Lactobacillus as Growth Promoter: A Meta-Analysis of Performance, Histology and Microbiota on Broiler Tract Digestive
Research Article
Lactobacillus as Growth Promoter: A Meta-Analysis of Performance, Histology and Microbiota on Broiler Tract Digestive
Bambang Hartoyo1*, Tri Rachmanto Prihambodo1,2, Wahyuningsih3, Sri Rahayu1, Fransisca Maria Suhartati1, Muhamad Bata1, Efka Aris Rimbawanto1
1Animal Science Faculty, Jenderal Soedirman University, Purwokerto 53123, Indonesia; 2Animal Feed Nutrition and Modelling Research Group, Animal Science Faculty, IPB University, Bogor, 16680, Indoenesia; 3Bogor Agricultural Development Polytechnic, Bogor, 16119, Indonesia.
Abstract | Meta-analysis of lactobacillus was built to evaluate the performance, histology and microbiota of lactobacillus on digestive tract of broilers. A database was built from previously published article from internet reporting lactobacillus as feed additives in broilers. Articles were strictly selected according to evaluation of title, abstract and parameter which used in the study. Database collected was statistically analyzed using the mixed model method with different study as random effect and level of lactobacillus as fixed effect using SAS program. Lactobacillus as potential feed additive had significant influence (p<0.05) to improve performance such as average daily gain (50.28 g), average feed intake (93.57 g) and feed conversion ratio (1.91) of broilers in ameliorate condition of digestive tract by decreasing the amount of Escherichia coli. Due to pathogen bacteria decrease, the histologic structures of digestive tract encounter improvement through minimizing damage of villus. In conclusion, lactobacillus supplementation in broilers increase performance due to improvement in the digestive tract and decrease in pathogenic bacteria with 5 x 10-7 cfu log-1 Lactobacillus population recommendation.
Keywords | Intestinal, Microorganism, Feed efficiency, Mix model, Systematic review
Received | February 27, 2023; Accepted | April 20, 2023; Published | May 03, 2023
*Correspondence | Bambang Hartoyo, Animal Science Faculty, Jenderal Soedirman University, Purwokerto 53123, Indonesia; Email: bambang.hartoyo@unsoed.ac.id
Citation | Hartoyo B, Prihambodo TR, Wahyuningsih, Rahayu S, Suhartati FM, Bata M, Rimbawanto EA (2023). Lactobacillus as growth promoter: A meta-analysis of performance, histology and microbiota on broiler tract digestive. Adv. Anim. Vet. Sci. 11(6):919-927.
DOI | https://dx.doi.org/10.17582/journal.aavs/2023/11.6.919.927
ISSN (Online) | 2307-8316
Copyright: 2023 by the authors. Licensee ResearchersLinks Ltd, England, UK.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
INTRODUCTION
The European nation aim to develop production that efficiently uses such as feed and renewable energy. Determination of novel solution meet the animal feed requirement balancing of production animals is key to development of animal industry in future trends (Adli, 2021). Indonesia facing several problems such as availability of the raw materials, and producing healthy meat (Adli et al., 2022). One of regulation has been established by government were prohibition of the use of antibiotic growth promoters on broiler chicken production. Prohibition on the use of Antibiotic Growth Promoter (AGP) as feed additives is stipulated in Minister of Agriculture regulation in 2017 concerning the classification of veterinary drugs. The regulation clarifies the mixing of veterinary drugs in feed for therapy based on instructions and under the supervision of a veterinarian.
Prohibition on the use of AGP on broilers can reduce the productivity. However, there is actually a way to keep the broilers performing well by using ingredients derived from nature. Natural ingredients do not cause any side effects on the host and researchers are looking for them to replace antibiotics. Resistance problem has become huge clinical and public health problem nowadays and will face multiresistant disease (Levy, 2002). Phytochemicals (Lillehoj et al., 2018; Prihambodo et al., 2022), probiotics and their derivate (Silva et al., 2020) and other metabolites are potential as antibiotics.
Probiotics are good bacteria with many types. Lactobacillus is one of the types of with abundant amount in fermentation products. Every fermentation product mostly produce Lactobacillus and it works optimally with the presence of a material providing an optimal environment. This condition results in an increase in the number/population of probiotics in the gastrointestinal tract. An increase in the Lactobacillus population results in an increase in the digestibility and absorption of nutrients resulting in an increase in performance. Actually, Lactobacillus has various mechanisms to improve performance, but the principal mechanism of Lactobacillus is by working anaerobically so pH of the digestive tract drops, and inhibits the development and growth of pathogenic bacteria.
Even mechanism of Lactobacillus as alternative antibiotics seems promising, but another report shows different results. Systematic review such as meta-analysis helps researchers find out inconsistency from several studies to conclude. Meta-analysis refers to a quantitative and methodical strategy creating a continuous analysis of previous studies (Hidayat et al., 2021). Meta-analysis can also be used to quantitatively verify the type of findings in a study. Therefore, this study aimed to evaluate, using a meta-analysis of previously published articles, the effects of Lactobacillus on the performance and intestine condition of broiler.
MATERIALS AND METHODS
Database development
Database established for this meta-analysis were collected from published articles in multiple search engines for scientific paper such as Google Scholar, Scopus and Science Direct using keywords “lactobacillus” and “broiler”. 48 articles have been collected discussing Lactobacillus as feed additives for broilers but only 38 articles were chosen as potential articles based on its title and abstract. Diagram flow of article selection in the meta-analysis using Systematic Review Centre for Laboratory Animal Experimentation (SYRCLE) method was reported in Figure 1. The parameters chosen were (1) productivity of broilers: average daily gain (ADG), average daily feed intake (ADFI) and feed conversion ratio (FCR), (2) histologic structure of intestine: villus height, crypt depth and ratio of villus height and crypt depth and (3) gastrointestinal microbiota specifically in caecum and ileum.
After strict evaluation of 38 articles, 17 articles were selected and reported in Table 1 based on their numerical results, confirmed specific species and dosage of the Lactobacillus. All parameters have been concluded based on descriptive method and reported in Table 2. All parameters have equal units as a requirement of meta-analysis such as average daily gain and feed intake were expressed in g/day, FCR was g/g, villus height and crypt depth were µm, microbiota population were log cfu/g, and dosage of Lactobacillus was in cfu/g.
Table 1: Articles included in the meta-analysis.
|
No |
Article |
Lactobacillus |
Broiler |
||
|
Species |
Dosage (cfu/g) |
Breed |
Age (d) |
||
|
1 |
Wu et al. |
L. acidophilus |
0 – 1 x 109 |
Cobb |
1 – 21 |
|
2 |
Wang et al. |
L. plantarum |
0 – 2 x 106 |
Cobb |
1 – 42 |
|
3 |
Peng et al. |
L. plantarum |
0 – 2 x 109 |
Arbor Acres |
1 – 42 |
|
4 |
Li et al. |
L. acidophilus |
0 – 4 x 106 |
Arbor Acres |
1 – 21 |
|
5 |
Jahromi et al. |
Mixed Lactobacillus |
0 – 1 x 109 |
Cobb |
1 – 35 |
|
6 |
Shokyazdan et al |
L. salivarius |
0 – 1 x 109 |
Cobb |
1 – 42 |
|
7 |
Qing et al. |
L. johnsonii |
0 – 1 x 106 |
Cobb |
1 – 28 |
|
8 |
Chen et al. |
Mixed Lactobacillus |
0 – 1 x 106 |
Arbor Acres |
1 – 35 |
|
9 |
Wang et al. |
L. plantarum |
0 – 1 x 108 |
Arbor Acres |
1 – 42 |
|
10 |
Vantsawa et al. |
L. acidophillus |
0 – 1 x 109 |
Arbor Acre+ |
1 – 42 |
|
11 |
Wu et al. |
L. acidophillus |
0 – 1 x 1010 |
Arbor Acres |
1 – 42 |
|
12 |
Yang et al. |
L. plantarum |
0 – 1 x 109 |
Cobb |
1 – 42 |
|
13 |
Cholis et al. |
Mixed Lactobacillus |
0 – 1 x 108 |
Lohmann |
1 – 42 |
|
14 |
Wang et al. |
L. johnsonii |
0 – 1 x 106 |
Cobb |
1 – 42 |
|
15 |
Wang et al. |
L. plantarum |
0 – 1 x 108 |
Arbor Acres |
1 – 42 |
|
16 |
Vineetha et al. |
L. plantarum |
0 – 1 x 108 |
Caribo Dhanraja |
1 – 35 |
|
17 |
Liu et al. |
L. plantarum |
0 – 1 x 108 |
Cobb |
1 – 21 |
Table 2: Descriptive statistics of the chosen article of meta-analysis.
|
Response parameter |
Unit |
Mean |
SD |
Min |
Max |
|
Performance |
|||||
|
Starter ADG |
g/day |
32.97 |
10.52 |
20.81 |
79.19 |
|
Starter ADFI |
g/day |
47.96 |
11.99 |
33.65 |
80.48 |
|
Starter FCR |
g/g |
1.500 |
0.162 |
1.280 |
1.860 |
|
Finisher ADG |
g/day |
64.15 |
20.67 |
27.40 |
89.23 |
|
Finisher ADFI |
g/day |
129.3 |
35.71 |
58.09 |
171.9 |
|
Finisher FCR |
g/g |
1.843 |
0.203 |
1.460 |
2.170 |
|
Total ADG |
g/day |
50.22 |
10.55 |
29.97 |
63.32 |
|
Total ADFI |
g/day |
92.53 |
16.00 |
63.54 |
111.5 |
|
Total FCR |
g/g |
1.880 |
0.402 |
1.340 |
3.280 |
|
Intestine histology |
|||||
|
Duodenum villus height |
µm |
1017 |
169.1 |
790.5 |
1316 |
|
Duodenum crypt depth |
µm |
182.8 |
40.23 |
144.6 |
236.0 |
|
Duodenum V: H |
µm/µm |
5.826 |
1.756 |
4.450 |
9.100 |
|
Jejunum villus height |
µm |
890.9 |
228.2 |
527.9 |
1247 |
|
Jejunum crypt depth |
µm |
154.6 |
50.97 |
95.56 |
237.9 |
|
Jejunum V: H |
µm/µm |
6.035 |
0.953 |
4.650 |
8.130 |
|
Ileum villus height |
µm |
687.1 |
200.8 |
349.4 |
968.0 |
|
Ileum crypt depth |
µm |
155.5 |
53.67 |
52.78 |
223.8 |
|
Ileum V: H |
µm/µm |
4.822 |
1.243 |
3.470 |
4.822 |
|
Intestine microbiota |
|||||
|
Caecum Lactobacillus |
Log cfu/g |
8.577 |
0.308 |
7.900 |
9.010 |
|
Caecum E. coli |
Log cfu/g |
6.587 |
0.450 |
5.920 |
7.280 |
|
Ileum caecum |
Log cfu/g |
8.251 |
0.514 |
7.350 |
8.980 |
|
Ileum E. coli |
Log cfu/g |
5.301 |
1.258 |
4.180 |
7.180 |
ADG, average daily gain; ADFI, average daily feed intake; FCR, feed conversion ratio.
Statistical analysis
Database were processed for statistical analysis using mixed model procedure in linear and quadratic model for meta-analysis (Sauvant et al., 2008; Prihambodo et al., 2021). Statistical analysis was conducted using SAS on demand for academic with PROC MIXED procedure. Lactobacillus addition dosage was used as fixed effect, while the studies were as random effect. The significance value was set as p<0.05.
Lactobacillus dosage was used as continuous predictor in which the response variables were regressed using the mathematical model:
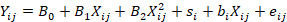
Where, Yij = dependent variable, B0= overall intercept across all studies (fixed effect), B1= linear regression coefficient of Y on X (fixed effect), B2= quadratic regression coefficient of Y on X (fixed effect), Xij = value of the continuous predictor variable (Lactobacillus addition level), si= random effect of study i, bi= random effect of study i on the regression coefficient of Y on X in study i, eij= the unexplained residual error while a low akaike information criteria (AIC) value indicates that the model is better at describing the observed data. The smaller the difference in AIC values between the two models, the smaller the difference in model quality between the two. Meanwhile, intercept is used to assist in understanding how the independent variable affects the dependent variable. The intercept can also provide information about the baseline or initial value of the dependent variable before the independent variable influences it.
Table 3: Lactobacillus addition effect on performance of broiler chicken.
|
Response parameter |
Model |
n |
Parameter estimates |
Model estimates |
||||
|
Intercept |
SE intercept |
Slope |
SE slope |
P-value |
AIC |
|||
|
Starter |
||||||||
|
ADG |
Q |
49 |
32.94 |
2.460 |
-0.001 |
0.001 |
<0.001 |
338.7 |
|
0.010 |
0.018 |
<0.001 |
327.9 |
|||||
|
ADFI |
Q |
52 |
48.08 |
2.620 |
-0.001 |
0.001 |
<0.001 |
316.5 |
|
0.002 |
0.008 |
<0.001 |
304.3 |
|||||
|
FCR |
Q |
52 |
1.512 |
0.034 |
0.001 |
0.001 |
<0.001 |
-58.90 |
|
-0.001 |
0.001 |
<0.001 |
-76.70 |
|||||
|
Finisher |
||||||||
|
ADG |
Q |
49 |
66.11 |
4.281 |
-0.002 |
0.002 |
<0.001 |
367.8 |
|
0.018 |
0.026 |
<0.001 |
358.1 |
|||||
|
ADFI |
Q |
46 |
128.3 |
7.914 |
-0.010 |
0.002 |
<0.001 |
390.5 |
|
0.012 |
0.034 |
<0.001 |
380.4 |
|||||
|
FCR |
Q |
46 |
1.866 |
0.045 |
0.001 |
0.001 |
<0.001 |
0.200 |
|
-0.001 |
0.001 |
<0.001 |
-17.00 |
|||||
|
Total |
||||||||
|
ADG |
Q |
37 |
49.78 |
2.403 |
-0.001 |
0.001 |
<0.001 |
247.1 |
|
0.019 |
0.017 |
<0.001 |
236.7 |
|||||
|
ADFI |
Q |
40 |
91.28 |
4.117 |
0.001 |
0.001 |
<0.001 |
271.5 |
|
0.003 |
0.013 |
<0.001 |
260.4 |
|||||
|
FCR |
Q |
40 |
1.881 |
0.087 |
0,001 |
0,001 |
<0.001 |
46.70 |
|
-0.001 |
0.001 |
<0.001 |
31.60 |
|||||
SE, standard error; AIC, akaike information criteria; Q, quadratic; ADG, average daily gain; ADFI, average daily feed intake; FCR, feed conversion ratio.
RESULTs AND DISCUSSION
In fact, all parameters had significant influence (p<0.05) due to Lactobacillus addition in positive way. Statistical analysis of this study was reported in Table 3. In this study, broilers gained good FCR score in starter, finisher and total phase. Average FCR of its respective phase were 1.5 ± 0.162, 1.843 ± 0.203 and 1.88 ± 0.402 g/g. Those FCR supported ADG in highest weight, namely 32.97 ± 10.52, 64.15 ± 20.67 and 50.22 ± 10.55 g, respectively. It can’t be separated due to escalation of intestine histology reported in Table 4.
Good condition in productivity of broilers cannot be separated from well-maintained intestinal ecosystem. Based on Table 5, Lactobacillus addition boosted Lactobacillus population and reduced Escherichia coli population. The population of Lactobacillus in intestine especially in caecum and ileum was significantly boosted (p<0.05) due to Lactobacillus addition in feed to 9.010 and 8.980 log cfu/g, respectively. Opposite result showed by Escherichia coli, the addition of Lactobacillus suppressed its population to support the performance of broilers.
The histologic structures of organs in digestive tract were significantly affected (p<0.05) due to Lactobacillus addition both in quadratic and linear model. Duodenum and ileum support the performance improvement in broilers even though jejunum did not. Duodenum and ileum construct good condition in intestine with average unit of 1017 ± 169.1 and 687.1 ± 200.8 µm, respectively and 5.826 ± 1.756 and 4.822 ± 1.243 µm/m for their villus height. Jejunum had a minimum trend due to Lactobacillus specifically in its villus height and ratio of villus and crypt depth due to linear decrease in crypt depth.
The results above have demonstrated the effect of Lactobacillus addition to broilers specifically in their performance based on their histologic structures and gastrointestinal microbiota. These results are in line with (Jahromi et al., 2017; Wang et al., 2017b; Fesseha et al., 2021), both in mixed or single Lactobacillus species. The capability to boost performance is inseparable from the power of Lactobacillus to modify or modulate such as regulate the microbial population in the digestive tract thereby influencing the immune response to efficiently absorb nutrients. Lactobacillus acidophilus, Lactobacillus plantarum, Lactobacillus johnsonii, Lactobacillus salivarius and mixed Lactobacillus were used in this study with each bacterium has its own mechanism to encourage the performance of broilers. Lactobacillus acidophilus has a mechanism by directly fermenting nutrients in the stomach (Jin et al., 2000),
Table 4: Lactobacillus addition effect on intestine histology of broiler chicken.
|
Response parameter |
Model |
n |
Parameter estimates |
Model estimates |
||||
|
Intercept |
SE intercept |
Slope |
SE slope |
P value |
AIC |
|||
|
Duodenum |
||||||||
|
Villus height |
L |
49 |
1001 |
75.18 |
17.85 |
6.571 |
<0.001 |
112.5 |
|
Crypt depth |
Q |
52 |
180.1 |
18.14 |
-0.520 |
0.165 |
<0.001 |
78.20 |
|
-0.270 |
0.473 |
<0.001 |
82.50 |
|||||
|
V:C |
L |
52 |
5.750 |
0.553 |
0.042 |
0.042 |
0.002 |
37.30 |
|
Jejunum |
||||||||
|
Villus height |
Q |
49 |
865.4 |
74.47 |
2.621 |
1.297 |
<0.001 |
318.8 |
|
3.780 |
4.581 |
<0.001 |
324.1 |
|||||
|
Crypt depth |
L |
46 |
147.9 |
15.60 |
-0.047 |
1.129 |
<0.001 |
253.7 |
|
V:C |
Q |
46 |
6.090 |
0.284 |
0.018 |
0.018 |
<0.001 |
83.40 |
|
0.011 |
0.037 |
<0.001 |
77.80 |
|||||
|
Ileum |
||||||||
|
Villus height |
Q |
37 |
670.8 |
69.16 |
-0.590 |
0.608 |
<0.001 |
285.9 |
|
-0.165 |
3.104 |
<0.001 |
287.6 |
|||||
|
Crypt depth |
Q |
40 |
148.4 |
17.95 |
-0.010 |
0.125 |
<0.001 |
219.8 |
|
-0.041 |
0.494 |
<0.001 |
215.4 |
|||||
|
V:C |
Q |
40 |
4.958 |
0.421 |
-0.002 |
0.005 |
<0.001 |
74.10 |
|
-0.001 |
0.019 |
<0.001 |
63.80 |
|||||
SE, standard error; AIC, akaike information criteria; Q, quadratic; L, linear; V:C, ratio of villus height and crypt depth.
Table 5: Lactobacillus addition effect on microbiota population intestine of broiler chicken.
|
Response parameter |
Model |
n |
Parameter estimates |
Model estimates |
|||||
|
Intercept |
SE intercept |
Slope |
SE slope |
P-value |
AIC |
||||
|
Ileum |
|||||||||
|
Lactobacillus |
Q |
11 |
8.156 |
0.308 |
-0.001 |
0.001 |
<0.001 |
33.4 |
|
|
0.004 |
0.001 |
<0.001 |
20.2 |
||||||
|
E. coli |
Q |
11 |
5.679 |
0.574 |
0.001 |
0.001 |
<0.001 |
32.8 |
|
|
-0.003 |
0.001 |
0.001 |
22.9 |
||||||
|
Caecum |
|||||||||
|
Lactobacillus |
Q |
11 |
8.440 |
0.161 |
-0.001 |
0.001 |
<0.001 |
27.2 |
|
|
0.003 |
0.002 |
<0.001 |
13.8 |
||||||
|
E. coli |
Q |
11 |
6.787 |
0.158 |
0.001 |
0.001 |
<0.001 |
24.5 |
|
|
-0.007 |
0.002 |
<0.001 |
11.2 |
||||||
SE, standard error; AIC, akaike information criteria, Q, quadratic.
Lactobacillus plantarum stimulates protective immune responses (Wang et al., 2015), Lactobacillus johnsonii assesses changes in lipid metabolism, gut microbiota, gut development, and digestive abilities (Wang et al., 2017). As mentioned above, it can be theoretically meaningful that Lactobacillus can replace antibiotics.
Supplementing diets with probiotics is one of the promising methods for preventing and treating bacterial illnesses. It is necessary for lactobacilli to get past physical and chemical barriers, such as stomach acid and bile in the gut, in order to exert health-promoting probiotic effects. Stimulating and modifying digestive tract increase the performance of broilers. One of the indicators representing the optimization of feed to performance is feed conversion ratio (Homma et al., 2021). This study reported that of all phase in broilers with minimum trend in quadratic model, 5 x 107 cfug-1 was the best dosage of Lactobacillus. The Lactobacillus addition to FCR parameter was analogous with Huang et al. (2004) and Mountzouris et al. (2010). This meta-analysis also validated overall performance parameters such as ADG and ADFI in all phase. In quadratic model, negative slope indicates maximum trend of Lactobacillus in representing the feed intake increase of broilers which in line with previous studies (Abdel-Hafeez et al., 2017; Rehman et al., 2020). The increase in feed intake and palatability (Jia et al., n.d.) is due to natural fermentation products such as acetic acid and biogenic amine (Lee et al., 2020).
Higher ADFI and lower FCR with an increase in ADG at maximum point cannot be separated with the histologic structure of broilers and the capability of Lactobacillus to produce digestive enzymes. The growth performance is improved by the secreted amylolytic, cellulolytic, proteolytic, and lipolytic enzymes because they increase the digestibility of starch, protein, and fat components and release the most energy. Furthermore, overall histologic structure of gut showed better condition than control. The high villi of duodenum, jejunum and ileum and supported by low crypt depth are notable parts of digestive tract related to immune health (Wu et al., 2021), stress control (Wu et al., 2021) and nutrient absorption (Cholis et al., 2018). Villus height of duodenum and ileum showed improvement than control with the better results. The longer the villus, the less damage can be caused by external factors. Each Lactobacillus species has each capability to increase villus height such as Lactobacillus acidophillus by producing enzyme to stimulate small intestine peristalsis (Wu et al., 2021), Lactobacillus plantarum by affecting mucosal immunity and the gut barrier (Wang et al., 2015), Lactobacillus johnsonii by balancing gut microflora in small intestine thereby healing the damaged mucosa through the renewal of epithelial cells (Dvorak, 2010), Lactobacillus salivarius by supporting the gut to reduce the enterocytes damage and renew it (Perić et al., 2010).
All mechanisms of Lactobacillus in this study are associated with reducing damage of intestine by producing digestive enzyme (Dudley et al., 2018; Zijlstra et al., 1997; Kyoung et al., 1998; Fathima et al., 2022) due to renewal cell of intestine such as villus height. Table 2 shows the correlation of good intestinal villi and an increase in broiler performance. The primary elements involved in nutrient absorption in the small intestine are villi. Epithelium surface of intestine area is increased by high villi for better nutrient absorption (Loh et al., 2010). Normally, pathogen microflora in intestine invades villi surface (Ritchie et al., 2012; Fathima et al., 2022) by altering their permeability resulting in chronic inflammation of intestine epithelium which leads to a decrease in villi size (Loh et al., 2010). In addition, a defense mechanism against other undesired bacterial colonization from the cecum, or control ileal flora is the bacterial adhesion to the ileal epithelial wall (Khonyoung and Yamauchi, 2012).
In other way, metabolites of Lactobacillus producing bacteriocin and organic acids help the immune system of broilers to inhibit the growth of pathogen bacteria. Both in ileum and caecum, Lactobacillus reduce the amount of Escherichia coli and making them a natural probiotic. The potential use of lactic acid bacteria (LAB)-produced bacteriocins as a non-toxic and secure bio-preservative to increase food safety has garnered a lot of attention (Lv et al., 2018). Bacteriocin is stable in acidic condition (Iranmanesh et al., 2014) and inhibits the growth of Escherichia coli (O’Shea et al., 2012) by transporting small ions like K+ and Na+ as essential electrolytes through the bacterial cell membrane, promoting cell membrane activities, and maintaining correct enzyme activity. Increased electrolyte release will signify the disrupted permeability barrier (Diao et al., 2014; Iranmanesh et al., 2014). Along with Na+ and K+, adenosine triphosphate (ATP), and nucleic acids are ingredients of membrane constituents (Bajpai et al., 2013) to identify certain intracellular components. Leakage markers serve as a measure of the membrane’s resistance to a particular antimicrobial agent in comparison to untreated cells.
Due to the lipophilic character of their undissociated state, organic acids have the ability to permeate cell membranes and alter the amounts of related anion and proton in the cytoplasm. Genetic, age, and sperm factors also related to the cell membranes production in the cytoplasm (Kusumawati et al., 2019; Susilawati et al., 2017, 2020). As a result, purine bases and crucial enzymes are affected, and bacterial viability is reduced (Warnecke and Gill, 2005; Gómez-García et al., 2019). Escherichia coli is one kind of pathogen bacteria categorized as gram negative bacteria with its membrane cell Gómez-García et al. (2019) aims to form organic acids especially formic acid. In addition to the metabolites, the capability of Lactobacillus is one of main factor how Lactobacillus work as antibiotic. Alp and Kuleaşan (2019) reported some factors for Lactobacillus to bind with intestine such as (a) mucus binding protein; (b) lipoteichoic acid (c) extracellular polysaccharides and (d) flagella and pili. Intestinal mucus has main role as the protection of epithelial surfaces against pathogens by maintaining a favorable environment for digestion thereby allowing the movement of nutrients from the lumen to the underlying epithelium. Douillard et al. (2013) reported pili by Lactobacillus increased mucus-binding activity. However, findings about the adhesion mechanism of Lactobacillus have not clearly explained. The binding of epitopes on carbohydrate chains and type of several reason become an obstacle and need to be investigated in the future (Nishiyama et al., 2016).
CONCLUSIONS AND RECOMMENDATIONS
The present meta-analysis concludes that overall Lactobacillus addition in broilers can increase performance due to improvement in the digestive tract and decrease in pathogenic bacteria with 5 x 10-7 cfu log-1 Lactobacillus population recommendation. Future research in this area is required, specifically in separated Lactobacillus strain since different bacterial strains could produce different outcomes.
Novelty Statement
The current study, shows the best evaluation and dosage of the addition of various types of lactobacillus in broilers which are seen in performance, histology and digestive tract profile through a meta-analysis approach.
AUTHOR’S CONTRIBUTION
BH, TRP and WW conducted the experiments, analyzed the data, and drafted the article. TRP reviewed the data analysis and revised the draft article. BH and SR supervised the experiment. MB, EAR and FMS designed the experiment, reviewed the data analysis, and revised the article draft.
Ethical approval
Ethical approval is not required for meta-analysis study.
Conflict of interest
The authors have declared no conflict of interest.
REFERENCES
Abdel-Hafeez HM, Saleh ESE, Tawfeek SS, Youssef IMI, Abdel-Daim ASA (2017). Effects of probiotic, prebiotic, and synbiotic with and without feed restriction on performance, hematological indices and carcass characteristics of broiler chickens. Asian-Australas. J. Anim. Sci., 30(5): 672–682. https://doi.org/10.5713/ajas.16.0535
Adli DN, Sjofjan O, Irawan A, Utama DT, Sholikin MM, Nurdianti RR, Nurfitriani RA, Hidayat C, Jayanegara A, Sadarman S (2022). Effects of fibre-rich ingredient levels on goose growth performance, blood profile, foie gras quality and its fatty acid profile: A meta-analysis. J. Anim. Feed Sci., 31(4): 301–309. https://doi.org/10.22358/jafs/152621/2022
Adli DN (2021). The effect of replacing fish meal with Sago larvae meal (SLM) on egg production and quality of laying hens. Livest. Res. Rural Dev., 33(7): 1-8.
Alp D, Kuleaşan H (2019). October 1: Adhesion mechanisms of lactic acid bacteria: conventional and novel approaches for testing. World J. Microbiol. Biotechnol., 35: 156. https://doi.org/10.1007/s11274-019-2730-x
Bajpai V, Sharma A, Baek A (2013). Antibacterial mode of action of Cudrania tricuspidata fruit essential oil, affecting membrane permeability and surface characteristics of food-borne pathogens. Food Control, 32(2): 582-590. https://doi.org/10.1016/j.foodcont.2013.01.032
Cholis MA, Suthama N, Sukamto B (2018). Feeding microparticle protein diet combined with Lactobacillus sp. On existence of intestinal bacteria and growth of broiler chickens. J. Indones. Trop. Anim. Agric., 43(3): 265–271. https://doi.org/10.14710/jitaa.43.3.265-271
Diao WR, Hu QP, Zhang H, Xu JG (2014). Chemical composition, antibacterial activity and mechanism of action of essential oil from seeds of fennel (Foeniculum vulgare Mill.). Food Contr., 35(1): 109–116. https://doi.org/10.1016/j.foodcont.2013.06.056
Douillard FP, Ribbera A, Järvinen HM, Kant R, Pietilä TE, Randazzo C, Paulin L, Laine PK, Caggia C, von Ossowski I, Reunanen J, Satokari R, Salminen S, Palva A, de Vosa WM (2013). Comparative genomic and functional analysis of Lactobacillus casei and Lactobacillus rhamnosus strains marketed as probiotics. Appl. Environ. Microbiol., 79(6): 1923–1933. https://doi.org/10.1128/AEM.03467-12
Dudley MA, Wykes LJ, Dudley AW, Burrin DG, Nichols BL, Rosenberger J, Jahoor F, Heird WC, Reeds PJ, Rosen-berger J (2018). Parenteral nutrition selectively decreases protein synthesis in the small intestine.
Dvorak B (2010). Milk epidermal growth factor and gut protection. J. Pediatr., 156(2 Suppl.). https://doi.org/10.1016/j.jpeds.2009.11.018
Fathima S, Hakeem WG, Shanmugasundaram R, Selvaraj RK (2022). October 1: Necrotic enteritis in broiler chickens: A review on the pathogen, pathogenesis, and prevention. Microorganisms, https://doi.org/10.3390/microorganisms10101958
Fesseha H, Demlie T, Mathewos M, Eshetu E (2021). Effect of Lactobacillus species probiotics on growth performance of dual-purpose chicken. Vet. Med. Res. Rep., 12: 75–83. https://doi.org/10.2147/VMRR.S300881
Gómez-García M, Sol C, de Nova PJG, Puyalto M, Mesas L, Puente H, Mencía-Ares Ó, Miranda R, Argüello H, Rubio P, Carvajal A (2019). Antimicrobial activity of a selection of organic acids, their salts and essential oils against swine enteropathogenic bacteria. Porcine Health Manage., 5(1): https://doi.org/10.1186/s40813-019-0139-4
Hidayat C, Irawan A, Jayanegara A, Sholikin MM, Prihambodo TR, Yanza YR, Wina E, Sadarman S, Krisnan, R, Isbandi I (2021). Effect of dietary tannins on the performance, lymphoid organ weight, and amino acid ileal digestibility of broiler chickens: A meta-analysis. Vet. World, 14(6): 1405–1411. https://doi.org/10.14202/vetworld.2021.1405-1411
Homma C, Hirose K, Ito T, Kamikawa M, Toma S, Nikaido S, Satoh M, Uemoto Y (2021). Estimation of genetic parameter for feed efficiency and resilience traits in three pig breeds. Animal, 15(11): https://doi.org/10.1016/j.animal.2021.100384
Huang MK, Choi YJ, Houde R, Lee JW, Lee B, Zhao X (2004). Effects of Lactobacilli and an acidophilic fungus on the production performance and immune responses in broiler chickens. https://doi.org/10.1093/ps/83.5.788
Iranmanesh M, Ezzatpanah H, Mojgani N (2014). Antibacterial activity and cholesterol assimilation of lactic acid bacteria isolated from traditional Iranian dairy products. LWT, 58(2): 355–359. https://doi.org/10.1016/j.lwt.2013.10.005
Jahromi MF, Liang JB, Ebrahimi R, Soleimani AF, Rezaeizadeh A, Abdullah N, Shokryazdan P (2017). Protective potential of Lactobacillus species in lead toxicity model in broiler chickens. Animal, 11(5): 755–761. https://doi.org/10.1017/S175173111600224X
Jia Y, Yunsheng H, Angkanaporn K, Zhong W, Hu P, Liu HY, Hy L, Copyright fvets, Zhu C, Yao J, Zhu M, Zhu C, Yuan L, Li Z, Cai D, Chen S (n.d.). A meta-analysis of Lactobacillus based probiotics for growth performance and intestinal morphology in piglets.
Jin LZ, Ho YW, Abdullah N, Jalaludin S (2000). Digestive and bacterial enzyme activities in broilers fed diets supplemented with Lactobacillus cultures. https://doi.org/10.1093/ps/79.6.886
Khonyoung D, Yamauchi KE (2012). Effects of heat-killed Lactobacillus plantarum L-137 on morphology of intestinal villi and epithelial cells in broiler chickens. J. Appl. Anim. Res., 40(2): 140–147. https://doi.org/10.1080/09712119.2011.640208
Kusumawati ED, Isnaini N, Yekti APA, Luthfi M, Affandhy L, Pamungkas D, Kuswati RA, Sudarwati H, Rahadi S, Rahayu S, Susilawati T (2019). The motility and ratio of X and Y sperm filial ongole cattle using different sexed semen methods. Am. J. Anim. Vet. Sci., 14(2): 111-114. https://doi.org/10.3844/ajavsp.2019.111.114
Kyoung PY, Monaco MM, Donovan SM (1998). Delivery of total parenteral nutrition (TPN) via umbilical catheterization: Development of a piglet model to investigate therapies to improve gastrointestinal structure and enzyme activity during TPN. Biol. Neonate, 73: https://doi.org/10.1159/000013988
Lee CH, Song MH, Yun W, Lee JH, Kwak WG, Oh SY, Oh HJ, Kim HB, Cho JH (2020). Effects of fermented whole-crop wheat and barley with or without supplementing inoculant (probiotics) on palatability, growth performance, nutrient digestibility, fecal microbiota and blood constituents in finishing pigs. Indian J. Anim. Res., 54(11): 1373–1378. https://doi.org/10.18805/ijar.B-1021
Levy S (2002). Factors impacting on the problem of antibiotic resistance. J. Antimicrob. Chemother., 49: 25–30. https://doi.org/10.1093/jac/49.1.25
Lillehoj H, Liu Y, Calsamiglia S, Fernandez-Miyakawa ME, Chi F, Cravens RL, Oh S, Gay CG (2018). Phytochemicals as antibiotic alternatives to promote growth and enhance host health. Vet. Res., https://doi.org/10.1186/s13567-018-0562-6
Loh TC, Thanh NT, Foo HL, Hair-Bejo M, Azhar BK (2010). Feeding of different levels of metabolite combinations produced by Lactobacillus plantarum on growth performance, fecal microflora, volatile fatty acids and villi height in broilers. Anim. Sci. J., 81(2): 205–214. https://doi.org/10.1111/j.1740-0929.2009.00701.x
Lv X, Miao L, Ma H, Bai F, Lin Y, Sun M, Li J (2018). Purification, characterization and action mechanism of plantaricin JY22, a novel bacteriocin against Bacillus cereus produced by Lactobacillus plantarum JY22 from golden carp intestine. Food Sci. Biotechnol., 27(3): 695–703. https://doi.org/10.1007/s10068-017-0280-2
Mountzouris KC, Tsitrsikos P, Palamidi I, Arvaniti A, Mohnl M, Schatzmayr G, Fegeros K (2010). Effects of probiotic inclusion levels in broiler nutrition on growth performance, nutrient digestibility, plasma immunoglobulins, and cecal microflora composition. Poult. Sci., 89(1): 58–67. https://doi.org/10.3382/ps.2009-00308
Nishiyama K, Sugiyama M, Mukai T (2016). Adhesion properties of lactic acid bacteria on intestinal mucin. Microorganisms, 4(3). https://doi.org/10.3390/microorganisms4030034
O’Shea E, Cotter P, Snaton C, Ross R, Hill C (2012). Production of bioactive substances by intestinal bacteria as a basis for explaining probiotic mechanisms: Bacteriocins and conjugated linoleic acid. Int. J. Food. Microbiol, 152 (3): 189-205. https://doi.org/10.1016/j.ijfoodmicro.2011.05.025
Perić L, Milošević N, Žikić D, Bjedov S, Cvetković D, Markov S, Mohnl M, Steiner T (2010). Effects of probiotic and phytogenic products on performance, gut morphology and cecal microflora of broiler chickens. Arch. Tierzucht, 53: https://doi.org/10.5194/aab-53-350-2010
Prihambodo TR, Sholikin MM, Nahrowi N, Batubara I, Utomo DB, Jayanegara A (2022). Flavonoids as dietary additives in laying hens: A meta-analysis of production performance, egg quality, liver, and antioxidant enzyme profile. Poult. Sci. J., 10(1): 27–34. https://doi.org/10.5713/ajas.20.0379
Prihambodo TR, Sholikin MM, Qomariyah N, Jayanegara A, Batubara I, Utomo DB, Nahrowi N (2021). Effects of dietary flavonoids on performance, blood constituents, carcass composition and small intestinal morphology of broilers: A meta-analysis. Anim. Biosci., 34(3): 434–442. https://doi.org/10.5713/ajas.20.0379
Rehman A, Arif M, Sajjad N, Al-Ghadi MQ, Alagawany M, Abd El-Hack ME, Alhimaidi AR, Elnesr SS, Almutairi BO, Amran RA, Hussein EOS, Swelum AA (2020). Dietary effect of probiotics and prebiotics on broiler performance, carcass, and immunity. Poult. Sci., 99(12): 6946–6953. https://doi.org/10.1016/j.psj.2020.09.043
Ritchie JM, Rui H, Zhou X, Iida T, Kodoma T, Ito S, Davis BM, Bronson RT, Waldor MK (2012). Inflammation and disintegration of intestinal villi in an experimental model for vibrio parahaemolyticus-induced diarrhea. PLoS Pathog., 8(3). https://doi.org/10.1371/journal.ppat.1002593
Sauvant D, Schmidely P, Daudin JJ, St-Pierre NR (2008). Meta-analyses of experimental data in animal nutrition. Animal, 2(8): 1203–1214. https://doi.org/10.1017/S1751731108002280
Silva DR, Sardi JCO, Pitangui N de S, Roque SM, Silva ACB da, Rosalen PL (2020). October 1: Probiotics as an alternative antimicrobial therapy: Current reality and future directions. J. Funct. Foods, https://doi.org/10.1016/j.jff.2020.104080
Susilawati T, Kuswati, Rahayu S, Sudarwati H, Marjuki YAPA, Udrayana S (2017). Quality of Ongole bull sperm after storage in CEP-2 extender containing different extracellular cryoprotectans. Asian J. Microbiol. Biotechnol. Environ. Sci., 19(7): 268-273.
Susilawati T, Sholikah NU, Wahjuningsih S, Herwiyanti E, Yekti APA (2020). Relationship of scrotal circumference with spermatozoa production in various breed of Indonesian local bulls. Am. J. Anim. Vet. Sci., 15(2): 102-107. https://doi.org/10.3844/ajavsp.2020.102.107
Wang L, Liu C, Chen M, Ya T, Huang W, Gao P, Zhang H (2015a). Novel Lactobacillus plantarum strain P-8 activates beneficial immune response of broiler chickens. Int. Immunopharmacol., 29(2): 901–907.https://doi.org/10.1016/j.intimp.2015.07.024
Wang H, Ni X, Qing X, Zeng D, Luo M, Liu L, Li G, Pan K, Jing B (2017a). Live probiotic Lactobacillus johnsonii BS15 promotes growth performance and lowers fat deposition by improving lipid metabolism, intestinal development, and gut microflora in broilers. Front. Microbiol., 8(Jun): https://doi.org/10.3389/fmicb.2017.01073
Wang S, Peng Q, Jia HM, Zeng XF, Zhu JL, Hou CL, Liu XT, Yang FJ, Qiao SY (2017b). Prevention of Escherichia coli infection in broiler chickens with Lactobacillus plantarum B1. Poult. Sci., 96(8): 2576–2586. https://doi.org/10.3382/ps/pex061
Wang Y, Lv X, Li X, Zhao J, Zhang K, Hao X, Liu K, Liu H (2021). Protective effect of Lactobacillus plantarum P8 on growth performance, intestinal health, and microbiota in Eimeria-infected broilers. Front. Microbiol., 12. https://doi.org/10.3389/fmicb.2021.705758
Warnecke T, Gill RT (2005). Organic acid toxicity, tolerance, and production in Escherichia coli biorefining applications. Microb. Cell Factories, https://doi.org/10.1186/1475-2859-4-25
Wu Z, Yang K, Zhang A, Chang W, Zheng A, Chen Z, Cai H, Liu G (2021). Effects of Lactobacillus acidophilus on the growth performance, immune response, and intestinal barrier function of broiler chickens challenged with Escherichia coli O157. Poult. Sci., 100(9). https://doi.org/10.1016/j.psj.2021.101323
Zijlstra RT, Donovan SM, Odle J, Gelberg HB, Petschow BW, Gaskins HR (1997). Human and clinical nutrition protein-energy malnutrition delays small-intestinal recovery in neonatal pigs infected with rotavirus 1, 2. J. Nutr., Vol. 127. https://doi.org/10.1093/jn/127.6.1118
To share on other social networks, click on any share button. What are these?






