Molecular Identification and Prevalence of Blood Parasites and Rickettsia in Stray Dogs in DKI Jakarta Province
Research Article
Molecular Identification and Prevalence of Blood Parasites and Rickettsia in Stray Dogs in DKI Jakarta Province
Wirokartiko Satyawardana1,4, Umi Cahyaningsih2*, Fadjar Satrija2, Safika Safika3, Arifin Budiman Nugraha2
1Graduate Student of Animal Biomedical Science, School of Veterinary Medicine and Biomedical Science, Jl. Agatis, IPB Dramaga Campus, IPB University, Bogor, Indonesia; 2Division of Parasitology and Medical Entomology, School of Veterinary Medicine and Biomedical Sciences, Jl. Agatis, IPB Dramaga Campus, IPB University, Bogor, Indonesia; 3Division of Medical Microbiology, School of Veterinary Medicine and Biomedical Sciences, Jl. Agatis, IPB Dramaga Campus, IPB University, Bogor, Indonesia; 4Indonesian Quarantine Authority, Jl. Harsono RM No. 3, Jakarta.
Abstract | The population of stray dogs is an underestimated problem in several countries and serves as a critical reservoir for zoonotic parasites. Therefore, this study aimed to identify blood parasites and rickettsia in stray dogs in DKI Jakarta. It included 50 stray dogs that were statistically representative of the entire population used detect disease sampling technique from various cities across the province, where blood samples were collected and subjected to polymerase chain reaction (PCR) analysis, targeting parasites’ 18S and 16S RNA. PCR analysis showed that 19 samples were tested positive for blood parasites and rickettsia. Furthermore, the sequencing results identified two blood parasites and two infected rickettsia species, including Babesia canis vogeli, Hepatozoon canis, Anaplasma platys, and Ehrlichia canis. Using the t-test, data analysis, which covered numerous categories, comprising city of origin, sex, and age for each species of blood parasites and rickettsia, showed that only the male had a significant difference in Babesia canis vogeli infections with P-value of 0.023. In contrast to the other categories, there were no significant differences. Based on the construction of a phylogenetic tree of Neighbour Joining using pairwise distance, the sample isolates showed close relationships between several origins, including Babesia canis vogeli from Taiwan (JN717134), India (MN700646), and Brazil (MN823219), Hepatozoon canis from Uruguay (OR814232), Anaplasma platys from Egypt (MN227688), as well as Ehrlichia canis from Italy (GQ857078), Israel (U26740), the Philippines (JN121380), India (MK281328), America (MH620196), Brazil (EF195135), and Egypt (MG564254). This implies that parasites and rickettsia may have originated from a common ancestor.
Keywords | Anaplasma platys, Babesia canis vogeli, Ehrlichia canis, Hepatozoon canis, Haemoparasites
Received | July 16, 2024; Accepted | September 04, 2024; Published | October 24, 2024
*Correspondence | Umi Cahyaningsih, Division of Parasitology and Medical Entomology, School of Veterinary Medicine and Biomedical Sciences, Jl. Agatis, IPB Dramaga Campus, IPB University, Bogor, Indonesia; Email: umi-ch@apps.ipb.ac.id
Citation | Satyawardana W, Cahyaningsih U, Satrija F, Safika S, Nugraha AB (2024). Molecular identification and prevalence of blood parasites and rickettsia in stray dogs in DKI Jakarta province. Adv. Anim. Vet. Sci. 12(12): 2364-2375.
DOI | https://dx.doi.org/10.17582/journal.aavs/2024/12.12.2364.2375
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright: 2024 by the authors. Licensee ResearchersLinks Ltd, England, UK.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
INTRODUCTION
The population of stray dogs is an underestimated problem, with policies to address it frequently lacking or not properly enforced, particularly in developing countries. Globally, the animals are crucial reservoirs of zoonotic parasites (Otranto et al., 2017). Stray dogs are considered a complex problem in DKI Jakarta Province because they threaten residents and the surrounding environment (Tanderson and Chrissandy, 2019). The Ragunan Animal Health Center does not only provide health services to stray dogs and cats but also provides shelter for them (Putri and Merdekawati, 2019).
In recent years, a series of vector-borne diseases have spread to new geographical areas worldwide, several of which are caused by hemoparasites. Hemoparasites are pathogens that inhabit the bloodstream of the host and include microorganisms such as bacteria, mycoplasma, protozoa, and flagellates (Stuen, 2020). Thus, the rickets also fit this criterion.
Most of parasites and rickettsia found in dogs blood include Babesia canis vogeli, Anaplasma platys, and Ehrlichia canis (Otranto et al., 2015). In North Jakarta, these infections are prevalent in the blood of dogs and cats, with Anaplasma platys and Ehrlichia canis also being prevalent (Nasution, 2018). Faizal et al. (2019) found that 11.8% (6/51) of dogs were positive for Anaplasma platys from Prof. Dr. Soeparwi Animal Hospitals, animal clinics, and pet shops in the Yogyakarta area during March - November 2018. The two prevalent infections are generally transmitted by tick and do not show specific clinical symptoms, thereby requiring further tests to confirm the correct diagnosis (Santi, 2017). When examining the blood of stray dogs from Havana, Cuba, using real-time polymerase chain reaction (PCR), 107 dogs were tested, with 95 (89.09%) being infected. Another study found that a total of 41 (38.31 %), 66 (61.68 %), 28 (26.17 %), and 40 (37.38 %) stray dogs were infected with Anaplasma platys, Ehrlichia canis, Babesia canis vogeli, and Hepatozoon canis, respectively (Corona et al., 2018).
Although blood parasites and rickettsia are widely spread in Indonesia, particularly in DKI Jakarta Province, there is limited information regarding their prevalence and molecular identification, because molecular identification has never been carried out, only morphological identification has been performed. Therefore, this study aimed to identify the prevalence and risk factors of blood parasites and rickettsia in stray dogs in the province. Proper identification of such parasites is needed to develop effective control measures by interested parties.
MATERIALS AND METHODS
Sampling Area
Blood samples were collected from 50 stray dogs across six sampling areas, including North Jakarta (3 samples), West Jakarta (19 samples), Central Jakarta (8 samples), South Jakarta (10 samples), and Seribu Islands (1 sample). A total of 2 ml blood was collected from the cephalic antebrachial vein using a 3 ml syringe. Subsequently, the collected blood samples were placed in a vacutainer with K3 EDTA anticoagulant for PCR analysis. Blood smears were performed for Giemsa staining to determine the degree of parasitemia and observe the morphology of parasites (Ndungu et al., 2005).
The sampling technique used was Detect Disease (Sumiarto and Budiharta, 2021). Detect Disease is used to determine the sample size in areas that are free areas. The formula used is as follows:
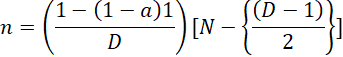
a= confidence level (usually 95% or 99%), N= population, n= sample, D= number of sick animals in the population.The number of samples taken from stray dogs based on 2015 population data was 15,000, assuming a prevalence of 12%; therefore, the number of samples taken was 48 samples.
DNA Extraction and PCR Amplification
DNA was extracted from stray dogs’ blood samples using a Genomic DNA miniKit for purification ® (Geneaid, Taiwan), in accordance with blood protocol method. The total DNA concentration was measured through the use of Nanodrop, and the extracted DNA was amplified using PCR method. PCR reaction was carried out in a total volume of 50 µL with a composition of 16 µL of nuclease-free water (NFW), 2 µl of each 10µM primer, 25 µl of My Taq HS Red Mix 2x ® (Bioline, UK), and 5 µL of template DNA. Amplification was performed using a pair of forward and reverse 18S rRNA primers, specifically Bab 7 and Bab 9 (Duarte et al., 2011), which were amplified at 490 bp (Table 1). This universal primer could detect several species of hemoparasite infections. The positive control uses Babesia gibsoni DNA while the negative control uses NFW.
Thermocycling conditions for the universal primer included pre-denaturation at 95°C for 1 min, followed by 35 cycles of denaturation at 95°C for 15 s, annealing at 51.5°C for 15 s, and elongation at 72°C for 10 s, with another elongation at 72°C for 1 min. In this study, a primer pair targeting 16S rRNA gene, specifically 16SANAF and 16SANAR primers (Table 1), was used to amplify a 421 bp (De la Fuente et al., 2006). For this primer, thermocycling conditions included pre-denaturation at 95°C for 1 min, followed by 35 cycles of denaturation at 95°C for 15 s, annealing at 57.5°C for 15 s, and elongation at 72°C for 10 s, with final elongation at 72°C for 1 min.
Table 1: Primers used in the PCR test to detect DNA of B. c. vogeli, H. canis, A. platys and E. canis in stray dogs in DKI Jakarta Province.
|
Pathogen |
Primer |
Sequence |
Product size |
Reference |
|
Hemoparasites |
Bab 7 |
5’-GGC TAC CAC ATC TAA GGA AG-3’ |
490 bp |
Duarte 2011 |
|
Bab 9 |
5’-CTA AGA ATT TCA CCT CTG ACA G- 3 |
|||
|
Rickettsia |
16 SANAF |
5’-CAG AGT TTG ATC CTG GCT CAG AAC G-3’ |
421 bp |
Fuente 2006 |
|
16 SANAR |
5’- GAG TTT GCC GGG ACT TCT TCT GTA-3’ |
Table 2: Prevalence of Blood Parasite and Rickettsia infections in stray dogs in DKI Jakarta Province.
|
Origin |
Number of samples |
B. c. vogeli |
H. canis |
A. platys |
B. c. vogeli + A. platys |
B. c. vogeli + E. canis |
Total infections |
|
North Jakarta |
3 |
1 |
- |
- |
- |
- |
1 |
|
West Jakarta |
19 |
3 |
- |
- |
2 |
1 |
6 |
|
Central Jakarta |
8 |
4 |
- |
- |
- |
- |
4 |
|
East Jakarta |
9 |
1 |
1 |
1 |
- |
- |
3 |
|
South Jakarta |
10 |
1 |
2 |
- |
1 |
- |
4 |
|
Seribu Islands |
1 |
- |
- |
1 |
- |
- |
1 |
|
Total |
50 |
10 (20%) |
3 (6%) |
2 (4%) |
3 (6%) |
1 (2%) |
19(38%) |
PCR analysis was visualized using electrophoresis on a 1% agarose gel in Tris-Acetate-EDTA (TAE) buffer (1X), followed by staining with 2 µL of FloroSafe DNA Stain® (1st BASE, Singapore). A 100 bp DNA ladder was used, and the expected sizes for 18S rRNA and 16S rRNA genes were approximately 490 and 421 bp, respectively.
DNA Sequencing Analysis
After PCR amplification, Sanger sequencing was performed to determine DNA composition of parasites. The sequence was carried out using two parties, namely PT. Genetic Science, Jakarta, Indonesia and Macrogen, Singapore to see consistent results. The results of the nucleotide sequences of parasites genes were adjusted to the database or library stored in GenBank, particularly NCBI (http://www.ncbi.nlm.nih.gov/blast) through BLAST program, and alignment is performed using ClustalW. To mitigate biases in the analysis, sequence alignment is performed using ClustalW. ClustalW is specifically designed to achieve high accuracy in multiple sequence alignment. The algorithm combines a robust heuristic approach with a progressive alignment method, enabling more precise alignment even when the sequences being aligned exhibit significant evolutionary differences. The phylogenetic construction was performed using MEGA.11. Phylogenetic analysis uses the Neighbor-Joining (NJ) method. Neighbor-Joining (NJ) is used in phylogenetics to construct evolutionary trees and infer evolutionary relationships based on sequence data obtained from BLAST results. To assess the quality of the phylogenetic tree, bootstrap analysis with 1000 replication was performed to measure the strength of the branch support in the tree.
Prevalence and Incidence Analysis
Data were analyzed using the Statistical Package for Social Sciences (SPSS, Chicago, IL, USA, and Version 20). The results were expressed as percentages and presented in the form of tables. Subsequently, the study design used in this research was detect disease with the chi-square test, and the odds ratio (OR) at a 95% confidence interval (CI) was used to assess risk factors, with statistical significance set at P < 0.05.
RESULTS AND DISCUSSION
From the 50 stray dogs’ blood samples collected from six areas in DKI Jakarta, 19 samples (38%) were tested positive for blood parasites and rickettsia through PCR and sequencing testing. The Nucleotide Basic Local Alignment Search Tool (BLAST) results were presented in Table 2, and the dominant single infection was Babesia canis vogeli, affecting 20% of the samples, followed by Hepatozoon canis (6%), and Anaplasma platys (4%). Furthermore, co-infections were observed, with 6% of the samples infected with the combinations of Babesia canis vogeli and Anaplasma platys, as well as 2% infected with Babesia canis vogeli and Ehrlichia canis.
Although there was no significant relationship between the variables of origin and age (P value > 0,05), sex must be considered as an important factor affecting haemoparasites and rickettsia infections in stray dogs in Jakarta. The incidence of these infections was significantly related to sex (P = 0.023), with male stray dogs having a 3.93 times higher risk of being infected than female counterparts (Table 3). The calculated P value was small (<0.05); therefore, there was a statistically significant relationship between the two classifications.
Table 3: Prevalence of Blood Parasite Infections and Rickettsiae in stray dogs in DKI Jakarta Province based on Category of Origin, Sex and Age.
|
Category |
Infections |
Prevalence |
P value |
OR |
|
|
(+) |
(-) |
% |
|||
|
Origin |
|||||
|
North Jakarta |
1 |
2 |
33.33 |
0.764 |
|
|
West Jakarta |
6 |
13 |
31.58 |
||
|
Central Jakarta |
4 |
4 |
50.00 |
||
|
East Jakarta |
3 |
6 |
33.33 |
||
|
South Jakarta |
4 |
6 |
40.00 |
||
|
Seribu Islands |
1 |
0 |
100.00 |
||
|
Sex |
|||||
|
Male |
13 |
11 |
54.17 |
0.023* |
3.93 |
|
Female |
6 |
20 |
23.08 |
||
|
Age |
|||||
|
≤ 3 years |
12 |
20 |
37.50 |
||
|
> 3 years |
7 |
11 |
38.89 |
0.92 |
1.06 |
|
19 |
31 |
38.00 |
|
|
|
* The calculated P value is small (<0.05), so there is a statistically significant relationship between the two classifications.
Table 4: Prevalence of Babesia canis vogeli infection in stray dogs in DKI Jakarta Province based on category of origin, gender and age.
|
Category |
Infections |
Prevalence |
P value |
OR |
|
|
(+) |
(-) |
% |
|||
|
Origin |
|||||
|
North Jakarta |
1 |
2 |
33.33 |
0.546 |
|
|
West Jakarta |
6 |
13 |
31.58 |
||
|
Central Jakarta |
4 |
4 |
50.00 |
||
|
East Jakarta |
1 |
8 |
11.11 |
||
|
South Jakarta |
2 |
8 |
20.00 |
||
|
Seribu Islands |
0 |
1 |
0.00 |
||
|
Sex |
|||||
|
Male |
9 |
15 |
37.50 |
0.135 |
2.64 |
|
Female |
5 |
22 |
18.52 |
||
|
Age |
|||||
|
≤ 3 years |
8 |
24 |
25.00 |
||
|
> 3 years |
6 |
13 |
31.58 |
0.611 |
1.38 |
|
Total |
14 |
36 |
28.00 |
|
|
In this study, the prevalence of Babesia canis vogeli reported for stray dogs (27.45%) was higher than the prevalence reported in pet dogs in Nigeria (10.8 %) (Obeta et al., 2020), Australia (3.6%) (Birkenheuer et al., 2020), India (10 %) (Manoj et al., 2020), Romania (3.3%) (Ciuca et al., 2021), and Chile (7.9%) (Di Cataldo et al., 2020). The low prevalence was attributed to the fact that pet dogs received insecticides more often than stray dogs. In addition, the prevalence of parasites tended to be higher in stray dogs when compared to pet dogs (Piratae et al., 2015). The animals that were the object of this study were stray dogs that did not receive adequate care, whereas in other studies, the animals that were the object of the research were pet dogs, which is why the prevalence rate in this study was much higher than that in several other studies.
Table 5: Prevalence of Hepatozoon canis infection in stray dogs in DKI Jakarta Province based on category of origin, gender and age.
|
Category |
Infections |
Prevalence |
P value |
OR |
|
|
(+) |
(-) |
% |
|||
|
Origin |
|||||
|
North Jakarta |
0 |
3 |
0.00 |
0.319 |
|
|
West Jakarta |
0 |
19 |
0.00 |
||
|
Central Jakarta |
0 |
8 |
0.00 |
||
|
East Jakarta |
1 |
8 |
11.11 |
||
|
South Jakarta |
2 |
8 |
20.00 |
||
|
Seribu Islands |
0 |
1 |
0.00 |
||
|
Sex |
|||||
|
Male |
2 |
22 |
8.33 |
0.514 |
2.27 |
|
Female |
1 |
25 |
3.85 |
||
|
Age |
|||||
|
≤ 3 years |
2 |
30 |
6.25 |
0.921 |
1.13 |
|
> 3 years |
1 |
17 |
5.56 |
||
|
Total |
3 |
47 |
6.00 |
|
|
The analysis results showed that P-values for the variables of origin, sex, and age were 0.546, 0.135, and 0.611, respectively. This indicated that there was no significant relationship between the incidence of Babesia canis vogeli and the three variables (Table 4). However, male stray dogs had a 2.64 times higher chance of being infected with such infections than their female counterparts. This suggested that sex might also influence the occurrence of babesiosis. Additionally, the temperature and hormonal status of male dogs could be considered important factors, as they contributed to limiting the attention and care provided by the owner. This was attributed to differences in environmental exposure, such as increased roaming behavior, or sex-related genetic or hormonal influences. Male dogs were more susceptible to flea infestations due to their higher tendency to wander around in search of mates and establish territories compared to female dogs, particularly those with puppies, who might receive better care from the owner (Obeta et al., 2020).
Table 6: Prevalence of Anaplasma platys infection in lying dogs in DKI Jakarta Province based on category of origin, gender and age.
|
Category |
Infections |
Prevalence |
P value |
OR |
|
|
(+) |
(-) |
% |
|||
|
Origin |
|||||
|
North Jakarta |
0 |
3 |
0.00 |
0.068 |
|
|
West Jakarta |
2 |
17 |
10.53 |
||
|
Central Jakarta |
0 |
8 |
0.00 |
||
|
East Jakarta |
1 |
8 |
11.11 |
||
|
South Jakarta |
1 |
9 |
10.00 |
||
|
Seribu Islands |
1 |
0 |
100.00 |
||
|
Sex |
|||||
|
Male |
5 |
19 |
20.83 |
0.072 |
14.94 |
|
Female |
0 |
26 |
0.00 |
||
|
Age |
|||||
|
≤ 3 years |
3 |
29 |
9.38 |
||
|
> 3 years |
2 |
16 |
11.11 |
0.844 |
1.2 |
|
Total |
5 |
45 |
10.00 |
|
|
In this study, stray dogs aged more than 3 years had a 1.38 times higher chance of being infected with Babesia canis vogeli. The incidence of babesiosis in dogs increased with age, reaching a peak between 3 and 5 years, before decreasing. In some protozoan infections, neonates from chronically infected mothers showed higher immunity to homologous parasites (Obeta et al., 2020).
Table 7: Prevalence of Ehrlichia canis infection in stray dogs in DKI Jakarta Province based on category of origin, gender and age.
|
Category |
Infections |
Prevalence |
P value |
OR |
|
|
(+) |
(-) |
% |
|||
|
Origin |
|||||
|
North Jakarta |
0 |
3 |
0.00 |
0.893 |
|
|
West Jakarta |
1 |
18 |
5.26 |
||
|
Central Jakarta |
0 |
8 |
0.00 |
||
|
East Jakarta |
0 |
9 |
0.00 |
||
|
South Jakarta |
0 |
10 |
0.00 |
||
|
Seribu Islands |
0 |
1 |
0.00 |
||
|
Sex |
|||||
|
Male |
0 |
24 |
0.00 |
||
|
Female |
1 |
25 |
3.85 |
0.523 |
2.88 |
|
Age |
|||||
|
≤ 3 years |
0 |
32 |
0.00 |
||
|
> 3 years |
1 |
17 |
5.56 |
0.3 |
5.57 |
|
Total |
1 |
49 |
2.00 |
|
|
The analysis results showed that P-values for the variables of origin, sex, and age were 0.319, 0.514, and 0.921, respectively (Table 5). This indicated that there was no significant relationship between the incidence of Hepatozoon canis and the three variables. Male stray dogs had a 2.27 times higher chance of being infected with such infections than female counterparts. Additionally, stray dogs between the ages of 3 years and below had a 1.13 times higher chance of being infected with Hepatozoon canis than those above 3 years.
The study conducted by Pacifico et al. (2020) showed that there was no significant relationship between Hepatozoon canis and sex. However, male dogs experienced a slight increase in the risk of contracting such infections. Male dogs might have a higher environmental exposure to tick-borne infections due to their roaming behavior. Although the rates of Hepatozoon canis were not significantly associated with age in the hunting dogs population, the prevalence was higher in adult dogs, possibly due to their longer duration of exposure to the vector.
The analysis results showed that P-values for the variables of origin, sex, and age were 0.068, 0.072, and 0.844, respectively (Table 6). This indicated that there was no significant relationship between the incidence of Anaplasma platys and the three variables. Male stray dogs had a 14.94 times higher chance of being infected with the infections than female counterparts. Additionally, stray dogs over the age of 3 years had a 1.2 times higher chance of being infected with Anaplasma platys when compared to those aged 3 years and below.
The study results showed that P-values for the variables of origin, sex, and age were 0.893, 0.523, and 0.3, respectively (Table 7). This indicated that there was no significant relationship between the incidence of Ehrlichia canis and the three variables. Female stray dogs had a 2.88 times higher chance of being infected with such infections than male counterparts. Additionally, stray dogs over the age of 3 years had a 5.57 times greater chance of being infected with Ehrlichia canis.
The analysis results showed that sex, race, and health status were not considered risk factors for Ehrlichia canis or Anaplasma platys. This suggested that other environmental factors, beyond immunological, ecological, and social factors might influence the risk of infection and they should be explored in future investigations. Positive results for Ehrlichia canis were more frequently observed in older dogs than in younger ones, due to their long exposure to infections in life. Furthermore, lower immune system competence in older dogs, which made them prone to infections, such as cancer, might contribute to a higher likelihood of infection (Perez-Machi et al., 2019). The most important risk factors for infection by hemoparasites include living in rural areas, kennels, animal shelters, or regions endemic for the infection, season of the year (which is associated with increased tick activity), infestation with ticks, and lack of treatment with acaricides (Zygner et al., 2023).
Table 8: Distance of the first clade between Babesia canis vogeli isolates from Jakarta and Taiwan isolates.
|
JN717134.1_ Uncultured_ Babesia_isolate_ Taiwan |
B._c._ vogeli_ isolate_ Central_ Jakarta-A5 |
B._c._ vogeli_ isolate_ Central_ Jakarta-A13 |
B._c._ vogeli _isolate_ Central_ Jakarta-A9 |
|
|
JN717134.1_Uncultured_Babesia_isolate_Taiwan |
||||
|
B._c._vogeli_isolate_Central_Jakarta-A5 |
0.00000 |
|||
|
B._c._vogeli_isolate_Central_Jakarta-A13 |
0.00000 |
0.00000 |
||
|
B._c._vogeli_isolate_Central_Jakarta-A9 |
0.00000 |
0.00000 |
0.00000 |
A phylogenetic tree construction was used to examine the evolutionary relationships between strains, using comparative strain sequences originating from National Center for Biotechnology Information (NCBI) database. The relationships were presented in a neighbor-joining (NJ) tree, as shown in Figures 1-4. NJ method identified sequences that best calculate the closest branch length, indicating the distance between sequences. The confidence level of a phylogenetic tree could be estimated using bootstrap values of 100–1000 replications (Hall, 2001).
Based on Figure 1, a phylogenetic tree showed that Jakarta isolates belonged to the same group as Babesia canis vogeli. This indicated that the sample isolates were in a monophyletic group with other Babesia canis vogeli strains. A monophyletic group, also called a clade, was a group of organisms that descended from a common ancestor, forming monophyletic taxa or clades. This term was also used by a molecular phylogenetic to determine a particular group of DNA (Deoxyribo Nucleic Acid), RNA (Ribonucleic Acid), or protein sequences in a phylogenetic tree (Moreira and Lopez, 2011). The sample isolates were closely related to Babesia canis vogeli, originating from Taiwan (JN717134), with Anaplasma phagocytophilum (OR287091) serving as the outgroup.
In this study, the partial sequence analysis of 14 Babesia canis vogeli 18S rRNA gene fragments showed the same identity as Babesia canis vogeli strains from the GenBank database, representing different geographic regions. The phylogenetic tree (Figure 1) indicated that Babesia canis vogeli isolates from Central Jakarta (sample codes A5, A13, and A9) and uncultured Babesia isolates (JN717134) from Taiwan were in the same cluster and had very close pairwise distances. As shown in Table 8, there were no DNA differences among these four isolates.
Babesia canis vogeli isolates from Central Jakarta (A8), West Jakarta (sample codes 3, 6, and 8), South Jakarta (sample codes A16 and A7BLOKB), East Jakarta (A6BLOKB), and North Jakarta (A18) showed the same cluster and had a very close pairwise distance, with no DNA differences among these eight isolates (Table 9). From the total samples, only Babesia_canis_vogeli_isolat_West_Jakarta-12 had a pairwise distance with a value of 0.1 (Table 10).
The analysis indicates that the Jakarta isolates are part of a monophyletic group with other B. canis vogeli strains. A monophyletic group, or clade, comprises organisms that descend from a common ancestor and includes all of its descendants. In this case, the Jakarta isolates and the B. canis vogeli strains from Taiwan share a common ancestor and have inherited traits from this ancestral lineage.
Table 9: Distance of the second clade of Babesia canis vogeli isolates from Jakarta.
|
B._c ._vogeli_ isolate_ West_ Jakarta-3 |
B._c._ vogeli_ isolate_ South_ Jakarta-A16 |
B._c._ vogeli_ isolate_ East_ Jakarta- A6BLOKB |
B._c._ vogeli_ isolate_ Central_ Jakarta-A8 |
B._c._ vogeli_ isolate_ South_ Jakarta- A7BLOKB |
B._c._ vogeli_ isolate_ North_ Jakarta-A18 |
B._c._ vogeli_ isolate_ West_ Jakarta-8 |
B._c._ vogeli_ isolate_ West_ Jakarta-6 |
|
|
B._c._ vogeli_ isolate_ West_ Jakarta-3 |
||||||||
|
B._c._ vogeli_ isolate_ South_ Jakarta-A16 |
0.00000 |
|||||||
|
B._c._ vogeli_ isolate_ East_ Jakarta-A6BLOKB |
0.00000 |
0.00000 |
||||||
|
B._c._ vogeli_ isolate_ Central_ Jakarta-A8 |
0.00000 |
0.00000 |
0.00000 |
|||||
|
B._c._ vogeli_ isolate_ South_ Jakarta-A7BLOKB |
0.00000 |
0.00000 |
0.00000 |
0.00000 |
||||
|
B._c._ vogeli_ isolate_ North_ Jakarta-A18 |
0.00000 |
0.00000 |
0.00000 |
0.00000 |
0.00000 |
|||
|
B._c._ vogeli_ isolate_West_ Jakarta-8 |
0.00000 |
0.00000 |
0.00000 |
0.00000 |
0.00000 |
0.00000 |
||
|
B._c._ vogeli_ isolate_ West_ Jakarta-6 |
0.00000 |
0.00000 |
0.00000 |
0.00000 |
0.00000 |
0.00000 |
0.00000 |
The classification of the Jakarta isolates within the same monophyletic group as other B. canis vogeli strains supports the conclusion that they belong to the same evolutionary lineage. This finding provides valuable insights into the evolutionary history and genetic diversity of B. canis vogeli. Overall, the phylogenetic tree offers a clear depiction of the evolutionary relationships between the Jakarta isolates and other B. canis vogeli strains, affirming that the Jakarta isolates are part of the same evolutionary lineage as these strains.
A phylogenetic tree construction (Figure 2) showed that Jakarta isolates belonged to the same group as Hepatozoon canis. This indicated that the sample isolates were part of a monophyletic group with other Hepatozoon canis strains. The isolates were closely related to Hepatozoon canis from Uruguay (OR814232), India (JN584477), Taiwan (EU289222), Thailand (DQ519358), Israel (MK091085), and Spain (AY461378), with Anaplasma phagocytophilum (OR287091), serving as the outgroup.
The phylogenetic tree showed that the isolates from South Jakarta (A21 and A19) and East Jakarta (A3) had the same cluster with isolates from Uruguay (OR814232), India (JN584477), Taiwan (EU289222), Thailand (DQ519358), Israel (MK091085), and Spain (AY461378). All these isolates had a very close pairwise distance with a value ranging from 0.000-0.004 (Table 11).
The Jakarta isolates show a close relationship with Hepatozoon canis strains from various geographic locations. This relationship indicates that Hepatozoon canis has a widespread global distribution or that these strains share a common evolutionary origin, confirming that the Jakarta isolates are part of the same evolutionary lineage. This finding provides insights into the global distribution and evolutionary history of this pathogen, as well as potential transmission routes or migration patterns.
As presented in Figure 3, the phylogenetic tree construction showed that Jakarta isolates belonged to the Anaplasma platys group. This indicated that the sample isolates were part of a monophyletic group with other Anaplasma platys strains. In this analysis, the outgroup included Babesia vogeli (MN823219). The phylogenetic tree showed that Anaplasma platys isolates from West Jakarta (8) and Kepulauan Seribu (15) were in the same cluster, with a pairwise distance close to a value of 0.00000 (Table 12).
Based on Figure 4, the construction of a phylogenetic tree showed that Jakarta isolates belonged to the same group as
Ehrlichia canis. This indicated that the sample isolates were part of a monophyletic group with other Ehrlichia canis strains. The isolates were closely related to Ehrlichia canis from Italy (GQ857078) and Egypt (MG564254), with Babesia vogeli (MN823219) serving as the outgroup. The phylogenetic tree showed that Ehrlichia canis isolates from West Jakarta (12) were closely related to the isolates from Italy (GQ857078) and Egypt (MG564254) with pairwise distances of 0.00671 and 0.00696, respectively (Table 13).
The pairwise distance results for each blood parasite and rickettsia showed that the blood parasites and rickettsia that infect stray dogs in the DKI Jakarta Province area are closely related to several comparative blood parasites and rickettsiae from several countries. This is likely to occur as a result of dog traffic from these countries carrying diseases such as blood parasites and rickettsia which then infect local dogs in the DKI Jakarta province.
CONCLUSIONS AND RECOMMENDATIONS
In conclusion, blood parasites and rickettsia that infected stray dogs in DKI Jakarta Province areas included Babesia canis vogeli, Hepatozoon canis, Anaplasma platys, and Ehrlichia canis. The prevalence of these infections was 38%, with a significant difference in Babesia canis vogeli rates between male and female stray dogs (p = 0.023). The sample isolates showed close relationships between origins, comprising Babesia canis vogeli from Taiwan (JN717134), Hepatozoon canis from Uruguay (OR814232), India (JN584477), Taiwan (EU289222), Thailand (DQ519358), Israel (MK091085), and Spain (AY461378), Anaplasma platys from Portugal (KX180944) and Mexico (KT357373), as well as Ehrlichia canis from Italy (GQ857078) and Egypt (MG564254). This implies that parasites and rickettsia may have originated from a common ancestor. This study shows that stray dogs have a high prevalence of blood parasitic diseases and rickettsiae, and thus, have the potential to infect pet dogs. Therefore, policies aimed at increasing the number of animal shelters are necessary. proposed a program to the government to prevent and control parasites in dogs, especially blood parasites. For further research, a larger sample size and different regions can be considered. This study has several limitations, including time constraints and the investigation of tick species that
have the potentialto transmit blood parasites to dogs. It would be better if, in future studies, the research time can be extended and ectoparasites that have the potential as vectors can be investigated.
ACKNOWLEDGEMENTS
The authors are grateful to the Ministry of Agriculture of Indonesia for the support provided. This study received funding from the Agency of Agricultural Counseling and Human Resources Development, Ministry of Agriculture, Indonesia, with a study assignment scholarship number 491/KPTS/KP.320/A/08/2021.
Novelty Statement
This study aimed to identify the presence of blood parasites and ricketsia in dogs using a molecular approach and phylogenetic analysis. from the DKI Jakarta Province, Indonesia
AUTHOR’S CONTRIBUTIONS
Wirokartiko Satyawardana contributed to data collection, data analysis, and the preparation of the original manuscript. Umi Cahyaningsih, Fadjar Satrija, Safika, and Arifin Budiman Nugraha were included in the study design, manuscript revision, and supervision. The authors have read and approved the final version of the manuscript.
Ethical Approval
The Animal Ethics Committee School of Veterinary Medicine and Biomedical Sciences, Bogor Agricultural University, carefully reviewed and approved the study protocol under Number 055/KEH/SKE/XII/2022 in order to ensure compliance with animal welfare standards.
Conflict of Interest
The authors declare no conflict of interest with any financial, personal, or other relationships with people or organizations related to the material discussed in the manuscript.
REFERENCES
Birkenheuer AJ, Buch J, Beall MJ, Braff J, Chandrashekar R (2020). Global distribution of canine Babesia species identified by a commercial diagnostic laboratory. Veterinary Parasitology: Reg. Stud. Rep., 22: 100471. https://doi.org/10.1016/j.vprsr.2020.100471
Ciuca L, Martinescu G, Miron LD, Roman C, Acatrinei D, Cringoli G, Rinaldi L, Maurelli MP (2021). Occurrence of Babesia Species and Co-Infection with Hepatozoon Canis in Symptomatic Dogs and in Their Ticks in Eastern Romania. Pathogens,10: 1339. https://doi.org/10.3390/pathogens10101339
Corona BG, Díaz-Sánchez AA, Meli ML, Cañizares EV, Arias LR, Dorta YL, Rivero EL, Hofmann-Lehmann R (2018). Occurrence of tick-borne pathogens in stray dogs from Havana Cuba. Acta Biomed. Sci., 3(4): 158-159. https://doi.org/10.29413/ABS.2018-3.4.25
De la Fuente J, Torina A, Naranjo V, Nicosia S, Alongi A, La Mantia F, Kocan KM (2006). Molecular characterization of Anaplasma platys strains from dogs in Sicily Italy. BMC Vet. Res., 2: 1-5. https://doi.org/10.1186/1746-6148-2-24
Di Cataldo S, Ulloa‐Contreras C, Cevidanes A, Hernández C, Millán J (2020). Babesia vogeli in dogs in Chile. Transboundary Emerg. Dis., 67(6): 2296-2299. https://doi.org/10.1111/tbed.13609
Duarte SC, Parente JA, Pereira M, Soares CMDA, Linhares GFC (2011). Phylogenetic characterization of Babesia canis vogeli in dogs in the state of Goiás Brazil. Revista Brasileira de Parasitologia Veterinária; 20: 274-280. https://doi.org/10.1590/S1984-29612011000400004
Faizal MD, Haryanto A, Tjahajati I. (2019). Diagnosis and molecular characterization of Anaplasma platys in dog patients in Yogyakarta area, Indonesia. Indonesian Journal of Biotechnology, 24(1): 43-50.
Hall BG (2001). Phylogenetic trees made easy: a how-to manual for molecular biologists. Sinauer Associates Inc. Sunderland Massachusetts USA.
Manoj RRS, Iatta R, Latrofa MS, Capozzi L, Raman M, Colella V, Otranto D (2020). Canine vector-borne pathogens from dogs and ticks from Tamil Nadu India. Acta trop., 203: 105308. https://doi.org/10.1016/j.actatropica.2019.105308
Moreira D, Lopez-Garcia P (2011). Monophyletic. In: Gargaud (eds) Encyclopedia of Astrobiology. Springer Berlin Heidelberg. https://doi.org/10.1007/978-3-642-11274-41022 https://doi.org/10.1007/978-3-642-11274-4_1022
Nasution AYA (2018). Study of parasitosis in pet dogs and cats coming to a veterinary clinic in North Jakarta. MSc thesis Institut Pertanian Bogor Bogor Indonesia.
Ndungu SG, Brown CGD, Dolan TT (2005). In vivo comparison of susceptibility between Bos indicus and Bos Taurus cattle types to Theileria parva infection. Onderstepoort J. Ve.t Res., (72): 13-22. https://doi.org/10.4102/ojvr.v72i1.220
Obeta SS, Ibrahim B, Lawal IA, Natala J (2020). A Ogo N. I Balogun E. O. Prevalence of canine babesiosis and their risk factors among asymptomatic dogs in the federal capital territory Abuja Nigeria. Parasite Epidemiology and Control, 11: e00186. https://doi.org/10.1016/j.parepi.2020.e00186
Otranto D, Cantacessi C, Pfeffer M, Dantas-Torres F, Brianti E, Deplazes P, Genchi C, Guberti V, Capelli G (2015). The role of wild canids and felids in spreading parasites to dogs and cats in Europe: Part I: Protozoa and tick-borne agents. Vet. Parasitol., 213(1-2): 12-23. https://doi.org/10.1016/j.vetpar.2015.04.022
Otranto D, Dantas-Torres F, Mihalca AD, Traub RJ, Lappin M, Baneth G (2017). Zoonotic parasites of sheltered and stray dogs in the era of the global economic and political crisis. Trends Parasitol., 33(10): 813-825. https://doi.org/10.1016/j.pt.2017.05.013
Pacifico L, Braff J, Buono F, Beall M, Neola B, Buch J, Sgroi G, Piantedosi D, Santoro M, Tyrrell P, Fioretti A, Breitschwerdt EB, Chandrashekar R, Veneziano V (2020). Hepatozoon canis in hunting dogs from Southern Italy: Distribution and risk factors. Parasitol. Res., 119: 3023-3031. https://doi.org/10.1007/s00436-020-06820-2
Pérez-Macchi S, Pedrozo R, Bittencourt P, Müller A (2019). Prevalence molecular characterization and risk factor analysis of Ehrlichia canis and Anaplasma platys in domestic dogs from Paraguay. Comp. Immunol. Microbiol. Infect. Dis., 62: 31-39. https://doi.org/10.1016/j.cimid.2018.11.015
Piratae S, Pimpjong K, Vaisusuk K, Chatan W (2015). Molecular detection of Ehrlichia canis Hepatozoon canis and Babesia canis vogeli in stray dogs in Mahasarakham province Thailand. Ann. Parasitol., 61(3).
Putri DR, Merdekawati A (2019). Web-Based Product Sales Information System and Donation Distribution for Animal Shelters. JTIM: Jurnal Teknologi Informasi dan Multimedia; 1(3): 269-278. https://doi.org/10.35746/jtim.v1i3.44
Santi GP (2017). The severity of dogs that are positive for Rickettsia infection using the Polymerase Chain Reaction (PCR) test in the Yogyakarta area via hematology examination. PhD thesis Universitas Gadjah Mada Yogyakarta Indonesia.
Stuen S (2020). Haemoparasites-Challenging and Wasting Infections in Small Ruminants: A Review. Animals (Basel).,10(11):2179. doi: 10.3390/ani10112179. PMID: 33266365; PMCID: PMC7700527. https://doi.org/10.3390/ani10112179
Sumiarto B, Budiharta S (2021). Analytical Veterinary Epidemiology. UGM PRESS.
Tanderson D, Chrissandy R (2019). Designing a Digital Promotional Video for the Jakarta Animal Aid Network on Social Media. Rupaka, 2(1).
Zygner W, Gójska-Zygner O, Bartosik J, Górski P, Karabowicz J, Kotomski G, Norbury LJ (2023). Canine babesiosis caused by large Babesia species: global prevalence and risk factors—a review. Animals, 13(16): 2612. https://doi.org/10.3390/ani13162612
To share on other social networks, click on any share button. What are these?






