for protein expression in E.coli. Panel a, plasmid pRSET/PAG1 encodes the recombinant protein boPAG1, with a decahistidine tag (10xHis tag) at the N-terminus. Panel b, plasmid pRSET/Trx-PAG1 encodes a fusion protein comprising E. coli thioredoxin (Trx) in the N-terminal part and boPAG1 in the C-terminal part. Genetic elements such as T7 promoter, ribosome binding site (RBS), T7 terminator, etc. are shown. Also shown are reading frames for the recombinant proteins boPAG1, Trx-boPAG1 and for a marker of antibiotic resistance (AmpR). Restriction sites used for cloning XbaI, NcoI, NheI are indicated.
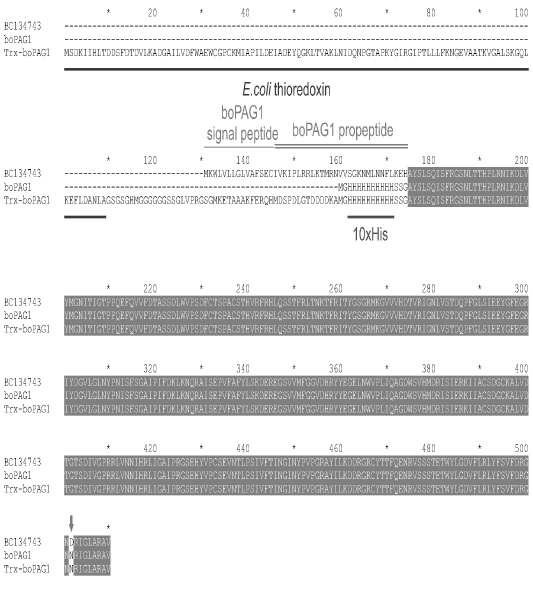
Amino acid sequences of the recombinant proteins boPAG1 and Trx-boPAG1 as compared to a prototype from Genbank (BC134743). Identical positions in the alignment are shown against a dark background. Missing sequences in non-homologous regions are indicated by dashes. For the Genbank sequence, the export signal (single horizontal line) and propeptide (double horizontal line) are marked. The export signal and propeptide are absent in the recombinant proteins. Thioredoxin is marked with a line under the alignment as well as a protein purification tag (10xHis). Aspartic acid-372 in BC134743 is replaced by asparagine in recombinant proteins because a mini-gene obtained in this work differs in this amino acid.
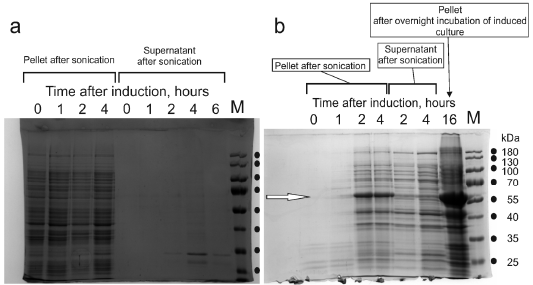
Results of experiments on the recombinant expression of boPAG1 (Panel a) and Trx-boPAG1 (Panel b). Samples of bacterial cultures were collected at time points indicated in the figures. Cell biomass was disrupted and separated into a soluble supernatant and insoluble precipitate. Protein contents in supernatants and precipitates were analyzed by SDS-PAGE. Photographs present stained SDS-PAGE gels. In Panel b, the white arrow points to the band of 56.2 kDa compatible with the Trx-boPAG1 protein. Lanes “M”, loaded with PageRuler Mw marker, 10-180 kDa (ThermoScientific 26616). The molecular masses of the marker bands are indicated.
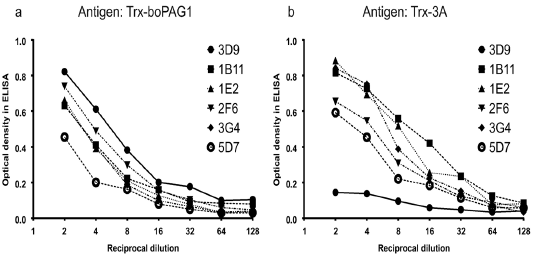
Panel a, a panel of six purified monoclonal antibodies react with the antigen Trx-boPAG1 which was used for immunization. Panel b, the same six mAbs were tested for reactivity against an irrelevant Trx-containing antigen. The mAb 3D9 reacts with the antigen used for immunization (Panel a) but not with the irrelevant antigen (Panel b).
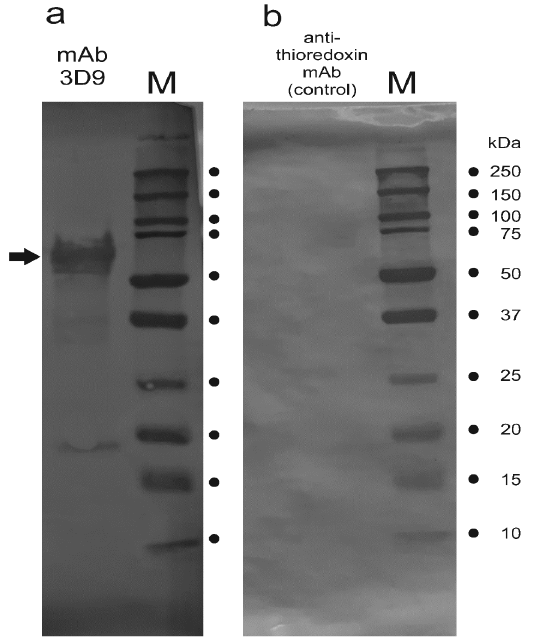
The mAb 3D9 reacts with a protein extracted from cow’s placenta. Results of an immunoblot are shown. Placenta-extracted proteins were separated in SDS-PAGE and blotted with the 3D9 mAb (Panel a) or a different control antibody as described in the text (Panel b). In Panel a, the arrow points to the band which was cut out from the membrane and used for the confirmation of native PAG1 in the immune complex using mass spectrometry. Lanes “M”, marker Precision Plus Protein Dual Color Standards (Biorad Cat. 1610394). The molecular masses of the marker bands are indicated.
Sequence of the plasmid pRSET/PAG1
Sequence of the plasmid pRSET/Trx-PAG1
Results of mass-spectroscopic (MASCOT) identification of the recombinant protein extracted from a gel slice
Results of mass-spectroscopic (MASCOT) identification of the recombinant protein present in a band on a blotting membrane which reacts with an anti-PAG1 antibody
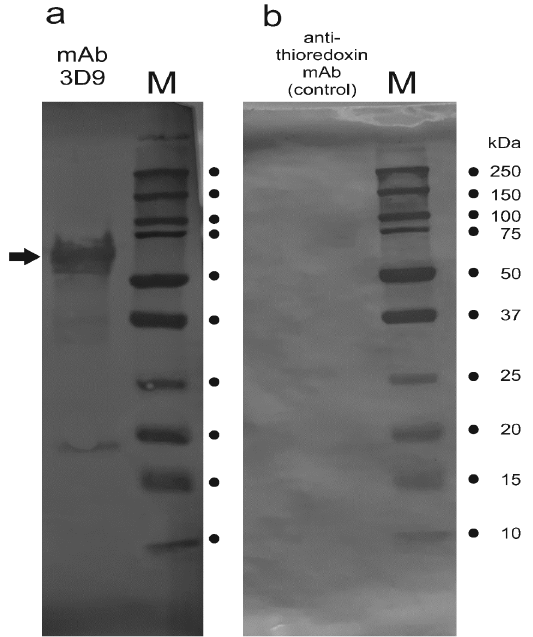
The mAb 3D9 reacts with a protein extracted from cow’s placenta. Results of an immunoblot are shown. Placenta-extracted proteins were separated in SDS-PAGE and blotted with the 3D9 mAb (Panel a) or a different control antibody as described in the text (Panel b). In Panel a, the arrow points to the band which was cut out from the membrane and used for the confirmation of native PAG1 in the immune complex using mass spectrometry. Lanes “M”, marker Precision Plus Protein Dual Color Standards (Biorad Cat. 1610394). The molecular masses of the marker bands are indicated.