Micronuclei Assay: A Suitable Tool for Evaluating the Heavy Metals Induced Genotoxicity in Fish, Labeo rohita
Micronuclei Assay: A Suitable Tool for Evaluating the Heavy Metals Induced Genotoxicity in Fish, Labeo rohita
Abeera Razzaq1, Sajid Abdullah1, Huma Naz2*, Khalid Abbas1, Laiba Shafique3 and Qingyou Liu3
1Department of Zoology, Wildlife and Fisheries, University of Agriculture, Faisalabad
2Department of Zoology, Cholistan University of Veterinary and Animal Sciences, Bahawalpur
3State Key Laboratory for Conservation and Utilization of Subtropical Agro-Bioresources, Guangxi University, Nanning, Guangxi 530005, PR China
ABSTRACT
Increasing load of heavy metals from industrial, agricultural and commercial chemicals discharged into aquatic habitats could pose a serious threat to the aquatic life like fish. These metals have ability to induce the production of reactive oxygen species (ROS). These ROS can interact with nucleic acids and cause oxidation of DNA. The potential of ROS to damage DNA has become a topic of significant interest for environmental toxicology studies. The present research was conducted to assess the genotoxic potential of cobalt (Co) and chromium (Cr) mixture to fish Labeo rohita by using micronuclei assay. Fish were exposed to the four sub-lethal doses (26.61, 13.30, 19.98 and 7.98 mgL-1) of Cr+Cr mixture for 28 days and blood was sampled after 7 days interval. A group (n=10) of fish were also kept in clean water (negative control=NC) and cyclophasphamid (positive control=PC), separately. Blood from caudal vein of fish was collected to see the micronuclei (MN) and de-shape nuclei (DN). Results showed that test dose 26.61 mgL-1 induced significantly higher mean MN followed by 13.30 mgL-1, 19.98 mgL-1, 7.98 mgL-1, PC and NC. However the result of DN showed minor difference. The frequency of DN in RBCs was maximum in fish exposed to 26.61 mgL-1 dose followed by the order: 13.30 mgL-1> 19.98 mgL-1> PC > 7.98 mgL-1> NC. Micronuclei and deshaped nuclei frequency differ with the exposure period as 28>21>14>7 days. This study concluded that metals present in mixture in aquatic environment can induce DNA damage in fish.
Article Information
Received 25 March 2019
Revised 22 May 2019
Accepted 11 September 2019
Available online 19 August 2021
Authors’ Contribution
AR conducted the research. SA planned the project. HN and KA helped in experimental work. LS and QL helped in writing the manuscript.
Key words
Metals mixture, DNA damage, Sub-lethal exposure, Fish, Blood
DOI: https://dx.doi.org/10.17582/journal.pjz/20190325130338
* Corresponding author: [email protected]; [email protected]
0030-9923/2021/0005-1997 $ 9.00/0
Copyright 2021 Zoological Society of Pakistan
The presence of genotoxic compounds in aquatic ecosystem raises the question about their impact on existing and future aquatic life (DaSilva-Souza and Fontanetti, 2006). Therefore, when evaluating genotoxicity in aquatic fauna especially fish, metals considered an important group of toxicants due their persistency, amassment in animals, water and sediments and also have strong impact on stability of aquatic environment (Has-Schon et al., 2006).
Chromium is broadly used in manufacturing of dyes and pigments. Chromium is listed as a toxic metal due to its ability to amass in fish body (Avenant-Oldewage and Marx, 2000). Cobalt is an indispensable metal and plays an important role in biochemical functions but its higher quantity in water becomes lethal to fish as it alter the enzyme activity (Yaqub and Javed, 2012). It is also reported as a potential carcinogenic compound to humans (I.A.R.C., 2003).
Genotoxicity induced by metals can be successfully evaluated in aquatic environment by the application of useful techniques, like Micronucleus assay used for quantify DNA damage, on exposed sentinel species (Frenzilli et al., 2009; Bolognesi and Hayashi, 2011). Now, micronucleus test has been widely used to detect both type of genotoxic substances such as clastogens and aneugens, in field and laboratory (Rajan et al., 2012; Obiakor et al., 2010a, b; Barsiene et al., 2006). Micronuclei are fragment of chromosome that lack centromere or whole chromosomes that lag behind at anaphase during cell division (Rajan et al., 2012; Fenech, 2007; Fenech, 2002). The concurrent appearance of morphological erythrocytic nuclear abnormalities (NAs) together with micronuclei has gained much interest of researchers. The mechanism behind the formation of NAs have not been understood however, these all are also be used as valuable marker for assessing the genotoxicity in complement to micronuclei (Cavas and Ergene-Gozukara 2005a, b; Fenech and Crott 2002; ). Therefore, this work was conducted to assess the genotoxic potential of metals mixture on erythrocytes of Labeo rohita.
Materials and methods
Labeo rohita was selected for this experiment. Fish were procured from Fish Seed Hatchery, Faisalabad and live transferred to wet laboratory at Fisheries Research Farm, University of Agriculture Faisalabad. Fish were initially acclimatized to laboratory environment for a period of 14 days and then fish (n=10) of 120-day age (average weight, 16.21±0.35; length,115.41±2.26) were shifted to 100-L glass aquarium. The LC50 of chromium(Cr)+cobalt(Co) mixture for 96-hr for L. rohita was calculated as 39.92 mgL-1 (Batool and Javed, 2015). Fish were treated with different sub-lethal doses viz. 2/3rd, 1/ 3rd,1/4th and 1/5th of LC50 of Cr+Co mixture calculated as 26.61, 13.30, 19.98 and 7.98 mgL-1, respectively. Water was partially changed throughout the study period. Fish were treated for one month and blood samples were collected after seven days interval. Fish (n=10) were maintained in clean water considered as negative control (NC) (Kousar and Javed, 2015). The cyclophasphamid was used as a positive control (PC). Fish were fed with diet available commercially at 2% body weight. The water quality parameters such as water hardness (225 mgL-1), temperature (28ᵒC), pH (7.0) and dissolve oxygen (5ppm) were monitored for whole study.
Blood from the fish caudal vein was collected and instantly smeared a drop on slide. The smears were immediately fixed in methanol for 10 minute and left over for air dry, and finally stained with wright-giemsa stain for 8 minutes (Barsiene et al., 2004). Scoring of micronuclei and deshape nuclei were performed (per 1,000 cell) on coded slides using a binocular microscope according to the criteria described by Fenech et al. (2003). Following formulae was used to calculate the MN frequency.
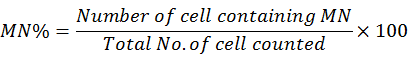
The experiment was performed with three independent replicates for each treatment. Data were expressed as mean (±SE) and analyzed by non-parametric Mann-Whitney U-test (Steel et al., 1996).
Results
Results showed that test dose 26.61 mgL-1 induced significantly higher mean MN followed by 13.30 mgL-1, 19.98 mgL-1, 7.98 mgL-1, PC and NC (Fig. 1C). However, the result of DN showed minor difference. The frequency of DN in RBCs was maximum in fish exposed to 26.61 mgL-1 dose followed by the order: 13.30 mgL-1> 19.98 mgL-1> PC > 7.98 mgL-1> NC (Fig. 1B). Micronuclei and deshaped nuclei frequency differ with the exposure period as 28>21>14>7 days (Table I).
Discussion
Aquatic environment has been contaminated due to presence of various toxicants released from industries and agricultural activities (Isani et al., 2009). Aquatic pollutants include heavy metals which may enter in aquatic ecosystem through natural and anthropogenic sources. Sub-lethal exposure of these metals to aquatic organisms results in amassment of metals in tissues that cause unfavorable effects not only in the exposed animal, but also in humans through food chain (IARC, 1993). Various toxicants in contaminated water can induce damage to genetic material of aquatic life and produce geno-toxic effects. Aquatic animals especially, fish is a good indicator to study the heavy metals produced genotoxic and mutagenic damage such as DNA strand breakages (Pruski and Dixon, 2002).
In recent investigation, the ability of Cr+Co mixture to produce micronuclei (MN) and deshape nuclei in erythrocytes of L. rohita varied due to test doses and exposure period. The formation of MN and deshaped nuclei increased during first 21-day, after that it was decreased. These nuclear abnormalities can be rapidly defeated by DNA repair mechanisms (Mateuca et al., 2006). Mixture of Cd+Zn significantly increased the formation of MN and nuclear abnormalities (NA) in the erythrocyte of O. niloticus in a duration dependent manner (Abu-Bakar et al., 2014). Similarly, Jiraungkoorskul et al. (2007) also observed the time-dependent effects in RBCs of fish exposed to cadmium, copper, lead, and cadmium chloride. Potential of cadmium and mercury to induce MN and NA in Phoxinus phoxinus was recorded by Ayllon and Gracia-Vazquez (2000).
Several authors reported the Cd induced MN in RBCs of fish like C. paleatus, C. carpio and C. gibelio (Cavas et al., 2005) and A. anguilla (Sanchez-Galan et al., 2001). According to Udroiu (2006) the production of micronucleus in blood varies due to species and exposure period. A significantly positive correlation between metal
Table I. Cr+Co mixture induced nuclear abnormalities in Labeo rohita.
|
Parameters |
Exposure duration |
Doses |
|||||
|
NC |
PC |
7.98 mgL-1 |
19.98 mgL-1 |
13.30 mgL-1 |
26.61mgL-1 |
||
|
Micronuclei |
7-day |
1.00Df |
22.00De |
27.00Dd |
42.00Dc |
48.00Db |
50.00Da |
|
14-day |
2.00Cf |
27.00Ce |
30.00Cd |
46.00Cc |
53.00Cb |
58.00Ca |
|
|
21-day |
3.00Bf |
31.00Be |
34.00Bd |
51.00Bc |
60.00Bb |
67.00Ba |
|
|
28-day |
2.00Af |
32.00Ae |
37.00Ad |
59.00Ac |
64.00Ab |
70.00Aa |
|
|
Micronuclei frequency (%) |
7-day |
0.05±0.02Df |
1.1±0.04De |
1.35±0.03Dd |
2.20±0.05Dc |
2.40±0.40Db |
2.70±0.19Da |
|
14-day |
0.10±0.01Cf |
1.35±0.02Ce |
1.50±0.01Cd |
2.30±0.01Cc |
2.65±0.02Cb |
2.90±0.02Ca |
|
|
21-day |
1.15±0.02Bf |
1.55±0.06Be |
1.70±0.06Bd |
2.55±0.23Bc |
3.00±0.19Bb |
3.35±0.12Ba |
|
|
28-day |
0.10±0.01Af |
1.60±0.10Ae |
1.85±0.10Ad |
2.95±0.10Ac |
3.20±0.02Ab |
3.50±0.10Aa |
|
|
De-shaped |
7-day |
2.00Df |
6.00Dd |
4.00De |
7.00Dc |
9.00Db |
13 .00Da |
|
14-day |
2.00 Cf |
10.00Cd |
7.00Ce |
8.00Cc |
11.00Cb |
15.00Ca |
|
|
21-day |
3.00Bf |
11.00Bd |
9.00Be |
11.00Bc |
16.00Bb |
19.00Ba |
|
|
28-day |
4.00Af |
14.00Ad |
12.00Ae |
13.00Ac |
19.00Ab |
21.00Aa |
|
|
De-shaped frequency (%) |
7-day |
0.10±0.01Df |
0.30±0.10Dd |
0.20±0.05De |
0.35±0.4Dc |
0.40±0.20Db |
0.60±0.10Da |
|
14-day |
0.10±0.01Cf |
0.50±0.03Cd |
0.35±0.01Ce |
0.40±0.02Cc |
0.55±0.04Cb |
0.75±0.10Ca |
|
|
21-day |
0.15±0.02Bf |
0.55±0.10Bd |
0.45±0.10Be |
0.55±0.02Bc |
0.80±0.02Bb |
0.95±0.10Ba |
|
|
28-day |
0.20±0.01Af |
0.70±0.03Ad |
0.60±0.01Ae |
0.65±0.04Ac |
0.95±0.02Ab |
1.05±0.30Aa |
|
Small alphabet superscripts show the difference between treatments within the same row While capital alphabet shows significant (P < 0.05) among different durations of exposure within the same column.
concentration and frequency of NA in O. niloticus was noted by Summak et al. (2010). A dose (arsenic) reliant raise in MN and NA frequency in Channa punctatus and Carassius auratus was observed by Kumar et al. (2013). Kousar and Javed (2016) also reported the arsenic induced micronuclei and de-shaped nuclei in fish species viz. L. rohita, C. idella, C. catla and C. mrigala. Arsenic, mercury and copper also can induced genotoxicity in Channa punctata (Yadav and Trivedi, 2009). Kousar et al. (2018) observed the concentration dependent higher micronuclei frequency in RBCs of Cirrhina mrigala. Chromium induced genotoxicity in term of micronuclei in L. rohita was observed by Parveen et al. (2011). The abattoir effluent also induced the frequency of MN in a time reliant manner was observed by Alimba et al. (2015). Rasal et al. (2011) also reported the chromium induced genotoxicity in Labeo rohita.
Conclusion
The present study indicated that Cr+Co mixture is a genotoxic agent for Labeo rohita under different concentrations. Micronucleus test is a sensitive and rapid method to detect the effect of heavy metal pollution in aquatic environment.
Statement of conflict of interest
The authors declare there is no conflict of interest.
References
Abu-Bakar, S.N.N., Ashriya, A., Shuib, A.S. and Razak, S.A., 2014. Sains Malay., 43: 1053-1059.
Alimba, C.G., Ajayi, E.O., Hassan, T., Sowunmi, A.A. and Bakare, A.A., 2015. Chinese J. Biol., 1-6. https://doi.org/10.1155/2015/624524
Avenant-oldewage, A. and Marx, H.M., 2000. Water S.A., 26: 621-640
Ayllon, F. and Gracia-Vazquez, E., 2000. Mutat. Res., 467: 177-186. https://doi.org/10.1016/S1383-5718(00)00033-4
Barsiene, J., Dedonyte, V., Rybakovas, A., Andreikenaite, L. and Andersen, O.K., 2006. Aquat. Toxicol., 78: 99-104. https://doi.org/10.1016/j.aquatox.2006.02.022
Barsiene, J., Lazutka, J., Syvokiene, J., Dedonyte, V., Rybakovas, A., Bjornstad, A. and Andersen, O.K., 2004. Environ. Toxicol., 19:365-371. https://doi.org/10.1002/tox.20031
Batool, U. and Javed, M., 2015. Pakistan J. Zool., 47: 617-623.
Bolognesi, C. and Hayashi, M., 2011. Mutagenesis, 26: 205-213. https://doi.org/10.1093/mutage/geq073
Cavas, T. and Ergene-Gozukara, S., 2005a. Aquat. Toxicol., 74: 264-271. https://doi.org/10.1016/j.aquatox.2005.06.001
Cavas, T. and Ergene-Gozukara, S., 2005b. Environ. Mol. Mutagen., 46: 64-70. https://doi.org/10.1002/em.20130
Cavas, T., Garanko, N.N. and Arkhipchuk, V.V., 2005. Fd. Chem. Toxicol., 43: 569-574. https://doi.org/10.1016/j.fct.2004.12.014
DaSilva-Souza, T. and Fontanetti, C.S., 2006. Mutat. Res., 60: 587-593.
Fenech, M., 2002. Drug. Discov. Today, 7: 1128-1137. https://doi.org/10.1016/S1359-6446(02)02502-3
Fenech, M., 2007. Nat. Protoc., 2: 1084-1104. https://doi.org/10.1038/nprot.2007.77
Fenech, M., Chang, W.P., Kirsch-Volders, M., Holland, N., Bonassi, S. and Zeiger, E., 2003. Mutat. Res., 534: 65-75. https://doi.org/10.1016/S1383-5718(02)00249-8
Fenech, M. and Crott, J.W., 2002. Mutat. Res., 504: 131-136. https://doi.org/10.1016/S0027-5107(02)00086-6
Frenzilli, G., Nigro, M. and Lyons, B.P., 2009. Mutat. Res./Rev. Mutat. Res., 681: 80-92. https://doi.org/10.1016/j.mrrev.2008.03.001
Has-Schon, E., Bogut, I. and Strelec, I., 2006. Arch. environ. Contam. Toxicol., 50: 545-551. https://doi.org/10.1007/s00244-005-0047-2
I.A.R.C., 2003. Monographs on the evaluation of carcinogenic risks to humans. Vol. 86, Lyon, France.
IARC, 1993. IARC scientific publications, Lyon, France. 58: 119-238.
Isani, G., Andreani, G., Cocchioni, F., Fedeli, D., Carpene, E. and Falcioni, G., 2009. Ecotoxicol. Environ. Safe., 72: 224-230. https://doi.org/10.1016/j.ecoenv.2008.04.015
Jiraungkoorskul, W., Kosai, P., Sahaphong, S. and Kirtputra, P., 2007. Res. J. environ. Sci., 1: 56-63.
Kligerman, D., 1982. Mutagenicity: New horizons in genetic technology. Acad. Press, New York, USA, pp. 435-456. https://doi.org/10.1016/B978-0-12-336180-6.50020-X
Kousar, S. and Javed, M., 2015. Turk. J. Fish. aquat. Sci., 15: 879-886. https://doi.org/10.4194/1303-2712-v15_4_11
Kousar, S. and Javed, M., 2016. J. Anim. Plant. Sci., 26: 1501-1506.
Kousar, S., Javed, M., Ambreen, F., Ashraf, A., Ilyas, R., Batool, M. and Azmat, H., 2018. Pakistan J. Zool. Suppl. Ser., 13: pp. 65-73.
Kousar, S. and Javed. M., 2015. Turk. J. Fish. aqua. Sci., 15: 879-886. https://doi.org/10.4194/1303-2712-v15_4_11
Kumar, A., Vibudh, P.K. and Parimal, K.K., 2013. BioMetals, 26: 337-346. https://doi.org/10.1007/s10534-013-9620-8
Mateuca, R., Lombaert, N., Aka, P.V., Decordier, I. and Kirsch-Volders, M., 2006. Biochimie, 88: 1515-1531. https://doi.org/10.1016/j.biochi.2006.07.004
Obiakor, M.O., Ezeonyejiaku, C.D., Ezenwelu, C.O. and Ugochukwu, G.C., 2010. Am.-Eurasian J. toxicol. Sci., 2: 196-202.
Obiakor, M.O., Okonkwo, J.C. and Ezeonyejiaku, C.D., 2010. J. appl. Sci. environ. Manage., 14: 59-64. https://doi.org/10.4314/jasem.v14i3.61468
Parveen, N. and Shadab, G.G.H.A., 2011. Int. J. Sci. Nat., 2: 625-631.
Pruski, A.M. and Dixon, D.R., 2002. Aquat. Toxicol., 57: 127-137. https://doi.org/10.1016/S0166-445X(01)00192-8
Rajan, A.P., Nathiya, T. and Alphonse, M.A., 2012. Int. J. Res. Ayurveda. Pharm., 3: 105-108.
Ramsdorf, W.A., Ferraro, M.V.M., Oliveiraribeiro, C.A., Costa, J.R.M. and Cestari, M.M., 2009. Environ. Monit. Assess., 158: 77-85. https://doi.org/10.1007/s10661-008-0566-1
Rasal, K., Rasal, A. and Makwana, N., 2011. Asian J. Anim. Sci., 6: 32-34.
Sanchez-Galan, S., Linde, A.R., Ayllon, F. and Garcia-Vazquez, E., 2001. Ecotoxicol. environ. Safe., 49: 139-143. https://doi.org/10.1006/eesa.2001.2048
Steel, R.G.D., Torrie, J.H. and Dinkkey, D.A. 1996. Principles and procedures of statistics (3rd Ed.) McGraw Hill Book Co., Singapore.
Summak, S., Aydemir, N.C., Vatan, O., Yilmaz, D., Zorlu, T. and Bilaloglu, R., 2010. Fd. Chem. Toxicol., 48: 2443-2447. https://doi.org/10.1016/j.fct.2010.06.007
Udroiu, I., 2006. Aquat. Toxicol., 79: 201-204. https://doi.org/10.1016/j.aquatox.2006.06.013
Yaqub, S. and Javed, M., 2012. Int. J. agric. Biol., 14: 276-280.
To share on other social networks, click on any share button. What are these?









