Liquid Culture System for In Vitro Propagation of Physalis minima L. a Threatened Medicinal Plant
Muhammad Akram¹,²*, Rashid Mahmood³, Fahim Arshad4 and Umer Farooq Gohar5
¹Department of Biology, Government Shalimar Graduate College, Lahore, Punjab Higher Education Department, 54000 Lahore, Pakistan; ²Institute of Botany, University of the Punjab, Lahore-54590, Pakistan; ³Department of Botany, Government MAO College, Lahore, Punjab Higher Education Department, 54000 Lahore, Pakistan; 4Department of Botany, University of Okara, Okara-56300, Pakistan; 5Institute of Industrial Biotechnology, Government College University, Lahore-54000, Pakistan.
*Correspondence | Muhammad Akram, Department of Biology, Government Shalimar Graduate College, Lahore, Punjab Higher Education Department, 54000 Lahore, Pakistan; Email: m.akramks@gmail.com
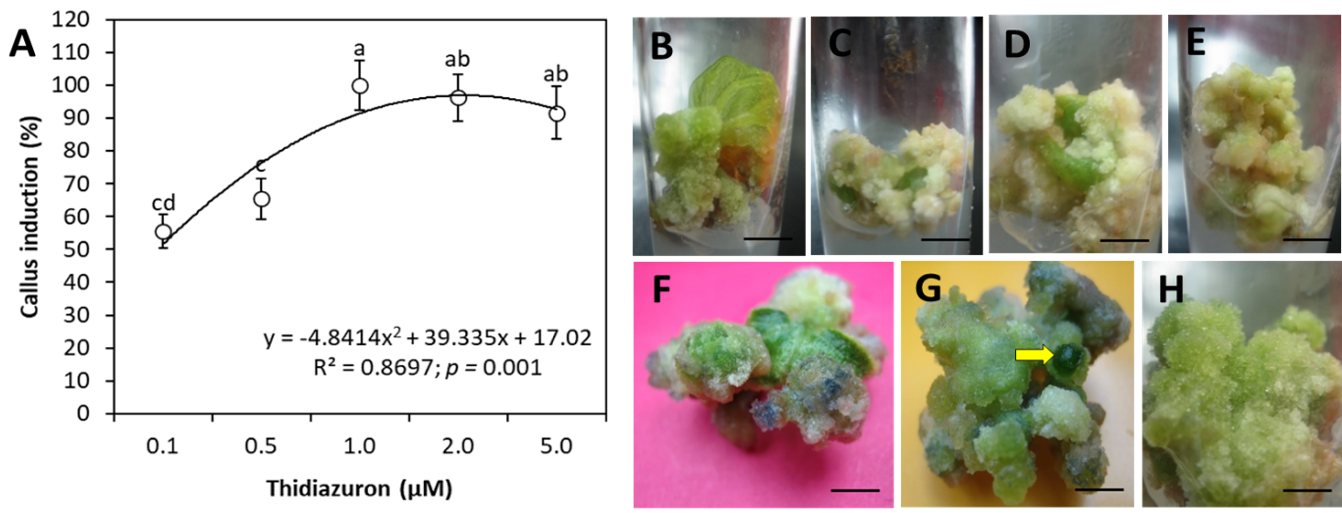
Figure 1:
Callus induction on MS medium from leaf explants of wild P. minima.
(A) Vertical bars above and below the circles are ± SE of the mean. Letters in the superscript in rows represent significant difference amongst the means of the two groups evaluated by the ANOVA followed by DMR post hoc test. Y in equation indicates the values of dependent variable and R2 is the Pearson coefficient of determination describing the covariance of the two variables (callus induction and TDZ concentration) divided by the product of their standard deviations. (B) Callus from cut margins of leaf explant on 0.1 µM TDZ Bar = 11 mm. (C, D) Off white loose callus at 0.5 µM TDZ after 10 days of culture Bar = 10 and 11 mm, respectively. (E) Subsequent development of callus mentioned in ‘C’ Bar = 10 mm. (F, G) Development of green nodules (yellow arrow) on green regenerating callus at 14 days of culture containing 1.0 µM TDZ Bar = 7 mm and 5 mm. (H) Granular greenish callus formation in 2.0 µM TDZ medium after 10 days Bar = 6 mm.
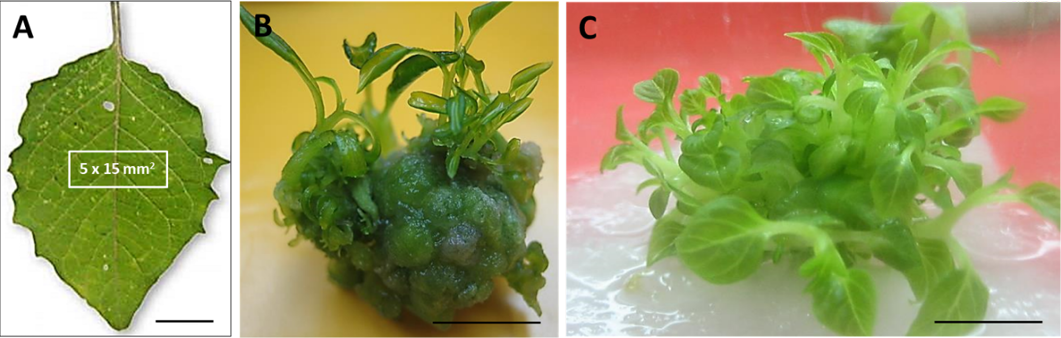
Figure 2:
Shoot organogenesis from leaf of wild P. minima.
(A) Leaf explant Bar=10 mm. (B) Five-day old shoots formed from callus tissue previously cultured on TDZ (1.0 µM) Bar=7 mm. (C). After 15 days on the same medium shoots were further proliferated. Bar = 5 mm.
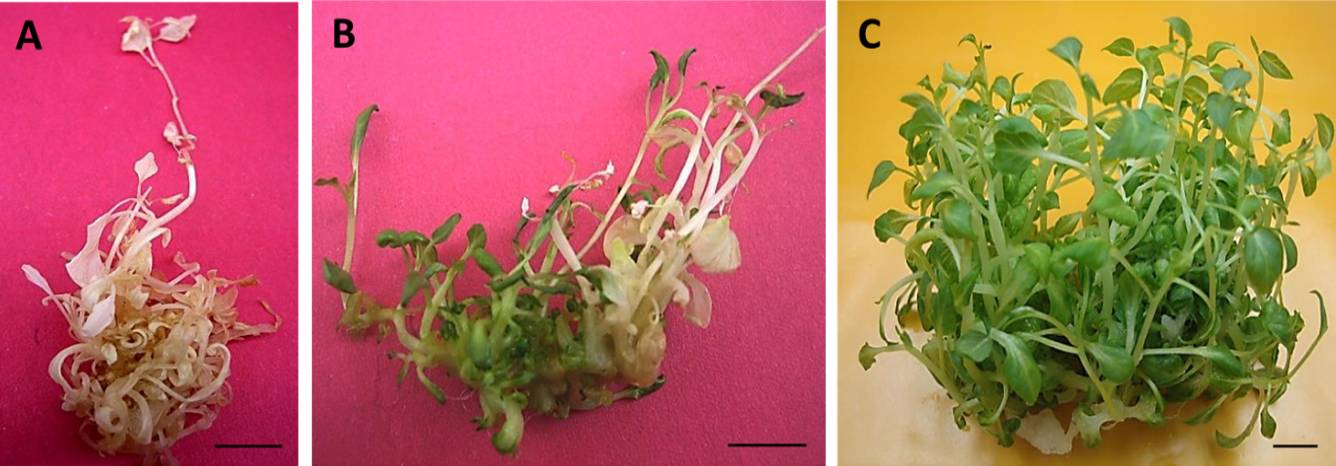
Figure 3:
Regeneration potential of the albino shoots derived from the 1.0 µM TDZ primed calli.
(A) A representative photograph displaying emergence of the albino shoot phenotype when medium was not refreshed within 15 days. (B) A representative photograph of re-greening albino shoots with invigoration with fresh MS medium for 10 days. (C) A representative photograph of the albino shoot culture displaying high frequency of shoot multiplication on the MS liquid medium. Bar = 15 mm.
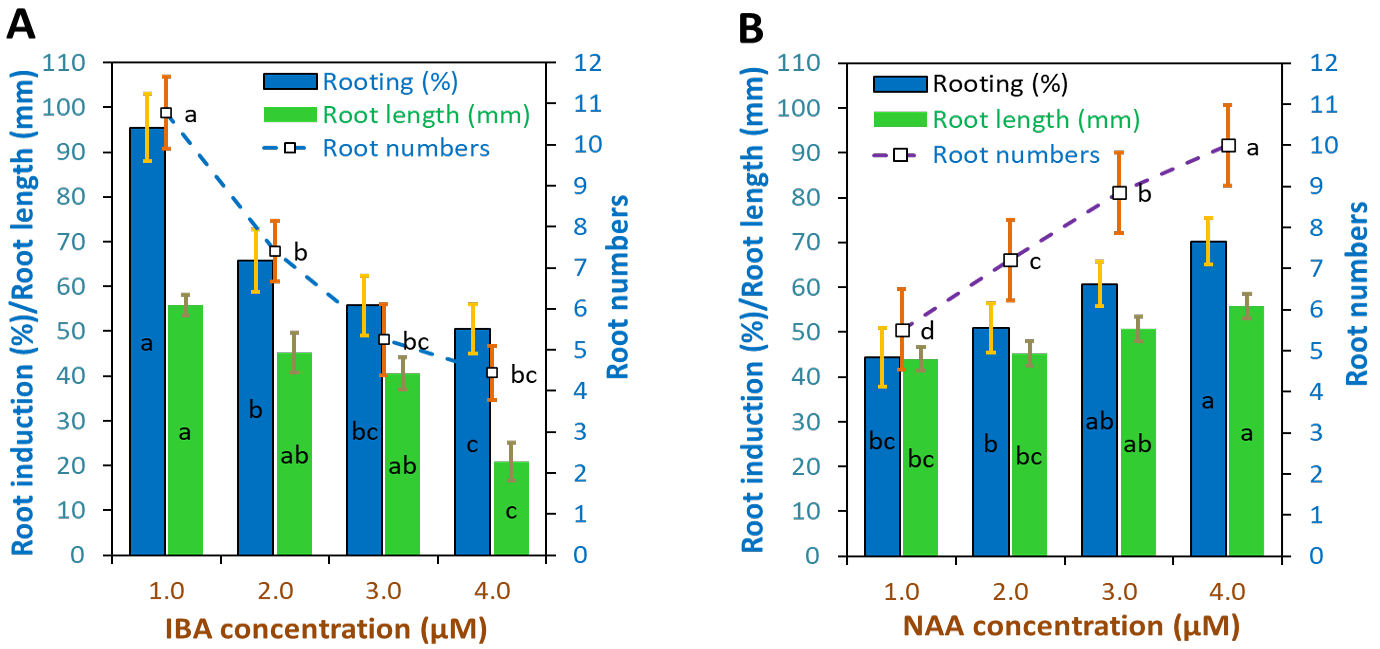
Figure 4:
In vitro rooting and survival of P. minima plantlets in half strength MS medium.
(A) Percentage of rooting, root length and root number obtained with 1, 2, 3, and 4 µM IBA. (B) Percentage of rooting, root length in mm and root numbers with NAA @ 1, 2, 3, and 4 µM after 10 days of culture. Bar charts represent the means and the vertical bars over bar charts represent ±SE.
Figure 5:
Plantlet showing roots and fully acclimatized plants in ½ MS medium.
(A) Representative photographs of a proliferating shoot displaying successful rooting with IBA @ 1.0 µM. Bar = 15 mm. (B) Representative pool of the 35-days old adult P. minima plant propagated via currently established protocol. The plants successfully produced flowers and fruits (circles). Bar = 15 mm.