Molecular and Immunological Effect of Propofol in Relieving Myocardial Ischemia-Reperfusion Injury in Type 2 Diabetic Patients
Molecular and Immunological Effect of Propofol in Relieving Myocardial Ischemia-Reperfusion Injury in Type 2 Diabetic Patients
Caiping Duan
Department of Anesthesiology, Ordos Central Hospital, Ordos 017000, China
ABSTRACT
This study aimed to explore the clinical mechanism of propofol in relieving myocardial ischemia-reperfusion injury in type 2 diabetic patients. In the present study, 369 patients suffering from myocardial ischemia-reperfusion injury who were diagnosed with type 2 diabetes in our hospital from January 2018 to May 2019 were selected as study subjects and randomly divided into low-dose propofol group (n=123; intravenously injected with 25 mg before angiography), high-dose propofol group (n=123; intravenously injected with 50 mg before angiography) and control group (n=123; receiving local anesthesia before angiography, subcutaneously injected with 2% lidocaine 2.5 ml at puncture point). After coronary angiography in each group, the serum nitric oxide (NO), endothelin-1 (ET-1) and cardiac troponin (cTnT) contents in different treatment groups were detected by enzyme-linked immunosorbent assay. Coronary blood samples were obtained during coronary angiography. Expressions of interleukin-1β (IL-1β), interleukin-6 (IL-6) and tumor necrosis factor-α (TNF -α) were detected by Western blotting (WB). The protein contents of B-cell lymphoma factor 2 (Bcl-2), apoptosis-related genes Bax and caspase-3 were detected by WB. The contents of lactic dehydrogenase (LDH), creatine kinase (CK), superoxide dismutase (SOD) and malondialdehyde (MDA) were detected by enzyme-linked immunosorbent assay. Results showed that compared with the control group, the low-dose propofol group had increased NO, decreased ET-1 and cTnT contents (P<0.05), and high-dose propofol group had further increased NO and further decreased ET- 1 and cTnT contents compared with low-dose propofol group (P<0.05). Compared with the control group, the low-dose propofol group had reduced IL-1β, IL-6 and TNF-α expressions (P<0.05), and high-dose propofol group had further reduced IL-1β, IL-6 and TNF-α expressions compared with low-dose propofol group (P<0.05). Compared with the control group, the low-dose propofol group had increased Bcl-2 protein content, decreased Bax and Caspase-3 protein contents (P<0.05); compared with low-dose propofol group, high-dose propofol group had further increased Bcl-2 protein content, further decreased Bax and Caspase-3 protein contents (P<0.05). Compared with the control group, low-dose propofol group had decreased SOD content, increased LDH, CK, and MAD contents (P<0.05). Compared with low-dose propofol group, high-dose propofol group had further decreased SOD content, further increased LDH, CK and MAD contents (P<0.05). We conclude that, Propofol has a protective effect on myocardial ischemia-reperfusion injury in type 2 diabetic patients, the mechanism of which is to up-regulate serum NO, Bcl-2 and SOD, and down-regulate serum ET-1, cTnT, IL-1β, IL-6, TNF-α, BAX, LDA, CK, MAD, thus protecting cardiomyocytes.
Article Information
Received 05 August 2020
Revised 13 October 2020
Accepted 15 December 2020
Available online 18 May 2022
(early access)
Published 14 March 2023
Key words
Myocardium, Ischemia-reperfusion injury, Type 2 diabetes, Propofol, Nitric oxide, Endothelin-1.
DOI: https://dx.doi.org/10.17582/journal.pjz/20200805170856
* Corresponding author: zhyxbj12@163.com
0030-9923/2023/0003-1167 $ 9.00/0
Copyright 2023 by the authors. Licensee Zoological Society of Pakistan.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Introduction
Type 2 diabetic patients have a high incidence of cardiovascular complications, and cardiovascular accidents are considered as a high-risk cause of perioperative death in diabetic patients (Wang et al., 2017). Diabetic patients with acute myocardial infarction after ischemia-reperfusion injury have several times higher mortality than non-diabetic patients (Chen et al., 2017). The release of inflammatory mediators is closely related to type 2 diabetes and myocardial ischemia-reperfusion injury (Zykov et al., 2018). In the pathophysiological process of reperfusion, damage and dysfunction of endothelial cells in the coronary arteries may be an important early event that leads to subsequent apoptosis of myocardial cells during ischemia-reperfusion (Yu et al., 2018). Endothelial dysfunction is characterized by reduced NO bioavailability, which is an important cause of reperfusion injury (Hou et al., 2019; Paelestik et al., 2017). Propofol is a widely used intravenous anesthetic that has been used in various experimental models to reduce oxidative stress, protect mitochondrial function, and reduce inhibitory effect of apoptosis on I/R injury (Bradic et al., 2019). It is reported that propofol provides cardioprotection against I/R injury in type 1 diabetes (Hao et al., 2017; Suchal et al., 2017). Whether propofol has a protective effect on myocardial ischemia/reperfusion injury in type 2 diabetes remains unclear as this disease has high clinical incidence, and the potential mechanism of this protective effect waits to be determined. This study is designed to evaluate the mechanism of action of isopropofol on myocardial ischemia-reperfusion injury in type 2 diabetic patients.
Materials and Methods
A total of 246 patients diagnosed with type 2 diabetes + myocardial ischemia-reperfusion injury in our hospital from January 2018 to May 2019 were selected as subjects and randomly divided into low-dose propofol group (n=123) and high-dose propofol group (n=123). Patients in the low-dose propofol group underwent coronary angiography under local anesthesia; patients in the high-dose propofol group underwent coronary angiography under propofol anesthesia (50 mg intravenously injected before angiography).Meanwhile, healthy examinees receiving coronary angiography in our hospital were selected as the control group (n=87).
Inclusion criteria: patients with prior myocardial ischemia or one or more coronary artery stenosis ≥50% during coronary angiography of percutaneous coronary intervention or coronary artery bypass grafting; type 2 diabetes is defined as the case where fasting blood glucose exceeds 7 mmol / l, or blood glucose exceeds 11 mmol / l two hours after oral administration of glucose 75 g. The oral glucose tolerance test confirmed the lack of type 2 diabetes in the CAD group.
Exclusion criteria: age> 80 years; acute myocardial infarction or unstable angina within 3 months before the experiment; Raynaud phenomenon in the forearm; arteriovenous shunt or other vascular abnormalities; any protocol that hinders completion of the study, participation in other study or unwillingness to participate in this study.
Medical ethics issues: This study was approved by the hospital ethics committee, and all participants signed informed consent.
Coronary angiography implementation plan
Perioperative coronary angiography detection
Clinical information obtained during the hospitalization (ECG, cTnT and hemoglobin serial laboratory measurements, perioperative and intraoperative vital signs monitoring, echocardiography, cardiac pressure and angiography detection of coronary artery disease) were followed. All perioperative myocardial injury met the cTn standard, so at least one of the following conditions was met: ischemic symptoms, new important ST-T wave changes or new left bundle branch block, pathological Q wave in ECG, loss of new viable myocardium or new regional wall motion abnormalities as indicated by imaging evidence, 30-day mortality rate in outcome end-point associated with primary event of coronary thrombosis identification, and 1-year mortality rate in the secondary outcome end-point. Death is classified into cardiovascular or non-cardiovascular death. Cardiovascular death includes death attributable to acute myocardial infarction, sudden cardiac death, heart failure, stroke, cardiovascular surgery, cardiovascular hemorrhage (e.g., ruptured or dissected aortic aneurysm), and pulmonary embolism. Unless there is evidence of non-cardiovascular causes, all deaths are assumed as cardiovascular deaths. Non-cardiovascular deaths include all deaths caused by clearly recorded non-cardiac and non-vascular factors.
Coronary angiography treatment procedure
If the absolute increase of cTnT exceeds the preoperative level ≥14ng/L, the structured response includes the assessment of possible symptoms associated with perioperative myocardial injury in patients diagnosed with perioperative myocardial injury. The researchers recorded the 12-leads ECG. In the pre-determined perioperative myocardial injury management plan, continuous guidance is given to all cardiologists who provide cardiac consultation after perioperative myocardial injury. All treatment decisions regarding perioperative myocardial injury are made jointly by the attending physician and the cardiologist.
Blood sample collection
A coronary blood sample (3ml) was obtained during coronary angiography and placed in a common plastic tube. 1.8 ml of this venous blood was transferred to an anticoagulant tube containing 0.2 ml of 3.8% sodium citrate. The sample was centrifuged (1200×g) for 10 min in 1 h, serum was extracted and stored in a 0.5 ml Eppendorf tube at a temperature of -30°C to be used within 1 month.
Determination of NO, ET-1 and cTnT
Nitric oxide (NO), endothelin-1 (ET-1) and cardiac troponin (cTnT) were determined by ELISA. All reagents in this study were from Wuhan Bosite Bioengineering Co., Ltd. (Wuhan, China) and were used according to the manufacturer’s instructions.
Detection of IL-1β, IL-6, TNF-α, Bcl-2, Bax, Caspase-3 protein contents
The blood sample was centrifuged at 12000 ×g, 4°C for 30 min, and the supernatant was aspirated, diluted with equal amount of 5 × SDS loading buffer at 1:1 (V/V), boiled at 100°C for 5 min. The protein concentration of the sample was measured by BCA protein concentration determination kit. Take 15 μL of the sample to be tested for loading {glyceraldehyde-3- phosphate dehydrogenase (GAPDH) monoclonal antibody (1:500) as the standard for protein loading amount}, and the total protein loading amount in each lane is 30 μg. SDS-polyacrylamide gel electrophoresis (SDS-PAGE) was performed. The protein strips were charged and transferred to Immun-blot PVDF membrane by wet process, blocked with 50 g/L skim milk powder at 20°C for 3 h, and the corresponding antibodies were added (mouse anti-rat IL-1β monoclonal antibody,1:400, mouse anti-rat IL-6 monoclonal antibody, 1:400, mouse anti-rat TNF-α monoclonal antibody, 1:400, rabbit anti-rat Bcl-2 polyclonal antibody,1:400, rabbit anti-rat Bax, 1:400 polyclonal antibody, rabbit anti-rat Caspase-3, 1:400 polyclonal antibody for incubation at 4°C overnight. The strips were rinsed for 10 min×3 times with TBS buffer (Tris 50 mmol/L, NaCl 100 mmol/L, pH 7.5), incubated at 37°C for 2 h using the corresponding horseradish enzyme-labeled IgG (1:1000), and rinsed for 10 min×3 times. After luminescence development, the image was scanned with a Mieroteck scanner, and integrated optical density analysis was performed using Quantity-One software.
Detection of LDH, CK, SOD and MAD expression in coronary blood samples
Colorimetric detection kit was used to detect the expression levels of LDH, CK, SOD and MAD in the coronary blood samples; the experimental steps of the colorimetric detection followed the instructions.
Statistical analysis
This study adopted SPSS20.0 statistical analysis software (IBM, USA). The measurement data was expressed as “mean ± standard deviation” (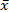 ± s). One-way analysis of variance or repeated measurement data analysis of variance was used for comparison between groups, LSD-t test was used for pairwise comparison. The count data was expressed as percentage (%), and χ2 analysis was used for comparison between groups. P<0.05 indicates statistically significant difference.
± s). One-way analysis of variance or repeated measurement data analysis of variance was used for comparison between groups, LSD-t test was used for pairwise comparison. The count data was expressed as percentage (%), and χ2 analysis was used for comparison between groups. P<0.05 indicates statistically significant difference.
Results
Table I shows the general characteristics of the participants. In comparison between the control group and low-dose propofol group, age, gender, BMI, total cholesterol, high-density lipoprotein and low-density lipoprotein do not differ statistically (P>0.05). The low-dose propofol group has higher systolic pressure, diastolic pressure, MAP, hemoglobin, and glucose levels than the control group (P<0.05). All subjects well tolerated the experimental protocol. During the ischemia, the subjects felt forearm numbness, but no patient felt any pain or discomfort.
Table II shows the NO, ET-1 and cTnT contents by ELISA, IL-1β, IL-6, TNF –α, Bcl2, Bax and Caspase-3 detected by western blotting and expression of LDH, CK, SOD and MAD in the blood of type 2 diabetic patients. Compared with the control group, the low-dose of propofol causes an increase in NO and Bcl2, and decrease in all the remaining parameters (P<0.05). The high-dose of propofol further intensifies the effect of propofol (P<0.05).
Table I.- Statistics of basic characteristics.
|
Item |
Control group (n=87) |
Low-dose propofol group (n=123) |
High-dose propofol group (n=123) |
F value |
P value |
|
Age |
58.73±5.82 |
58.24±6.17 |
57.18±5.53 |
4.875 |
0.178 |
|
Gender (Male: Female) |
41:46 |
58:65 |
54:69 |
6.142 |
0.654 |
|
Systolic pressure (mmHg) |
115.34±4.51 |
134.68±12.93 |
130.52±11.71 |
37.516 |
0.016 |
|
Diastolic pressure (mmHg) |
75.22±6.18 |
83.15±8.26 |
81.26±9.05 |
15.135 |
0.013 |
|
MAP |
91.37±7.28 |
101.58±11.18 |
98.65±8.66 |
11.764 |
0.028 |
|
BMI(kg/m2) |
26.14±3.54 |
26.23±2.72 |
27.53±2.32 |
2.376 |
0.463 |
|
Hemoglobin (%) |
32.23±4.53 |
47.62±2.85 |
45.39±3.15 |
24.183 |
0.007 |
|
Glucose (mmol/L) |
0.86±0.11 |
1.87±0.16 |
1.79±0.21 |
12.227 |
0.016 |
|
Total cholesterol (mmol/L) |
4.35±0.16 |
4.78±0.14 |
4.68±0.37 |
0.186 |
0.568 |
|
High density lipoprotein (mmol/L) |
1.38±0.15 |
1.26±0.13 |
1.32±0.10 |
3.563 |
0.429 |
|
Low density lipoprotein (mmol/L) |
2.46±0.43 |
2.37±0.23 |
2.56±0.19 |
5.187 |
0.172 |
Table II.- Effect of low and high doses of propofol on levels of NO, ET-1 and cTnT contents detected by ELISA, IL-1β, IL-6, TNF –α, Bcl2, Bax and Caspase-3 proteins detected by western blotting and expression of LDH, CK, SOD and MAD using colorimetric detection kits in the blood of type 2 diabetic patients.
|
Control / Propofol Group |
Control (n=87) |
Low-dose (n=123) |
High-dose (n=123) |
F value |
p value |
|
NO (μmol/L) |
58.73±10.15 |
75.34±9.26 |
87.54±5.37 |
35.347 |
0.012 |
|
ET-1 (μg/L) |
63.75±0.32 |
55.56±0.37 |
37.14±0.22 |
12.175 |
0.024 |
|
cTnT (μg/L) |
12.132±0.12 |
9.145±2.25 |
5.748±1.08 |
13.145 |
0.018 |
|
IL-1β (integrated optical density) |
1324.57±36.28 |
1155.46±50.47 |
902.94±25.22 |
33.267 |
0.014 |
|
IL-6 (integrated optical density) |
1623.22±39.53 |
1259.17±42.24 |
915.05±50.53 |
55.187 |
0.008 |
|
TNF-α (integrated optical density) |
1589.13±59.26 |
1059.17±36.34 |
785.86±62.35 |
66.175 |
0.001 |
|
Bcl-2 (integrated optical density) |
5.88±1.26 |
8.04±1.11 |
12.06±2.22 |
15.264 |
0.012 |
|
Bax (integrated optical density) |
2.87±0.34 |
1.98±0.24 |
1.25±0.31 |
12.524 |
0.008 |
|
Caspase-3 (integrated optical density) |
3.85±0.14 |
2.95±0.24 |
2.05±0.16 |
14.485 |
0.013 |
|
LDH (U/L) |
1861.52±232.61 |
1652.57±150.54 |
1472.15±127.37 |
12.534 |
0.012 |
|
CK (U/L) |
190.22±19.16 |
145.26±25.28 |
121.84±29.23 |
11.256 |
0.024 |
|
SOD (μmol/mL) |
68.11±1.25 |
43.54±0.37 |
26.22±0.54 |
15.137 |
0.033 |
|
MAD (nmol/L) |
15.13±2.87 |
10.37±2.24 |
7.22±1.48 |
10.185 |
0.008 |
Figure 1 shows the expression of IL-1β, IL-6 and TNF-α. Figure 2 shows the IL, Bcl-2, Bax and Caspase-3 protein contents in coronary blood samples by western blotting. Compared with the control group, the low-dose propofol group has reduced IL-1β, IL-6 and TNF-α expressions, Bax and Caspase-3 protein contents (P<0.05), and high-dose propofol group has further reduced expression compared with low-dose propofol group (P<0.05).
Table II also shows level of LDH, CK, SOD and MAD in coronary blood samples. Compared with the control group, low-dose propofol has decreased all the parameters (P<0.05). High-dose propofol group has further decreased the exzume levels (P<0.05).
Discussion
IR injury involves destruction and metabolic disorders of tissue structure. In clinical cardiac surgery, infarction after coronary artery ligation was observed in the case of myocardial ischemia-reperfusion injury. Type 2 diabetes is one of the most common endocrine and metabolic diseases (Li et al., 2018). This study indicates that propofol improves heart function in type 2 diabetic patients, increases serum NO, and reduces the content of ET-1 and inflammatory mediators in myocardial ischemia-reperfusion injury.
There is evidence that intravenous narcotic propofol may inhibit lipid peroxidation, improve mitochondrial function, protect myocardium and reduce myocardial ischemia-reperfusion injury in patients (Fan and Yang, 2017). Several clinical and biochemical indicators can be used in diagnosis of myocardial injury. cTnT is considered as the “gold standard” for the diagnosis of myocardial injury (Huynh et al., 2019). In this study, propofol reduced cTnT concentration in the serum and improved heart function. During myocardial ischemia, changes in NO levels are controversial. Previous studies have shown that NO release is increased during myocardial ischemia of coronary artery (Sheng et al., 2017). By contrast, other authors found that NO release was reduced. Endothelial function plays an important role in maintaining stability and normal hemodynamics. The key factor to its function is NO (Zhang et al., 2017). NO exerts an anti-inflammatory effect by inhibiting the adhesion of neutrophilic granulocyte to endothelial cells and reducing the release of inflammatory factors. Appropriate amount of NO can protect cardiomyocytes, reduce damage, inhibit intimal hyperplasia and improve heart function after IR injury. As an important regulator of cardiovascular function, ET plays an important role in maintaining vascular tone and cardiovascular system stability. Epinephrine, thromboxane, angiotensin, insulin, inflammatory factors and hypoxia stimulate the synthesis of ET-1, while inhibitors of ET-1 synthesis include NO, PGI2, atrial natriuretic peptide and heparin (Jia et al., 2017). This experiment shows that propofol can increase NO levels and reduce serum ET-1 concentration, thereby exerting cardioprotective effects against myocardial ischemia-reperfusion injury in type 2 diabetic patients.
Type 2 diabetes and myocardial ischemia-reperfusion injury concern inflammatory mediators. Inflammatory factors may cause myocardial damage in type 2 diabetic patients. Therefore, inhibiting the release of inflammatory cytokines is an important strategy for protecting the heart of type 2 diabetic patients against myocardial damage. As an important inflammatory cytokine, TNF-α mainly produced by the activation of monocytes/macrophages is involved in certain autoimmune diseases (Liu et al., 2019). Therefore, it can stimulate NO synthase (i-NOS) synthesis to release large amounts of NO (Eroglu et al., 2017). This will exacerbate peroxidation of membrane lipid and tissue damage. IL-1β is mainly produced by macrophages, and it is found that systemic response is a result of injection and secretion of large amounts of IL-1β (Yu et al., 2018). IL-6 plays an important role in combating infection by enhancing the effects of other cytokines and regulating immune response, acute phase response and hematopoiesis (Wu et al., 2017). Inflammatory factors play an important role in myocardial ischemia-reperfusion injury. Inflammation caused by myocardial ischemia and hypoxia prompts monocytes and macrophages to release large amounts of IL. As a highly specific neutrophil chemotactic factor, interleukin causes adhesion and aggregation of large numbers of leukocytes (an obstacle that hinders microcirculation), increases active oxygen and damages cardiomyocytes (Yu and Gao, 2017). Inflammatory factors cause neutrophils to release cytotoxicity, aggravate the inflammatory response, block capillaries and vasoactive substances, leading to acute tissue injury. This study indicates that propofol reduces the expression of inflammatory cytokines such as IL-1β, IL-6, and TNF-α in serum.
Under conditions of diabetes and ischemia, hypoxia leads to anaerobic respiration, resulting in lactic acid production, ATP consumption, and intracellular pH decline. These effects induce oxidative stress, intracellular Ca2+ overload and cell membrane damage, and cause direct oxidative damage to DNA and mitochondria (Zhang et al., 2018). All these factors can lead to cell death through apoptosis, which is considered as one of the major causes of myocardial ischemia and reperfusion injury. Caspase-3 is one of the most important enzymes that regulate and execute apoptosis, which acts as an early marker of apoptosis (Ge et al., 2017). Bcl-2 protein family, through its pro-apoptotic and anti-apoptotic members, plays a vital role in controlling apoptosis mechanism of mammalian cells (Li et al., 2017). The ratio of Bcl-2 to Bax determines the fate of the cell. When the ratio is reduced, the cell tends to undergo apoptosis. Conversely, when this ratio increases, cells tend to survive. In addition, increased apoptosis is shown in cardiomyocytes of diabetic patients and STZ-induced diabetic animals. Studies have shown that cardiomyocyte apoptosis in diabetic patients is about 85 times that of non-diabetic patients (Cooper et al., 2018). Recent studies have found that propofol has a protective effect against ischemia-reperfusion injury in brain I/RI patient models and myocardial I/RI patient models. Our results revealed that propofol treatment increased Bcl-2 protein content and decreased the Bax and Caspase-3 protein contents (Meng et al., 2018).
Conclusion
To conclude, propofol has a protective effect on myocardial ischemia-reperfusion injury in type 2 diabetic patients by improving cardiac function, increasing serum NO, Bcl-2, and SOD, and decreasing serum ET-1, cTnT, and IL- 1β, IL-6, TNF-α, BAX, LDA, CK, MAD.
Statement of conflict of interest
The authors have declared no conflict of interests.
References
Bradic, J., Jeremic, N., Petkovic, A., Jeremic, J., Zivkovic, V., Srejovic, I., Sretenovic, J., Matic, S., Jakovljevic, V. and Tomovic, M., 2019. Cardioprotective effects of Galium verum L. extract against myocardial ischemia-reperfusion injury. Arch. Physiol. Biochem., 11: 1-8. https://doi.org/10.1155/2019/4235405
Chen, L., Cai, P., Cheng, Z., Zhang, Z. and Fang, J., 2017. Pharmacological postconditioning with atorvastatin calcium attenuates myocardial ischemia/reperfusion injury in diabetic rats by phosphorylating GSK3β. Exp. Ther. Med., 14: 25-34. https://doi.org/10.3892/etm.2017.4457
Cooper, R., Newman, P. and Herachwati, N., 2018. RAPD molecular markers to analyze the DNA variation of the three Bruguiera species on Kemujan Island. CCAMLR Sci., 25: 209-214.
Eroglu, T., Bozkurt, M., Kapi, E., Selcuk, C.T., Kuvat, S.V., Tufek, A., Isik, F.B., Bozarslan, B.H., Firat, U. and Satici, O., 2017. A study on the effects of the use of propofol in experimental model inferior epigastric island flap on ischemia-reperfusion injury. J. Craniofac. Surg., 28: 2193-2198. https://doi.org/10.1097/SCS.0000000000004049
Fan, Z.X. and Yang, J., 2017. Exosomes-ADMS: A novel therapy thought in myocardial ischemia reperfusion injury. Int. J. Cardiol., 239: 11. https://doi.org/10.1016/j.ijcard.2017.01.084
Ge, M., Chen, H. and Zhu, Q., 2017. Propofol post-conditioning alleviates hepatic ischaemia reperfusion injury via BRG1-mediated Nrf2/HO-1 transcriptional activation in human and mice. J. cell. mol. Med., 21: 3693-3704. https://doi.org/10.1111/jcmm.13279
Hao, W., Zhao, Z.H., Meng, Q.T., Tie, M.E., Lei, S.Q. and Xia, Z.Y., 2017. Propofol protects against hepatic ischemia/reperfusion injury via miR-133a-5p regulating the expression of MAPK6. Cell Biol. Int., 41: 495-504. https://doi.org/10.1002/cbin.10745
Hou, J., He, H., Huang, S., Qian, M., Wang, J., Tan, X., Han, G., Song, Y., Xu, Z. and Liu, Y., 2019. A mitochondria-targeted nitric oxide donor triggered by superoxide radical to alleviate myocardial ischemia/reperfusion injury. Chem. Commun., 55: 1205-1208. https://doi.org/10.1039/C8CC07304J
Huynh, D.N., Elimam, H., Bessi, V.L., Ménard, L., Burelle, Y., Granata, R., Carpentier, A.C., Ong, H. and Marleau, S., 2019. A linear fragment of unacylated ghrelin (uag6-13) protects against myocardial ischemia/reperfusion injury in mice in a growth hormone secretagogue receptor-independent manner. Front. Endocrinol., 9: 798. https://doi.org/10.3389/fendo.2018.00798
Jia, L., Wang, F., Gu, X., Weng, Y., Sheng, M., Wang, G., Li, S., Du, H. and Yu, W., 2017. Propofol postconditioning attenuates hippocampus ischemia-reperfusion injury via modulating JAK2/STAT3 pathway in rats after autogenous orthotropic liver transplantation. Brain Res., 1657: 202-207. https://doi.org/10.1016/j.brainres.2016.12.015
Li, Q., Fan, Z.X., Yang, Y. and Yang, J., 2018. Ethyl pyruvate: A promising feasible therapeutic approach for myocardial ischemia-reperfusion injury under both normoglycemia and hyperglycemia. Int. J. Cardiol., 265: 38. https://doi.org/10.1016/j.ijcard.2018.02.089
Li, R., Fan, L., Ma, F., Cao, Y., Gao, J., Liu, H. and Li, Y., 2017. Effect of etomidate on the oxidative stress response and levels of inflammatory factors from ischemia-reperfusion injury after tibial fracture surgery. Exp. Ther. Med., 13: 971-975. https://doi.org/10.3892/etm.2017.4037
Liu, N.B., Wu, M., Chen, C., Fujino, M., Huang, J.S., Zhu, P. and Li, X.K., 2019. Novel molecular targets participating in myocardial ischemia-reperfusion injury and cardioprotection. Cardiol. Res. Pract., 2019: 6935147. https://doi.org/10.1155/2019/6935147
Meng, S.Y. and Young, B., 2018. Effects of vitamin D addition levels on growth performance, body composition and serum biochemical parameters of mid-term tilapia. CCAMLR Sci., 25: 97-105.
Pælestik, K.B., Jespersen, N.R., Jensen, R.V., Johnsen, J., Bøtker, H.E. and Kristiansen, S.B., 2017. Effects of hypoglycemia on myocardial susceptibility to ischemia–reperfusion injury and preconditioning in hearts from rats with and without type 2 diabetes. Cardiovasc. Diabetol., 16: 148. https://doi.org/10.1186/s12933-017-0628-1
Sheng, M., Huang, Z., Pan, L., Yu, M., Yi, C., Teng, L., He, L., Gu, C., Xu, C. and Li, J., 2017. SOCS2 exacerbates myocardial injury induced by ischemia/reperfusion in diabetic mice and H9c2 cells through inhibiting the JAK-STAT-IGF-1 pathway. Life Sci., 188: 101-109. https://doi.org/10.1016/j.lfs.2017.08.036
Suchal, K., Malik, S. and Khan, S.I., 2017. Protective effect of mangiferin on myocardial ischemia-reperfusion injury in streptozotocin-induced diabetic rats: role of AGE-RAGE/MAPK pathways. Scient. Rep., 7: 42027. https://doi.org/10.1038/srep42027
Wang, Y., Qi, X. and Wang, C., 2017, Effects of propofol on myocardial ischemia-reperfusion injury in rats with type-2 diabetes mellitus. Biomed. Rep., 6: 69-74. https://doi.org/10.3892/br.2016.805
Wu, H., Zhou, J., Ou, W., Li, Y., Liu, M. and Yang, C., 2017. TAK1 as the mediator in the protective effect of propofol on renal interstitial fibrosis induced by ischemia/reperfusion injury. Eur. J. Pharmacol., 811: 134-140. https://doi.org/10.1016/j.ejphar.2017.06.009
Yu, D.J. and Gao, H.Y., 2017. Effect of propofol on mitochondrial ATP content and ATPase activity in hippocampus of rats with cerebral ischemia-reperfusion injury. Saudi J. biol. Sci., 24: 246-250. https://doi.org/10.1016/j.sjbs.2016.09.007
Yu, W., Gao, D., Jin, W., Liu, S. and Qi, S., 2018. Propofol prevents oxidative stress by decreasing the ischemic accumulation of succinate in focal cerebral ischemia-reperfusion injury. Neurochem. Res., 43: 420-429. https://doi.org/10.1007/s11064-017-2437-z
Yu, X., Sun, X. and Zhao, M., 2018. Propofol attenuates myocardial ischemia reperfusion injury partly through inhibition of resident cardiac mast cell activation. Int. Immunopharmacol., 54: 267-274. https://doi.org/10.1016/j.intimp.2017.11.015
Zhang, L., Ruan, Z., Liang, J., Du, Y., Lu, Z., Feng, D., Cai, S., Zhang, X., Cai, W. and Hu, Z., 2018. Protective effect of propofol on ischemia-reperfusion injury detected by HPLC-MS/MS targeted metabolic profiling. Eur. J. Pharmacol., 833: 69-78. https://doi.org/10.1016/j.ejphar.2018.05.039
Zhang, Y., Chen, Z., Feng, N., Tang, J., Zhao, X., Liu, C., Xu, H. and Zhang, M., 2017. Protective effect of propofol preconditioning on ischemia-reperfusion injury in human hepatocyte. J. Thoracic. Dis., 9: 702-710. https://doi.org/10.21037/jtd.2017.02.80
Zykov, V.A., Tuchina, T.P. and Lebedev, D.A., 2018. Effects of glucagon-like peptide 1 analogs in combination with insulin on myocardial infarct size in rats with type 2 diabetes mellitus. World J. Diabetes, 9: 149-156. https://doi.org/10.4239/wjd.v9.i9.149
To share on other social networks, click on any share button. What are these?










