Influence of Honey on Sperm Traits in KUB Rooster Concerning Cold Storage
Research Article
Makruf Arif1*, Claude Mona Airin1, Dwi Sunu Datrianto2, Dinda Anggun Roro Sejati3
1Department of Physiology, Faculty of Veterinary Medicine, Universitas Gadjah Mada, Jl. Fauna No. 2, Karangmalang, Sleman, Yogyakarta 55281, Indonesia; 2Department of Internal Medicine, Faculty of Veterinary Medicine, Universitas Gadjah Mada, Jl. Fauna No. 2, Karangmalang, Sleman, Yogyakarta 55281, Indonesia; 3Undergraduate student, Faculty of Veterinary Medicine, Universitas Gadjah Mada, Jl. Fauna No. 2, Karangmalang, Sleman, Yogyakarta 55281, Indonesia.
Abstract | Damage due to oxidative stress during semen preservation can cause a decrease in motility, viability, and damage to plasma membranes that can reduce fertility rates. The use of antioxidants in semen preservation is very important to suppress lipid peroxidation so it can maintain the quality of the sperm. This study aimed to determine additional honey to the quality of KUB rooster sperm during cold storage. The semen with superior quality was diluted into four different treatments, lactate ringer egg yolk without honey as control (LREY0), LREY with 0.2% honey (LREY2), LREY with 0.4% honey (LREY4), and LREY with 0.6% honey (LREY6). Semen was stored at 5°C for 48 hours. The evaluation of semen quality includes an examination of spermatozoa motility using a microscope, the viability with the eosin-negrosin staining method, and membrane integrity using the hypoosmotic swelling test (HOST) at the fresh semen, 5th, 24th, and 48th hours post-cold storage. The results revealed that in the 5th hour, percentage of motility, viability, and membrane integrity were not significantly different. However, at the 24th and 48th-hour results revealed significant differences by adding honey (P < 0,05). The 0.4% honey supplementation was the best to maintain KUB rooster quality until the 48th hour with motility of up to 41.50±1.19%, the viability of up to 59.75±0.63%, and membrane integrity of up to 66.50±1.32%. It can be concluded from the current study that adding 0.4% honey can preserve the quality of KUB rooster sperm during cold storage.
Keywords | Antioxidant, Chilling storage, Honey, KUB rooster, Sperm membrane integrity
Received | December 03, 2022; Accepted | January 02, 2023; Published | January 26, 2023
*Correspondence | Makruf Arif, Department of Physiology, Faculty of Veterinary Medicine, Universitas Gadjah Mada, Jl. Fauna No. 2, Karangmalang, Sleman, Yogyakarta 55281, Indonesia; Email: makrufarif@ugm.ac.id
Citation | Arif M, Airin CM, Datrianto DS, Sejati DAR (2023). Influence of honey on sperm traits in KUB rooster concerning cold storage. Adv. Anim. Vet. Sci. 11(2):246-251.
DOI | https://dx.doi.org/10.17582/journal.aavs/2023/11.2.246.251
ISSN (Online) | 2307-8316
Copyright: 2022 by the authors. Licensee ResearchersLinks Ltd, England, UK.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
INTRODUCTION
Statistical data on livestock and animal health states that the consumption of non-purebred chicken meat and eggs in Indonesia from 2016 to 2017 has increased by 24,9% and 14,7% (Anonim, 2018). Ayam Kampung Unggul Badan Penelitian dan Pengembangan Pertanian (Kampong Chicken KUB) is known as one of the Indonesian selected-native chickens with a high-productivity of eggs and high-quality meats (Krista and Harianto, 2013; Udjianto, 2016) which can meet people’s needs efficiently. The increase in the efficiency and productivity of livestock can be achieved through artificial insemination (AI) technology. The AI implementation can improve the effectiveness and efficiency of superior males (Mohan et al., 2018). Rooster semen has a high concentration value, but its volume is low, so it needs to be diluted to increase the volume so that it can be used for AI in large quantities (Bebas and Laksmi, 2015; Hafez, 2000). The viability and quality of spermatozoa stored at room temperature cannot last long, which is necessary to carry out storage in cold temperatures (Kusumaningrum et al., 2002).
Rooster spermatozoa have a high arrangement of polyunsaturated fatty acids (PUFA), which are then easily damaged via lipid peroxidation and free radicals such as reactive oxygen species (ROS). Uncontrolled ROS production can damage the membrane and the mitochondria of spermatozoa (Shah et al., 2016). The addition of antioxidants into the diluent can eliminate free radicals to maintain sperm quality during the storage process (Herdis, 2007; Hendiyani et al., 2018). Antioxidants are molecules that can end the chain of free radical reactions before the occurrence of damage to main molecules (Burgos et al., 2006).
Honey, as an antioxidant, contains vitamin C and E, phenolic components, flavonoids, ascorbic acid, glucose, oxidase enzyme, and catalase enzyme (Hidayaturrahmah, 2007). The study of honey in semen extender had carried out on different species as turkeys (Sari et al., 2015), cattle (Yimer et al., 2015; Malik, 2018), stallions (Sheshtawy et al., 2016), and fish (Rahardhianto et al., 2012; Condro et al., 2012; Nainggolan et al., 2015; Martin et al., 2018). The addition of 3% honey can maintain the quality of turkey sperm (motility and viability) during storage at 5°C (Sari et al., 2015). In Arab stallions, the addition of honey can maintain the motility, viability, and membrane integrity of spermatozoa during cryopreservation (Sheshtawy et al., 2016). There are no reports on the sperm quality of KUB roosters during preservation at 5°C in an extender with honey supplementation as an antioxidant which is the main reason conducted the current study. The honey supplementation is used as an antioxidant for preserving the sperm quality of KUB roosters during storage at 5°C. We evaluated the sperm characteristics such as motility, viability, and membrane integrity.
MATERIALS AND METHODS
Animal trial and ethics
The procedures were approved by the Ethics Committee of Research at the Faculty of Veterinary Medicine, Universitas Gadjah Mada, Yogyakarta number 0041/EC-FKH/Int/2022. Five KUB roosters aged 48 weeks were placed in a 90 x 56 x 67 cm of individual cage. The roosters were daily fed with 150 grams of commercial feed (Multifeed BR 1-7 RT, Indonesia) and ad-libitum water. A poultry house, 3x9 meters, was used to place the individual cage with steady light and circulation. The rooster cages were cleaned and disinfected every day. The roosters were treated with anthelmintics before the study began, and the vitamins were given every two days. The semen collection habituation was done two weeks before the research to create an adaptable environment.
Extender preparation
The basic diluents used in this study were a lactated ringer and 20% egg yolk (LR-EY) with penicillin and streptomycin antibiotics. The lactate ringer and egg yolk are homogenized using a stirrer, then centrifuged for 15 min at 3000 rpm. Supernatants were harvested as a basic diluent, then penicillin 1000 IU/ml and streptomycin 1 mg/ml were added. The basic diluents were divided into four solutions then honey was added at rates of 0.2% (LREY2), 0.4% (LREY4), and 0.6% (LREY6) to the 1st, 2nd, and 3rd solutions respectively, while the 4th was kept without honey as a control (LREY0).
Semen collection and dilution
We collected semen from five healthy KUB roosters aged 48 weeks. KUB rooster semen was collected twice a week by using the dorso-abdominal massage method (Tabatabei et al., 2011), with a total of four times repetitions. Fresh semen was evaluated macroscopically (volume, color, pH, and consistency) and microscopically (motility, mass movement, concentration, and viability). Semen with spermatozoa motility of more than 70% and concentration of more than 2x109/ml was divided into four tubes. Each tube was diluted with a lactate ringer egg yolk extender with honey supplementation at rates of 0.2% (LREY2), 0.4% (LREY4), 0.6% (LREY6), and without honey as control (LREY0), then stored in a temperature of 5°C for 48 hours.
Spermatozoa quality evaluation
Semen was stored in four different storage conditions, fresh, 5th, 24th, and 48th hours post-storage. Straws were incubated in a water bath at 37 oC for 30 sec. Semen quality evaluation included motility (%), viability (%), and membrane integrity (%).
Sperm motilities
A coverslip slide was used to cover a glass object containing 20 μl of collected sperm and 80 μl of saline solution. Sperm motility was evaluated under a microscope (XSZ-107BN, BW Optics China) with a 40x magnification in five fields.
Sperm viabilities
The eosin-negrosin staining method was used to examine the viability (Telnoni et al., 2017). A bunsen burner was used to heat a mixture of 50 ml of eosin-negrosin staining (1:5) and 10 ml of semen on the microscope slide. The slides were evaluated under a microscope with a 40x a microscope (XSZ-107BN, BW Optics China) with a 40x magnification. In a sample stained with eosin-negrosin, morphometric measurements involved 200 sperm cells. A living sperm is characterized as a white non-colored head and a dead sperm revealed as a red head (Susilawati, 2011; Telnoni et al., 2017).
Membrane integrity. Following Akcay et al. (2012), the membrane integrity was evaluated using a modified version of the Hypoosmotic Swelling Test (HOST). A fixed volume of 10 ml of sperm was dissolved in 100 μl of HOST solution (0.9 g of fructose and 0.49 g of sodium citrate dissolve equates to a final volume of 100 ml) and incubated for 30 min at 37°C. Under a microscope with a microscope 40x magnification (XSZ-107BN, BW Optics China), 200 spermatozoa were counted after the mixture was spread on a glass object, dried, and fixed. Spermatozoa with a normal plasma membrane are characterized as swollen or curled tails, while the damaged membrane is revealed as a straight tail. Percentage calculation of the percentage of the normal plasma membrane (NPM) was carried out using the formula:
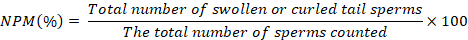
Data analysis
The motility (%), viability (%), and membrane integrity (%) of KUB rooster sperm were statistically processed by the Analysis of Variance (ANOVA) method and a Duncan post hoc multiple ranges to determine the differences between treatments.
RESULTS
Fresh semen evaluation of the KUB rooster indicated that the seminal quality was normal. The macroscopic evaluation revealed that the volume and pH were 0.38±0.01 ml and 7.25±0.14, respectively. Microscopic examination results recorded a concentration (2.38x109±0.087 cells/ml), motility (92.00±0.82%), viability (92.00±1.22%), and membrane integrity (93.75±1.11%). The motility percentage was revealed in Table 1, the viability in Table 2, and membrane integrity in Table 3. The results discovered in Figure 1, at 5th-hour storage did not show any significant differences, while honey supplementation in the 24th and 48th hours storage indicated a significant effect (P < 0.05). The recommended honey concentration for maintaining KUB rooster semen quality in cold storage until the 48th hour was 0.4% with motility (41.50±1.19%), viability (59.75±0.63%), and membrane integrity (66.50±1.32%).
Table 1: The motility (%) of KUB rooster semen on LREY extender with honey supplementation during cold storage for 48 hours.
|
Treatments |
Sperm motility (%) |
|||
|
Fresh semen |
5th hours |
24th hours |
48th hours |
|
|
LREY0 (without honey) |
92.00±0.82 |
73.75±6.57 |
55.50±1.55b |
33.50±1.55b |
|
LREY2 (0.2% of honey) |
92.00±0.82 |
78.25±5.45 |
56.75±0.95b |
35.50±1.04b |
|
LREY4 (0.4% of honey) |
92.00±0.82 |
79.25±4.78 |
61.25±1.65a |
41.50±1.19a |
|
LREY6 (0.6% of honey) |
92.00±0.82 |
75.00±5.97 |
56.00±1.22b |
35.50±2.22b |
a, b, c Different superscripts following the numbers within the same column indicate significant differences between treatments P<0.05.
Table 2: The viability (%) of KUB rooster semen on LREY extender with honey supplementation during cold storage for 48 hours.
|
Treatments |
Sperm viability (%) |
|||
|
Fresh semen |
5th hours |
24th hours |
48th hours |
|
|
LREY0 (without honey) |
92.00±1.22 |
84.00±2.35 |
64.75±1.18b |
50.00±2.68b |
|
LREY2 (0.2% of honey) |
92.00±1.22 |
85.25±1.80 |
69.25±1.11ab |
54.00±2.27ab |
|
LREY4 (0.4% of honey) |
92.00±1.22 |
83.75±2.02 |
73.00±0.91a |
59.75±0.63a |
|
LREY6 (0.6% of honey) |
92.00±1.22 |
81.50±2.63 |
67.25±2.87b |
50.25±2.66b |
a, b, c Different superscripts following the numbers within the same column indicate significant differences between treatments P<0.05.
Table 3: The membrane integrity (%) of KUB rooster semen on LREY extender with honey supplementation during cold storage for 48 hours.
|
Treatments |
Sperm membrane integrity (%) |
|||
|
Fresh semen |
5th hours |
24th hours |
48th hours |
|
|
LREY0 (without honey) |
93.75±1.11 |
81.50±3.86 |
73.50±0.87b |
59.50±0.87b |
|
LREY2 (0.2% of honey) |
93.75±1.11 |
82.25±1.54 |
74.25±1.25b |
63.25±1.70ab |
|
LREY4 (0.4% of honey) |
93.75±1.11 |
83.50±4.19 |
79.25±0.95a |
66.50±1.32a |
|
LREY6 (0.6% of honey) |
93.75±1.11 |
81.25±4.71 |
73.75±2.29b |
62.50±1.19ab |
a,b,c Different superscripts following the numbers within the same column indicate significant differences between treatments P<0.05.
DISCUSSION
During preservation, semen is highly susceptible to oxidative stress which causes biochemical and functional damage to the sperm. The sperm membrane plasma contains a high concentration of polyunsaturated fatty acids (PUFA) which maintains membrane stability and flexibility during the fertilization process. However, during cold storage, this plasma also makes the sperm more susceptible to lipid peroxidation through reactive oxygen species (ROS) (Sharideh et al., 2019; Vui et al., 2021). In physiological concentrations, ROS can help sperm function in hyperactivation, acrosome reaction, capacitation, and oocyte penetration, however high concentrations of ROS can cause lipid peroxidation, changes in membrane fluidity, and damage to sperm structure and death (Vui et al., 2021). Damaged membranes contribute to dead spermatozoa and disrupt membrane stability which plays an important role in fertilization (Ciftci and Aygun, 2018). This study’s findings were in line with the theory that preservation of KUB rooster semen at 5°C for 48 h negatively influences the spermatozoa and causes decreased membrane integrity, motility, and viability of the spermatozoa.
In the seminal plasma, some antioxidants can prevent or minimize the effects of oxidation damage by ROS. However, antioxidants in seminal plasma are not always available compared to ROS in semen which is greater during the preservation process, so the antioxidant capacity may not be able to fight lipid peroxidation (Amstrong et al., 1999; Vui et al., 2021). To maintain spermatozoa viability and fertility, the antioxidant system and the amount of ROS production needed to be in sync (Neuman et al., 2002). In this study, honey was used as an antioxidant in semen. Honey provides exogenous molecules that can prevent auto-oxidation reactions and neutralize free radicals through various mechanisms (Otmani et al., 2021), as well as the antioxidant properties can maintain the motility of the sperm, the viability, and the membrane integrity (Sheshtawy et al., 2016). Rochmi and Sofyan (2019) stated that spermatozoa motility is the primary determinant in pigeons, turkeys, and roosters’s fertility.
The membrane integrity, motility, and viability of KUB rooster spermatozoa in extender with various concentrations of honey at the 5th hour were not significantly different, this might have happened at the start of storage, but the antioxidant capacity in seminal plasma was still able to fight lipid peroxidation during brief preservation (Amstrong et al., 1999; Vui et al., 2021). At the 24th to 48th hour, honey supplementation had a significant effect (P < 0.05) on motility (41.50±1.19%), viability (59.75±0.63%), and membrane integrity (66.50±1.32%). Our results matched those reported on sperm from Pelung roosters (Hidayat et al., 2021), turkeys (Sari et al., 2015), bulls (Yimer et al., 2015; Malik, 2018), stallions (Sheshtawy et al., 2016), and fish (Rahardhianto et al., 2012; Condro et al., 2012; Nainggolan et al., 2015; Martin et al., 2018).
Honey, as an antioxidant, contains vitamins C and E, phenolic components, flavonoids, ascorbic acid, glucose oxidase enzyme, and catalase enzyme (Sari et al., 2015). Honey protein exerts its antioxidant activity as a reducing agent and free radical scavenger, transferring electrons (e-) or hydrogen atoms (H+) (Ramon-Sierra et al., 2022). Additionally, honey contains simple sugars (monosaccharides) like fructose and glucose, which are nutrients and cryoprotectants for spermatozoa that do not penetrate (Fuller, 2014; Sari et al., 2015). Honey is a strong antioxidant in defending cells against the damage caused by ROS (Erejuwa et al., 2012). Lestari et al. (2021) stated that antioxidants in honey significantly increase superoxide dismutase (SOD) levels in semen, which continues to counteract the increase in ROS caused by the preservation process. Minimal lipid peroxidation will protect the spermatozoa membrane from damage and in turn preserve the membrane integrity, motility, and viability of the spermatozoa. So, honey can be used as an alternative antioxidant to maintain the KUB rooster sperm quality during cold preservation at 5°C.
CONCLUSIONS
The 5th hour storage with honey supplementation had no significant effect on the spermatozoa quality. At the 24th and 48th hour significant differences were revealed and 0.4% honey was the recommended concentration in maintaining motility, viability, and membrane integrity compared to other treatments.
ACKNOWLEDGEMENTS
The author wishes to thank the Physiology Laboratory staff, Faculty of Veterinary Medicine, Universitas Gadjah Mada, for their cooperation in conducting this research. This work was supported by grants from Universitas Gadjah Mada, Indonesia.
Novelty Statement
Our study is the first report on the utilization of honey in semen extender to maintain spermatozoa quality of KUB roosters in cold storage. Our research demonstrated that the supplementation of 0.4% honey to the lactate ringer egg yolk can maintain the spermatozoa quality in cold storage (5 ℃) until 48 hours.
AUTHOR’S CONTRIBUTION
From the beginning of this study until the publications are published, the authors declared their participation.
Conflict of interests
The authors have declared no conflict of interest.
REFERENCES
Akcay E, Kulaksiz R, Daşkin A, Çebi Ç, Tekin K (2012). The effect of different dilution rates on post-thaw quality of ram semen frozen in two different egg yolk free extenders. Slovenian Vet. Res., 49: 97–102.
Anonim (2018). Statistik peternakan dan kesehatan hewan 2018. Jakarta: Direktorat Jenderal Peternakan dan Kesehatan Hewan Kementerian Pertanian RI.
Armstrong JS, Rajasekaran M, Chamulitrat W, Gatti P, Hellstrom WJ, Sikka SC (1999). Characterization of reactive oxygen species induced effects on human spermatozoa movement and energy metabolism. Free Radic. Biol. Med., 26(1999): 869–880. https://doi.org/10.1016/S0891-5849(98)00275-5
Bebas W, Laksmi D (2015). Viabilitas Spermatozoa Ayam hutan Hijau dalam pengencer Posfat Kuning Telur ditambah Laktosa pada Penyimpanan 5 derajat celcius. J. Vet., 16(1): 62-67.
Burgos S, Bohorquez DV, Burgos SA (2006). Vitamin deficiency induced nourological diseases of poultry. Poult. Sci., 5(9): 804–807. https://doi.org/10.3923/ijps.2006.804.807
Ciftci HB, Aygun A (2018). Poultry semen cryopreservation technologies. World’s Poult. Sci. J., 74(4): 699-710. https://doi.org/10.1017/S0043933918000673
Condro HS, Mubarak AS, Sulmartiwi L (2012). Pengaruh penambahan madu pada media pengencer nacl fisiologis dalam proses penyimpanan sperma terhadap kualitas sperma ikan komet (Carassius Auratus Auratus). J. Mar. Coastal Sci., 1(1): 1–12.
Erejuwa OO, Sulaiman SA, Wahab MSA (2012). Honey a novel antidiabetic agent. Int. J. Biol. Sci., 8(6): 913-934. https://doi.org/10.7150/ijbs.3697
Fuller BJ (2004). Cryoprotectants: The essential antifreezes to protect life in the frozen state. Cryo Lett., 25(6): 375-88.
Hafez B (2000). Reproduction in farm animals 7th edition. USA: Lippincot William. https://doi.org/10.1002/9781119265306
Hendiyani M, Bebas W, Budiasa MK (2018). Penambahan alfa tokoferol dalam pengencer terhadap motilitas dan daya hidup spermatozoa ayam pelung pada suhu 4oC. Indones. Med. Vet., 7(2): 168-176. https://doi.org/10.19087/imv.2018.7.2.168
Herdis (2007). Manfaat lesitin nabati pada preservasi dan kriopreservasi semen:suatu kajian pustaka. Anim. Prod., 9: 49–52.
Hidayat N, Ismoyowati, Hidayah CN, Nugroho AP (2021). Honey supplementation in lactate ringer-egg yolk extender on quality of Pelung chicken spermatozoa post-chilling. J. Kedokteran Hewan. 15(1): 7-10. https://doi.org/10.21157/j.ked.hewan.v15i1.18556
Hidayaturrahmah (2007). Waktu motilitas dan viabilitas spermatozoa ikan mas (Cyprinus carpiol) pada beberapa konsentrasi fruktosa. J. Biosci., 4(1): 9-18.
Krista B, Harianto B (2013). Ayam kampung petelur. Jakarta: Agromedia Pustaka, pp. 12.
Kusumaningrum DA, Situmorang P, Setioko AR, Sugiarti T, Triwulaningsih E, Siantu RG (2002). Pengaruh jenis dan aras krioprotektan terhadap daya hidup spermatozoa entog. J. Ilmu Ternak dan Vet., 7(4): 244-250.
Lestari S, Abinawato, Bowolaksono A, Lestari R, Dwiranti A, Gustiano R, Kristanto AH (2021). The use of honey as anti-oxidative agent: Hatching rate embryo of tor soro after 48h post-cold storage. J. Hunan Univ. (Nat. Sci.). 48(12).
Malik A (2018). Effects of honey supplementation into the extender on the motility, abnormality and viability of frozen thawed of Bali bull spermatozoa. Asian J. Anim. Vet. Adv., 13: 109-113. https://doi.org/10.3923/ajava.2018.109.113
Martin L, Watung JC, Kalesaran OJ, Ginting EL, Sinjal HJ, Salindeho IRN (2018). Penambahan madu dalam pengenceran sperma terhadap motilitas spermatozoa, fertilisasi dan daya tetas telur ikan Patin Siam, Pangasius hipophthalmus. Budidaya Perairan Mei., 6(2): 45-52. https://doi.org/10.35800/bdp.6.2.2018.20625
Mohan J, Sharma SK, Kolluri G, Dhama K (2018). History of artificial insemination in poultry, its components and significance. World’s Poult. Sci. J., 74: 475-488. https://doi.org/10.1017/S0043933918000430
Nainggolan R, Monijung RD, Mingkid W (2015). Penambahan madu dalam pengenceran sperma untuk motilitas spermatozoa, fertilisasi dan daya tetas telur ikan nila (Oreochromis niloticus). J. Budidaya Perairan Januari, 3(1): 131-140. https://doi.org/10.35800/bdp.3.1.2015.6948
Neuman SL, Orban JI, Lin TL, Latour MA, Hester PY (2002). The effect of dietary asam askorbat on semen traits and testis histology of male Turkey breeders. Poult. Sci., 81: 265–268. https://doi.org/10.1093/ps/81.2.265
Otmani A, Amessis-Ouchemoukh N, Birinci G, Yahiaoui S, Kolayli S, Rodriguez-Flores M, Escuredo O, Seijo MG, Ouchemoukh S (2021). Phenolic compounds and antioxidant and antibacterial activities of Alergian honeys. Food Biosci., 42: 1-11. https://doi.org/10.1016/j.fbio.2021.101070
Rahardhianto A, Abdulghani N, Trisyani N (2012). Pengaruh konsentrasi larutan madu dalam NaCl Fisiologis terhadap viabilitas dan motilitas spermatozoa ikan patin (Pangasius pangasius) selama Masa Penyimpanan. J. Sains Dan Seni Its, 1(1):
Ramon-Sierra JM, Villanueva MA, Yam-Puc A, Rodriguez-Mendiola M, Arias-Castro C, Ortiz-Vazquez E (2022). Antimicrobial and antioxidant activity of proteins isolated from Melipona beechii honey. Food Chem., X. 13: 1-7. https://doi.org/10.1016/j.fochx.2021.100177
Rochmi SE, Sofyan MS (2019). A diluent containing coconut water, fructose, and chicken egg yolk increases rooster sperm quality at 5°C. Vet. World, 12(7): 1116-1120. https://doi.org/10.14202/vetworld.2019.1116-1120
Sari NMDPS, Bebas W, Trilaksana IGNBT (2015). Madu meningkatkan kualitas semen kalkun selama penyimpanan. Bull. Vet. Udayana, 7(2): 164-171.
Shah SAH, Andrabi SMH, Ahmed H, Qureshi IZ (2016). Cryoprotection synergism between glycerol and dimethyl sulfoxide improves the mitochondrial transmembrane potential, plasmalemma, acrosomal and DNA integrities, and in vivo fertility of water buffalo (Bubalus bubalis) spermatozoa. Cytotechnology, 68: 2335-2344. https://doi.org/10.1007/s10616-016-0027-6
Sharideh H, Zhandi M, Zenioaldini S, Zaghari M, Sadeghi M (2019). The effect of coenzyme Q10 on rooster semen preservation in cooling condition. Theriogenology, 129: 103e109. https://doi.org/10.1016/j.theriogenology.2019.02.028
Sheshtawy RI, Badry DAE, Sisy GA, Nattat W (2016). Natural honey as a cryoprotectant to improve arab stallion post-thawing sperm parameters. Asian Pasific J. Reprod., 5(4): 331-334. https://doi.org/10.1016/j.apjr.2016.06.004
Susilawati T (2011). Spermatologi. Malang: UB Press, pp. 96-97.
Tabatabaei S, Batavani R, Ayen E (2011). Effects of vitamin E addition to chicken semen on sperm quality during in vitro storage of semen. Vet. Res. Forum, 2(2): 103-111.
Telnoni SP, Arifiantini RI, Yusuf TL, Darwati S (2017). SK Kedu semen cryopreservation in Beltsville poultry semen extender and lactated ringer’s-egg yolk extender using dimethyl sulfoxide. Asian J. Poult. Sci., 11: 14-19. https://doi.org/10.3923/ajpsaj.2017.14.19
Udjianto A (2016). Beternak Ayam Kampung KUB. Jakarta: Agromedia Pustaka, pp. 3-4.
Vui NV, Quyen NTK, Linh NT, Nhi NTM (2021). Effects of eugenol and vitamin e as a supplement to semen extender on chilled canine sperm quality. Adv. Anim. Vet. Sci., 9(7): 964-970. https://doi.org/10.17582/journal.aavs/2021/9.7.964.970
Yimer N, Muhammad N, Sarsaifi K (2015). Effect of honey supplementation into tris extender on cryopreservation of bull spermatozoa. Mal. J. Anim. Sci., 18(2): 47-54.
To share on other social networks, click on any share button. What are these?






