Induction of Heat Tolerance in Maize through Exogenous Application of Salicylic Acid, Ascorbic Acid and Hydrogen Peroxide in a Field Study
Induction of Heat Tolerance in Maize through Exogenous Application of Salicylic Acid, Ascorbic Acid and Hydrogen Peroxide in a Field Study
Muhammad Mazhar Iqbal1*, Ijaz Ahmad2, Shahzad Maqsood Ahmed Basra3, Abu Baker Ijaz4, Zahid Hassan Tarar5, Muhammad Ansar6, Umer Iqbal7, Tayyaba Naz4, Bilquees Fatima8, Muhammad Akram9, Allah Wasaya10, Asif Iqbal11, Shakeel Ahmed Anwar12, Khalid Saif Ullah Khan13, Azeem Khalid14, Asif Aziz15 and Rashid Mehmood16
1Soil and Water Testing Laboratory, Ayub Agricultural Research Institute, Chiniot, Punjab, Pakistan; 2Soil Conservation Group of Agriculture Department, Ratti, Gujrat, Punjab, Pakistan; 3Department of Agronomy, University of Agriculture, Faisalabad, Pakistan; 4Institute of Soil and Environmental Sciences University of Agriculture, Faisalabad, Pakistan; 5Soil and Water Testing Laboratory, Ayub Agricultural Research Institute, Mandi Bahauddin, Punjab, Pakistan; 6Department of Agronomy, PMAS Arid Agriculture University Rawalpindi, Pakistan; 7Crop Diseases Research Institute, National Agricultural Research Centre, Islamabad, Pakistan; 8Institute of Horticultural Sciences, University of Agriculture Faisalabad, Pakistan; 9Department of Environmental Sciences, COMSATS, Institute of Information Technology, Vehari, Pakistan; 10Department of Agronomy, Bahauddin Zakarya University Sub-Campus Layyah, Pakistan; 11On Farm Water Management, 21-Davis Road Lahore, Pakistan; 12Pulses Research Institute, Faisalabad, Pakistan; 13Department of Soil Science and Soil and Water Conservation, PMAS Arid Agriculture University Rawalpindi, Pakistan; 14Department of Environmental Sciences, PMAS Arid Agriculture University Rawalpindi, Pakistan; 15Department of Entomology, PMAS Arid Agriculture University Rawalpindi. Arid Agriculture University, Rawalpindi, Pakistan; 16Department of Plant Breeding and Genetics, PMAS Arid Agriculture University Rawalpindi, Pakistan.
Abstract | Temperature is a very important factor that affects crop yield. Maize, a monoecious plant, is adversely affected by high temperature during anthesis. Asynchronous fertilization and pollen desiccation reduce maize yield by reducing grain number and their size. Spray of hydrogen peroxide (H2O2), salicylic acid (SA) and ascorbic acid (AsA) may induce heat stress tolerance. Spray of SA, AsA, and H2O2 increased chlorophyll, relative water and nutrient contents, membrane stability index (MSI) and antioxidants activities in heat stress. Moreover, foliar application of chemicals during normal and late planting improved the grain yield by increasing both the grain number and size. Foliar spray of SA, AsA and H2O2 may induce heat tolerance by improving antioxidant activities which stabilized membrane and maintaining relative water, chlorophyll and nutrient content in ear leaves of maize during heat stress.
Received | February 02, 2020; Accepted | June 23, 2020; Published | August 01, 2020
*Correspondence | Muhammad Mazhar Iqbal, Soil and Water Testing Laboratory, Ayub Agricultural Research Institute, Chiniot, Punjab, Pakistan; Email: [email protected]
Citation | Iqbal, M.M., I. Ahmad, S.M.A. Basra, A.B. Ijaz, Z.H. Tarar, M. Ansar, U. Iqbal, T. Naz, B. Fatima, M. Akram, A. Wasaya, A. Iqbal, S.A. Anwar, K.S.U. Khan, A. Khalid, A. Aziz and R. Mehmood. 2020. Induction of heat tolerance in maize through exogenous application of salicylic acid, ascorbic acid and hydrogen peroxide in a field study. Pakistan Journal of Agricultural Research, 33(3): 585-593.
DOI | http://dx.doi.org/10.17582/journal.pjar/2020/33.3.585.593
Keywords | Heat tolerance, Maize, Growth, Physiology, Biochemistry, Salicylic acid, Ascorbic acid, Hydrogen Peroxide
Introduction
Maize (Zea mays L.) is an important cereal crop and cultivated under divergent climatic conditions of spring and summer seasons in Pakistan (Tariq et al., 2002). Being monoecious crop and very much sensitive to high temperatures (Ullah et al., 2020) which causes severe irreversible changes in the membranes which leading to destabilize them and leaked electrolytes (Ahmad et al., 2014). High temperature stress also decreases crop production by shortening the life cycle (Muchow et al., 1990), inducing pollen sterility (Mohammed and Tarpley, 2009), decreasing water content (Ahmad et al., 2014) and chlorophyll biosynthesis (Havaux, 1998). Ultimately, heat limits the photosynthetic capacity, light interception, carbon assimilation (Steven et al., 2002) and causes distinctive losses in yield (Rowhani et al., 2011). These yield losses may attribute to over-production of reactive oxygen species (ROS) during respiration process that results from exposure to high temperature and its negative effects on many plant physiological processes (Sairam and Tyagi, 2004).
Plants have adopted different protecting mechanisms of both enzymatic as well non-enzymatic in nature which counteract oxidative damage of ROS (Sairam and Tyagi, 2004). The enzymes like catalase (CAT), peroxidase (POD) and superoxide dismutase (SOD) while antioxidants, phytohormones and osmoprotectants are non-enzymatic in characteristics (Ahmad et al., 2013, 2014; Gill and Tuteja, 2010) may mitigate heat-induced damage by upregulating various scavenging mechanisms, including enzymatic and non-enzymatic antioxidants which detoxify ROS (Foyer and Noctor, 2003).
Different Non-enzymatic chemicals including SA, AsA, and low concentrations of H2O2 may alleviate the adversities of both temperature extremes on growth of maize through induction of enzymatic reactions which protect chlorophyll contents and membrane stability (Ahmad et al., 2012, 2014). Similarly, H2O2 is also a signalling molecule and its exogenous application at low concentration may induces the cold and heat stress by enhancing antioxidant activities, stabilizing cell membrane and protecting photosynthetic pigments in maize and mustard (Kumar et al., 2010; Ahmad et al., 2013). While at H2O2 higher levels, it damage the cellular system (Sairam and Tyagi, 2004; Kathiresan et al., 2006).
Based on this hypothesis, the present research work was planned to explore the impact of foliar spray of SA, AsA and H2O2 at anthesis to induce high temperature tolerance in maize and the mechanisms of high temperature stress tolerance of these chemicals in spring planted maize in diverse conditions.
Materials and Methods
This experimental study was performed at the research farm area, University of Agriculture, Faisalabad to explore response of foliar spray of SA, AsA and H2O2 on physiological, biochemical and agronomic attributes maize hybrid (Hi Sawn 9697) under divergent conditions of 22 February (normal planting; NP) and 15 March (late planting; LP). The research was carried out in split plot design, randomizing sowing dates in main plots while foliar applied chemicals at 20 mg L-1 at anthesis in the subplots. The 4.2 × 7.7 m net plot size was used. All crop production and protection practices were kept uniform throughout research span. After 60 days of crop growth, samples of ear leaves for different physiological and biochemical attributes were collected at tasselling while yield data were at harvesting.
For determination of relative water content (RWC), 0.5 g of fresh ear leaves (fresh weigh, FW) samples were rinsed in separate test tubes for each experimental unit until this attained turgidity and weighed through an electric balance (turgid weight, TW). The turgid leaf’s samples were air-dried and oven-dried for 24 h at 80 oC and then weighed (dry weight, DW). The RWC was calculated by formula following Reddy (2004).
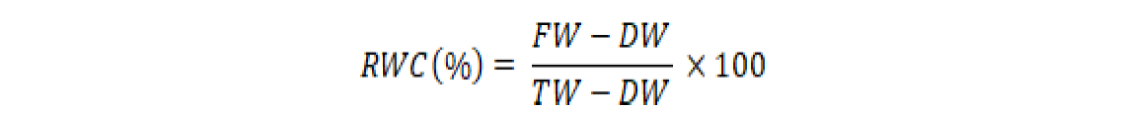
To determine the membrane stability index (MSI), leaf’s samples of 200 mg of each experimental unit were collected and placed in 2 sets of test tubes having doubled-distilled water (Sairam, 1994). One lot of test tubes of each treatment were heated at 40˚C for 30 minutes while other set were boiled in a water bath at 100˚C for 10 minutes. The electrical conductivity (EC) of each sample was measured using conductivity bridge.
For the determination of chlorophyll a and b (Chl a and Chl b) content, 0.5-cm segments of fresh ear leaf of each experimental unit were collected after 60 days of crop growth. These samples were extracted overnight via 80% acetone at -10˚C. These extracted samples were centrifuged at 14000×g for 5 minutes and absorbance of each supernatant sample was observed at 663 and 645 nm wavelength through spectrophotometer (T60). The chlorophyll a and b were intended via subsequent formula as described by Iqbal et al. (2020).
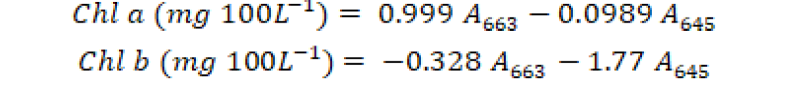
To extract antioxidant enzymes, 0.5 g of fresh leaf sample from each experimental unit were collected in test tube containing 8 mL of refrigerated phosphate buffer of 7 pH (1%, w/v) polyvinyl pyrrolidone. Each sample was ground with a tissue grinder and 0.2 g quartz sand was also poured in each sample. The homogenates of each experimental unit were centrifuged at 15000×g for 20 minutes at 4˚C and enzymatic activities were assayed by using supernatant.
The SOD was detected through inhibitition the photo-reduction of nitro blue tetrazolium (NBT) (Giannopolitis and Ries, 1977) while POD and CAT activities was measured by using the mode as described by Naz et al. (2019).
Data on yield and its attributes were recorded using standard procedures. Air temperature was recorded for 19 weeks starting from the planting date or under normal (22 February) and heat stress conditions (15 March) and the mean temperature after the week of sowing was calculated (Table 1).
The above collected data were computed through Fisher’s Analysis of Variance (ANOVA) technique and treatment means were contrasted via Least Significance Difference (LSD) test (Steel et al., 1997).
Results and Discussion
Air temperature during study
Maximum day temperatures of 31.4˚C, 36.1˚C, 40.5˚C, and 41.6˚C for maize sown on 22 February (normal planting, NP) and maximum day temperatures of 41.6˚C, 37.2˚C, 39.7˚C, and 34.1˚C for maize sown on 15 March (late planting, LP) were recorded during weeks 8, 9, 10 and 11 after sowing. Similarly, relative humidity (RH) values of 38.7%, 24.1%, 16.3%, and 15.0% for NP maize, and 15.9%, 32.9%, 31.7%, and 43.7% for LP maize were recorded during weeks 8, 9, 10, and 11 after sowing (Table 1).
Table 1: Meteorological data recorded of experimental site from sowing to harvesting.
| Weeks after sowing | Maximum Temp. (ºC) | Minimum Temp. (ºC) | Average Temp (ºC) | Relative humidity (%) | Rainfall (mm) | |||||
|
22nd Feb. |
15th March |
22nd Feb. |
15th March |
22nd Feb. |
15th March |
22nd Feb. |
15th March |
22nd Feb. |
15th March |
|
| 1 | 23.4 | 31.9 | 7.7 | 15.4 | 15.5 | 23.7 | 34.6 | 34.6 | 0.0 | 0.0 |
| 2 | 28.9 | 31.9 | 12.2 | 15.3 | 20.6 | 23.6 | 43.1 | 35.1 | 0.0 | 0.0 |
| 3 | 29.0 | 32.7 | 13.1 | 16.9 | 21.1 | 24.8 | 40.1 | 36.4 | 0.0 | 0.8 |
| 4 | 31.9 | 27.4 | 15.4 | 16.4 | 23.7 | 21.9 | 34.6 | 50.3 | 0.0 | 0.3 |
| 5 | 31.9 | 31.4 | 15.3 | 18.4 | 23.6 | 24.9 | 35.1 | 38.7 | 0.0 | 1.2 |
| 6 | 32.7 | 36.1 | 16.9 | 20.6 | 24.8 | 28.4 | 36.4 | 24.1 | 0.8 | 0.0 |
| 7 | 27.4 | 40.5 | 16.4 | 21.1 | 21.9 | 30.8 | 50.3 | 16.3 | 0.3 | 0.0 |
| 8* | 31.4 | 41.6 | 18.4 | 24.9 | 24.9 | 33.3 | 38.7 | 15.9 | 1.2 | 0.0 |
| 9 | 36.1 | 37.2 | 20.6 | 22.6 | 28.4 | 28.2 | 24.1 | 32.9 | 0.0 | 1.8 |
| 10 | 40.5 | 39.7 | 21.1 | 25.2 | 30.8 | 32.5 | 16.3 | 31.7 | 0.0 | 1.6 |
| 11 | 41.6 | 34.1 | 24.9 | 22.4 | 33.3 | 28.3 | 15.9 | 43.7 | 0.0 | 7.3 |
| 12 | 37.2 | 39.6 | 22.6 | 27.3 | 28.2 | 33.5 | 32.9 | 30.9 | 1.8 | 0.0 |
| 13 | 39.7 | 39.1 | 25.2 | 28.4 | 32.5 | 33.7 | 31.7 | 44.1 | 1.6 | 1.9 |
| 14 | 34.1 | 38.4 | 22.4 | 26.8 | 28.3 | 32.6 | 43.7 | 50.9 | 7.3 | 0.2 |
| 15 | 39.6 | 37.0 | 27.3 | 26.9 | 33.5 | 31.9 | 30.9 | 53.6 | 0.0 | 2.7 |
| 16 | 39.1 | 38.0 | 28.4 | 27.9 | 33.7 | 33.0 | 44.1 | 53.9 | 1.9 | 1.2 |
| 17 | 38.4 | 37.4 | 26.8 | 28.4 | 32.6 | 32.9 | 50.9 | 54.4 | 0.2 | 2.6 |
| 18 | 37.0 | 36.1 | 26.9 | 26.5 | 31.9 | 31.3 | 53.6 | 61.4 | 2.7 | 2.0 |
| 19 | 38.0 | 31.9 | 27.9 | 15.4 | 33.0 | 23.7 | 53.9 | 34.6 | 1.2 | 0.0 |
*: Highlighted indicate the high after 56 days and foliar spray at 58 days after sowing.
Physiological and biochemical attributes
The chlorophyll a and b contents were decreased with rise in temperature but application SA, AsA, and H2O2 significantly improved NP and LP planting conditions (Tables 2 and 4). The higher Chl a and Chl b were observed when AsA applied alone and when both SA and H2O2 were applied as the most prominent compounds during the NP and LP plantings, despite facing high temperatures of 31.6°C and 41.6oC during tasseling, respectively. Minimum Chl a and Chl b were detected in maize in controlled treatments under NP and LP dates (Table 4). The RWC was significantly increased with foliar applied SA, AsA, and H2O2 in both NP and LP maize (Table 4). The foliarly applied SA, H2O2 and AsA and improved the RWC by 4% in NP maize and by 7%, 6% and 6%, respectively, in LP maize. Similarly, exogenous spray of SA, AsA or H2O2 improved MSI by 5%, 5%, and 5%, respectively, in NP crops, and by 6%, 7%, and 6% in LP crops, respectively, compared with respective controls (Table 4). Similarly, high temperatures reduced SOD activity but exogenously applied SA, AsA or H2O2 improved SOD activity by 13%, 6% and 14% in NP maize and by 7%, 3% and 5% in LP maize (Tables 2 and 4). The H2O2 was more effective than SA or AsA during increased temperatures up to 41.6˚C. Likewise, high temperature reduced CAT activity but foliarly applied SA, AsA, or H2O2 improved these activities by 123%, 47% and 74% in NP maize and by 93%, 42% and 73% in LP maize, respectively (Tables 2 and 4). Likewise, peroxidase (POD) were augmented with exogenously applied SA, AsA, or H2O2 at the rate of 19%, 11% and 12% in NP and 9%, 11%, and 9% in LP maize, respectively (Tables 2 and 4). While heat stress reduced POD activities, spray of SA, AsA or H2O2 improved them to a larger amount in NP than in LP maize (Tables 2 and 4).
Yield and yield components
The maize yield and its components decreased with an increase in the temperature, but foliarly applied SA, AsA, or H2O2 increased those (Tables 3 and 5). Foliar spray of SA, AsA, or H2O2 improved cobs per plant by 2%, 3%, and 5% in NP and by 2%, 2%, and 4% in LP maize, respectively (Tables 3 and 5). Moreover, the number of grains declined with rise in the air temperature during anthesis but foliarly applied SA, AsA, or H2O2 enlarged grains per cob by 13, 13 and 15% in NP and 8, 9 and 10% in LP (TTables 3 and 5). Similarly, 100-grain weight was also significantly increased by SA, AsA, or H2O2 application. Lighter grains were produced in LP plants than in NP plants, however foliarly applied SA, AsA, or H2O2 amplified grain size by 3, 4 and 1% in NP and 5, 6 and 7% in LP, respectively (Tables 3 and 5). Similarly, grain yield was reduced with rise in temperature the greater grain yield of 4.99 Mg ha-1 was produced in NP than 4.12 Mg ha-1 in LP. The SA, AsA or H2O2 application increased yield by 5, 3 and 4% in NP and 16, 24 and 14% in LP, respectively (Tables 3 and 5). Relatively better response of chemicals was observed in LP than NP was linked with raise in grain number in NP while higher 100-grain weight in LP.
In present study, lower chlorophyll a and b contents were observed in LP maize than in NP of maize but spray of SA, AsA or H2O2 improved the chlorophyll content in both NP and LP maize (Table 4). The lessening in chlorophyll contents can be owing to greater deterioration of the photosynthetic apparatus by oxy-radicals produced during increased respiration in response to high temperatures in stressed LP than non-stressed conditions NP. A similar decrease in the chlorophyll content was observed previously in turf grass and heat stressed maize (Jiang and Huang, 2001; Robin et al., 2014). The exogenous application of SA, AsA, or H2O2, increased chlorophyll contents in both NP and LP in present study were results of increased antioxidants activities and stabilizing membranes in this study. These results were in consonance with the findings of He and Huang (2007) whom also reported that thermo-tolerance was improved with antioxidant defence systems under heat stress and Scandalios (1993) speculated that the intracellular membrane might be damaged by ROS production, which can break down pigment.
The relatively higher RWC and MSI in NP than LP was observed in this study indicated that high temperature may be due to damage membranous system in response to heat but SA, AsA or H2O2 increase RWC and MSI in NP and LP (Table 4). This increase in RWC can be associated with protective influence of exogenous molecules on the cell membrane against destruction by ROS produced during high temperatures in reaction with unsaturated fatty acids in phospholipids, which cause cell membrane damage. DaCosta and Huang (2007) and Robin et al. (2014) also reported that stress induces oxidative damage to membranes, but exogenous application of SA, AsA, or H2O2 may protect against lipid peroxidation through strengthening the antioxidant defence system.
High temperatures decreased the SOD activity but foliar spray of SA, AsA, or H2O2 improved SOD activity in NP and in LP in maize (Table 4).
The reduced SOD activity under heat stress conditions
Table 2: Mean square values for ear leaf chlorophyll a (Chl a), b (Chl b), and relative water contents (RWC), membrane stability index (MSI), superoxide dismutase (SOD), catalase (CAT) and peroxidase (POD) activity.
| Source of variation | DF |
Chl a (mg 100 mL-1) |
Chl b (mg 100 mL-1) |
MSI (%) | RWC (%) |
SOD activity (units mg-1 Pr.) |
CAT activity (units mg-1 Pr.) |
POD activity (units mg-1 Pr.) |
| Replication | 3 |
0.00004NS |
1.0084NS |
0.2599NS |
0.2599NS |
0.2056NS |
0.00181NS |
0.00068NS |
| Sowing date | 1 |
6.02045** |
11.2338* |
5.78** |
7.78** |
11.2931** |
4.11845** |
0.03315** |
| Error-I | 3 | 0.00001 | 2.0537 | 0.3344 | 0.3344 | 0.0161 | 0.00011 | 0.00001 |
| Foliar spray | 3 |
0.09147** |
7.8* |
26.5143** |
26.5143** |
4.462** |
1.18749** |
0.01799** |
| Sowing date × Foliar spray | 18 |
0.08336** |
4.96* |
0.7404** |
0.7404** |
0.9762* |
0.04861** |
0.00247** |
| Error-II | 31 | 0.00005 | 0.4097 | 0.0615 | 0.0615 | 0.2565 | 0.00241 | 0.00001 |
*: Significant at 0.05 p; **: highly significant at 0.05 p; NS: Non Significant at 0.05 p.
Table 3: Mean square values for cobs per plant, grains per cob, 100-grain weight and grain yield.
| Source of variation | DF | Cobs per plants | Grains per cob | 100-Grain weight (g) |
Grain Yield (Mg ha-1) |
| Replication | 3 |
0.00016NS |
4.3NS |
0.347NS |
0.02721NS |
| Sowing Date | 1 |
0.01051** |
77159.7** |
684.315** |
6.14251** |
| Error-I | 3 | 0.00087 | 73.8 | 0.001 | 0.01595 |
| Foliar Spray | 3 |
0.00373* |
4094.8** |
1.498* |
0.38562** |
| Sowing Date × Foliar spray | 18 |
0.00014NS |
362.8** |
1.379* |
0.18302* |
| Error-II | 31 | 0.001 | 42.6 | 0.161 | 0.03317 |
*: Significant at 0.05 p, **: highly significant at 0.05 p; NS: Non-Significant at 0.05 p.
Table 4: Comparison of chlorophyll a (Chl a), b (Chl b) and membrane stability index (MSI), relative water contents (RWC), superoxide dismutase (SOD), catalase (CAT) and peroxidase (POD) activity of spring sown maize as influenced by foliar application of ascorbic acid (AsA), salicylic acid (SA) and hydrogen peroxide (H2O2) when planted on 22nd Feb. and 15th March.
| Treatments |
Chl a (mg 100mL-1) |
Chl b (mg 100mL-1) |
Membrane stability index (%) | Relative water contents (%) |
SOD activity (units mg-1 Pr.) |
CAT activity (units mg-1 Pr.) |
POD activity (units mg-1 Pr.) |
|
| 22nd Feb.(NP) | 2.62 a | 2.56 | 61.50 a | 73.50 a | 15.25 a | 1.72 a | 0.87 a | |
| 15th March (LP) | 1.75 b | 1.38 | 60.65 b | 72.65b | 14.06 b | 0.99 b | 0.81 b | |
| LSD | 0.003 | NS | 0.65 | 0.651 | 0.143 | 0.012 | 0.004 | |
| Control | 2.19 c | 1.50 b | 58.35 c | 70.35 c | 13.74 c | 0.88 d | 0.77 c | |
| AsA | 2.04 d | 1.68 b | 61.87 b | 73.87b | 14.40 b | 1.26 c | 0.86 b | |
| SA | 2.30 a | 2.90 a | 62.17 a | 74.17a | 15.43 a | 1.78 a | 0.88 a | |
|
H2O2 |
2.20 b | 1.79 b | 61.90 b | 73.90 b | 15.05 a | 1.52 b | 0.86 d | |
| LSD | 0.075 | 0.67 | 0.261 | 0.261 | 0.532 | 0.052 | 0.003 | |
| 22nd Feb (NP). | Control | 2.41 d | 1.94 bc | 59.21 d | 71.21d | 13.94 cd | 1.13 e | 0.81 f |
| AsA | 2.75 b | 2.18 b | 62.21abc | 74.21 abc | 14.79 b | 1.61 c | 0.90 a | |
| SA | 2.79 a | 4.21 a | 62.32 a | 74.32 a | 16.36 a | 2.18 a | 0.88 c | |
|
H2O2 |
2.52 c | 1.92 bcd | 62.24 ab | 74.24 ab | 15.90 a | 1.95 b | 0.90 a | |
| 15th March (LP) |
Control |
1.65 h | 1.06 f | 57.49 e | 69.49 e | 13.53 d | 0.62 g | 0.73 g |
| AsA | 1.68 g | 1.19 e | 61.52 c | 73.52 c | 14.00 cd | 0.91 f | 0.81 f | |
| SA | 1.82 f | 1.59 de | 62.02 abc | 74.02abc | 14.50 bc | 1.38 d | 0.87 d | |
|
H2O2 |
1.86 e | 1.67 bcd | 61.56bc | 73.56 bc | 14.21 bcd | 1.08 e | 0.82 e | |
| LSD | 0.0106 | 0.45 | 0.368 | 0.175 | 0.752 | 0.073 | 0.004 | |
Means sharing same letter in a column do not differ significantly at 0.05 probability level.
might reflect higher O2- production in heat stressed maize but exogenous application at low concentration of H2O2 was more effective for maintaining SOD activity under heat stress by detoxifying superoxide (O2-). Maximal SOD activities may require protection against superoxide which is reduced in the LP conditions, in this study leading to more severe heat damage to the leaves in this study (Table 4). Previously, plants must protect themselves from these oxidative injuries by maintaining sufficient levels of SOD were also reported by Scandalios (1993).
The most important scavenging enzyme in organisms is CAT, which detoxifies H2O2 into O2-. In present research, the CAT and POD were observed in NP than LP but exogenous effectors molecules increased CAT and POD activities in this study was observed (Table 4). A reduction in CAT and POD activities at heat stress conditions was also reported in crop plants when exposed to heat stress (Dat et al., 1998; Foyer et al., 1997; Jiang and Huang, 2001; Sato et al., 2001). The essential role of CAT for scavenging H2O2 was established in chloroplasts, where CAT is absent (Asada and Takahashi, 1987). Although heat stress reduced POD activity, exogenous spray of SA, AsA, or H2O2 increased POD activity in both NP and LP maize. Moreover, these chemicals induced more POD activity in NP maize than in LP maize. The higher POD activity due to the application of SA, AsA, or H2O2 upon exposure to heat stress may be due to improved scavenging system, which protects the membranes from the deleterious effects of heat via converting superoxide radicals to H2O2 and H2O2 to O2-. Higher POD might enhance the H2O2 scavenging system by exogenous or foliar application of stress signalling molecules like AsA, SA, or a low concentration of H2O2 may impair the accumulation of ROS of H2O2, resulted lower heat-induced injury. These findings demonstrate the dual role of H2O2 as a ROS and as a signalling molecule in induction of heat stress. A decline in POD under heat stress conditions was also reported by Almeselmani et al. (2006). The heat induced a reduction in POD activity in both NP and LP maize, suggesting that POD is sensitive to high temperatures and SA, SA, H2O2. In addition, antioxidant enzyme activity is increased by AsA, SA, and H2O2 application in different crops under stress. The current research results are also in line with Khan et al. (2006), Ahmad et al. (2013, 2014) and Appu and Muthukrisnan (2014) whom concluded that antioxidant activity is improved by foliarly applied SA, AsA, and H2O2 at suboptimal temperature in maize.
The higher grain yield, number of cobs and grains as well as 100-grain weight was produced in NP than LP, but foliarly applied SA, AsA, or H2O2 enlarged under normal and stressful conditions of high temperature (Table 5) The grain yield is commutative effects of various yield attributes which started from pollen fertilization ended grain harvesting. The number of grains reduced in this study indicated that high may reduce pollen fertilization while poor grain development produce small grains size (Table 4). The reduction of grain yield owing to high temperature was pragmatic as a result lesser grains and poor grain number by Jones and Thornton (2003), Lobell et al. (2008), and Rowhani et al. (2011) who also reported lower as result of poor fertilization and grain development under heat stress. Application of exogenous effectors improved the physiological, biochemical, and yield attributes, ultimately resulting in increased maize grain yield even under high temperature (Table 5).
Table 5: Comparison of cobs per plant, grains per cob, 100-grain weight and grain yield of spring sown maize as influenced by foliar application of ascorbic acid, salicylic acid and hydrogen peroxide when planted on 22nd Feb. and 15th March.
| Treatments | Cobs per Plant | Grains per cob | 100-Grain weight (g) |
Grain Yield (Mg ha-1) |
|
|
22nd Feb (NP). |
1.32 a | 471.5 a | 31.05 a | 4.99 a | |
|
15th March (LP) |
1.29 b | 373.3 b | 21.80 b | 4.12 b | |
| LSD | 0.033 | 9.67 | 0.033 | 0.142 | |
| Control | 1.28 b | 388.8c | 26.40 b | 4.24 b | |
| AsA | 1.31 ab | 431.4b | 26.88 a | 4.75 a | |
| SA | 1.31 ab | 430.7b | 26.57 ab | 4.65 a | |
|
H2O2 |
1.33a | 438.5 a | 25.85 c | 4.58 a | |
| LSD | 0.033 | 6.85 | 0.421 | 0.191 | |
|
22nd Feb (NP). |
Control | 1.29bc | 428.0 b | 30.43 c | 4.85 a |
| AsA | 1.33ab | 482.5 a | 31.70 a | 4.99 a | |
| SA | 1.32ab | 484.7 a | 31.23 ab | 5.10 a | |
|
H2O2 |
1.35 a | 490.7 a | 30.83 bc | 5.03 a | |
| 15th March (LP) | Control | 1.26 c | 349.7 d | 20.87e | 3.63 d |
| AsA | 1.29 bc | 380.3c | 22.07 d | 4.51 b | |
| SA | 1.29 bc | 376.7c | 21.90 d | 4.20 c | |
|
H2O2 |
1.31ab | 386.3c | 22.37 d | 4.13 c | |
| LSD | 0.047 | 9.692 | 0.596 | 0.271 | |
Means sharing same letter in a column do not differ significantly at 0.05 probability level.
The results depicted that number of grains declined with rise temperature during anthesis, which might be due to pollen desiccation and failed fertilisation, but exogenous application of SA, AsA, or H2O2 increased grains per cob by ameliorating the adversities of high temperature (31.6˚C and 41.6˚C) on grains (Table 5). High temperature adversely affects grain number by increased pollen abortion, poor fertilization (Dupuis and Dumas, 1990; Talwar et al., 1999) and desiccating exposed pollen grains (Sinsawat et al., 2004), while SA, AsA or H2O2 application reduced these effects in both NP and LP maize exposed to an ambient temperature of 31.4˚C (normal) or heat stressed (41.6˚C) under late sown conditions during tasseling (8 weeks after sowing).
Lighter grains were produced in LP maize than in NP maize, but foliarly applied SA, AsA, or H2O2 increased grain size regardless of the planting date. Lighter grain during late planting when plants face high temperatures during anthesis and grain formation stage may be due to the adversities of heat stress on kernel development. Drastic impacts of high temperature on grain development owing to asynchronous fertilization and grain formation were also reported by Rahman et al. (2013). Comparable destructive influences of high temperature on grain development were quoted by Gibson and Paulsen (1999), Rahman et al. (2013) and Viswanathan and Chopra (2001).
Conclusions and Recommendations
In present study, foliar spray of SA, AsA, or H2O2 induced high temperature stress tolerance in maize by improving growth through protecting chlorophyll, increasing water and nutrient uptake, antioxidants activities and stabilising cell membranes These positive changes eventually resulted in increased maize yield by improving grain number and size under heat stress. Induction of these positive changes in the photosynthetic pigments and stabilisation of membranes through applied AsA, SA or H2O2, which exhibited differential responses in NP and LP maize exposed to heat stress, may lead to improvement in heat stress tolerance.
Author’s Contribution
Muhammad Mazhar Iqbal has written the main body of present manuscript. Ijaz Ahmad conducted and carried out the present research study. Shahzad Masood Ahmed Basra designed and supervised the present research. Abu Bakar Ijaz helped in literature citation and references. Zahid Hassan Tarar has contributed in data collection and laboratory analysis. Muhammad Ansar critically reviewed and edited the manuscript. Umer Iqbal has has helped in data collection and determination of different parameters in laboratory. Tayyaba Naz statistically analyzed the obtained data. Bilquees Fatima has significantly contributed in Discussion. Muhammad Akram considerably contributed in Introduction. Allah Wasaya facilitated in Materials and Methods. Asif Iqbal noticeably assisted in Results. Shakeel Ahmed Anwar helped in research experimentation, availability of inputs and data tabulation. Khalid Saif Ullah Khan and Azeem Khalid critically read and improved the manuscript for publication in Pakistan Journal of Agricultural Research. Asif Aziz helped in maize crop protection measures. Rashid Mehmood helped in agronomic production system of maize crop.
Acknowledgements
The authors are also thankful to Higher Education Commission, Islamabad for providing funds for this research study.
Conflict of interest
The authors declare that they have no conflict of interest.
References
Ahmad, I., S.M.A. Basra, I. Afzal, M. Farooq and A. Wahid. 2013. Growth improvement in spring maize through exogenous application of ascorbic acid, salicylic acid and hydrogen peroxide. Int. J. Agric. Biol. 15: 95-100.
Ahmad, I., S.M.A. Basra and A. Wahid. 2014. Exogenous application of ascorbic acid, salicylic acid and hydrogen peroxide improves the productivity of hybrid maize under at low temperature stress. Int. J. Agric. Biol. 16: 825-830.
Ahmad, I., T. Khaliq, A. Ahmad, S.M.A. Basra, Z. Hussain and A. Ali. 2012. Effect of seed priming with ascorbic acid, salicylic acid and hydrogen peroxide on emergence, vigor and antioxidant activities of maize. Afr. J. Biotech. 11: 1127-1132. https://doi.org/10.5897/AJB11.2266
Ahmad, I., S.M.A. Basra, S. Hussain, S.A. Hussain, Hafeez-ur-Rehman, A. Rehman and A. Ali. 2015. Priming with ascorbic acid, salicylic acid and hydrogen peroxide improves seedling growth of spring. J. Environ. Agric. Sci. 3:14-22.
Almeselmani, M., P.S. Deshmukh, R.K. Sairam, S.R. Kushwaha and T.P. Singh. 2006. Protective role of antioxidant enzymes under high temperature stress. Plant Sci. 171: 382-388. https://doi.org/10.1016/j.plantsci.2006.04.009
Appu, M. and S. Muthukrishnan. 2014. Foliar application of salicylic acid stimulates flowering and induce defense related proteins in finger millet plants. Universal J. Plant Sci. 2: 14-18.
Asada, K. and M. Takahashi. 1987. Production and scavenging of active oxygen in photosynthesis. in Photoinhibition: Topics of Photosynthesis, DJ Kyle, CB Osmond and CJ Arntzen, Eds, Elsevier, Amsterdam, 9th edition, The Netherlands. pp. 227–287.
Chance, M. and A.C. Maehly. 1955. Assay of catalases and peroxidases. Methods Enzymol. 2: 764-775. https://doi.org/10.1016/S0076-6879(55)02300-8
DaCosta, M. and B. Huang 2007. Changes in antioxidant enzyme activities and lipid peroxidation for bent grass species in response to drought stress. J. Am. Soc. Hort. Sci. 132: 417-422. https://doi.org/10.21273/JASHS.132.3.319
Dat, J.F., H. Lopez-Dalgado, C.H. Foyer and I.M. Scott. 1998. Parallel changes in H2O2 and catalase during thermotolerance induced by salicylic acid or heat acclimation in mustard seedlings. Plant Physiol. 116: 1351-1357. https://doi.org/10.1104/pp.116.4.1351
Dupuis, I. and Dumas, C. 1990. Influence of temperature stress on maize (in vitro) fertilization and heat shock protein synthesis in maize (Zea mays L.) reproductive tissue. Plant Physiol. 94: 665-670. https://doi.org/10.1104/pp.94.2.665
Foyer, C.H., H. Lopez-Delgado, J.F. Dat and I.M. Scott. 1997. Hydrogen peroxide- and glutathione-associated mechanisms of acclamatory stress tolerance and signaling. Physiol. Plant. 100: 241-254. https://doi.org/10.1034/j.1399-3054.1997.1000205.x
Foyer, C.H. and G. Noctor. 2003. Redox sensing and signaling associated with reactive oxygen in chloroplasts, peroxisomes and mitochondria. Physiol. Plant. 119: 355-364. https://doi.org/10.1034/j.1399-3054.2003.00223.x
Giannopolitis, C.N. and S.K. Ries. 1977. Superoxide dismutase. I. Occurrence in higher plants. Plant Physiol. 59: 309-314. https://doi.org/10.1104/pp.59.2.309
Gibson, L.R. and G.M. Paulsen. 1999. Yield components of wheat grown under high temperature stress during reproductive growth. Crop Sci. 39: 1841-1846. https://doi.org/10.2135/cropsci1999.3961841x
Gill, S.S. and N. Tuteja. 2010. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 48: 909-930. https://doi.org/10.1016/j.plaphy.2010.08.016
Havaux, M., 1998. Carotenoids as membrane stabilizers in chloroplasts. Trends Plant Sci. 3: 147-151. https://doi.org/10.1016/S1360-1385(98)01200-X
He, Y. and B. Huang. 2007. Protein changes during heat stress in three kentucky bluegrass cultivars differing in heat tolerance. Crop Sci. 47: 2513-2520. https://doi.org/10.2135/cropsci2006.12.0821
Iqbal, M.M., T. Naz, M.I. Zafar, M. Imtiaz, O. Farooq, A. Rehman, S. Ali, M. Rizwan, S. Hussain, W. Javed, G. Murtaza, M. Afzal, A. Mahmood, S.M. Mehdi, M.A. Sarwar and G. Du Laing. 2020. Green remediation of saline-sodic Pb-factored soil by growing salt-tolerant rice cultivar along with soil applied inorganic amendments. Paddy Water Environ. https://doi.org/10.1007/s10333-020-00807-6
Jiang, Y. and B. Huang. 2001. Effects of calcium on antioxidant activities and water relations associated with heat tolerance in two cool-season grasses. J. Exp. Bot. 52: 341-349. https://doi.org/10.1093/jexbot/52.355.341
Jones, P.G. and P.K. Thornton. 2003. The potential impacts of climate change on maize production in Africa and Latin America in 2055. Glob. Environ. Change. 13: 51-59. https://doi.org/10.1016/S0959-3780(02)00090-0
Kathiresan, A., H.R. Lafitte, J. Chen, L. Mansueto, R. Bruskiewich and J. Bennett. 2006. Gene expression microarrays and their application in drought stress research. Field Crops Res. 97: 101-110. https://doi.org/10.1016/j.fcr.2005.08.021
Khan, A., M. Sajid, A. Ahmad, H.R. Athar and M. Ashraf. 2006. Interactive effect of foliarly applied ascorbic acid and salt stress on wheat (Triticum aestivum L.) at the seedling stage. Pak. J. Bot. 38: 1407-1414.
Kumar, M., G. Sirhindi, R. Bhardwaj, S. Kumar and G. Jain. 2010. Effect of exogenous H2O2 on antioxidant enzymes of Brassica juncea L. seedlings in relation to 24-epibrassinolide under chilling stress. Indian J. Biochem. Biophys. 47:378-382.
Lobell, D.B., M.B. Burke, C. Tebaldi, M.D. Mastrandrea, W.P. Falcon and R.L. Naylor. 2008. Prioritizing climate change adaptation and needs for food security in 2030. Science. 319: 607-610. https://doi.org/10.1126/science.1152339
Mohammed, A.R. and L. Tarpley. 2009. High nighttime temperatures affect rice productivity through altered pollen germination and spikelet fertility. Agric. For. Meteorol. 149: 999-1008. https://doi.org/10.1016/j.agrformet.2008.12.003
Muchow, R.C., T.R. Sinclair and J.M. Bennett. 1990. Temperature and solar radiation effects on potential maize yield across locations. Agron. J. 82: 338-343. https://doi.org/10.2134/agronj1990.00021962008200020033x
Naz, T., J. Akhtar, M.M. Iqbal, M. Anwar-ul-Haq, G. Murtaza, N.K. Niazi, Atique-ur-Rehman, O. Farooq, M. Ali and B. Dell, 2019. Assessment of gas exchange attributes, chlorophyll contents, ionic composition and antioxidant enzymes of bread wheat genotypes in boron toxic, saline and boron toxic-saline soils. Int. J. Agric. Biol. 21: 1271‒1278.
Rahman, S., M. Arif, K. Hussain, S. Hussain, T. Mukhtar, A. Razaq and R.A. Iqbal. 2013. Evaluation of maize hybrids for tolerance to high temperature stress in Central Punjab. Am. J. Bioengr. Biotechnol. 1: 30-36. https://doi.org/10.7726/ajbebt.2013.1003
Reddy, S.R. 2004. Principles of Crop Production. 2nd Ed. Kalyani Publishers, New Dehli, India, pp. 354.
Robin, A.W., M.K. Sangha, S.S. Banga, A.K. Atwal and S. Gupta. 2014. Heat stress tolerance in relation to oxidative stress and antioxidants in Brassica juncea. J. Environ. Biol. 35: 383-387.
Rowhani, P., D.B. Lobell, M. Linderman and N. Ramankutty. 2011. Climate variability and crop production in Tanzania. Agric. Forest Meteorol. 151: 449-460. https://doi.org/10.1016/j.agrformet.2010.12.002
Sairam, R.K., 1994. Effect of moisture stress on physiological activities of two contrasting wheat genotypes. Indian J. Exp. Biol. 32: 594-597.
Sairam, R.K. and A. Tyagi. 2004. Physiological and molecular biology of salinity stress tolerance in plants. Curr. Sci. 86: 407-421.
Sato, Y., T. Murakami, H. Funatsuki, S. Matsuba, H. Saruyama and M. Tanida. 2001. Heat shock-mediated APX gene expression and protection against chilling injury in rice seedlings. J. Exp. Bot. 52: 145-151. https://doi.org/10.1093/jexbot/52.354.145
Scandalios, J.G., 1993. Oxygen stress and superoxide dismutases. Plant Physiol. 101: 7-12. https://doi.org/10.1104/pp.101.1.7
Sinsawat, V., J. Pandy, P. Leipner, P. Stamp and Y. Fracheboud. 2004. Effect of heat stress on the Photosynthetic apparatus in maize (Zea mays L.) grown at control and high temperature. Environ. Exp. Bot. 52: 123-129. https://doi.org/10.1016/j.envexpbot.2004.01.010
Steel, R.G.D., T.H. Torrie and D.A. Dickey. 1997. Principles and procedures of statistics. A Biometrical Approach 3rd Ed. McGraw Hill Book International Co. Inc., Singapore, pp. 204-232.
Steven, J., C. Brandner and M. Salvucci. 2002. Sensitivity of photosynthesis in C4 maize plant to heat stress. Plant Physiol. 129: 1773-1780. https://doi.org/10.1104/pp.002170
Talwar, H.S., H. Takeda, S. Yashima and T. Senboku. 1999. Growth and photosynthetic responses of groundnut genotypes to high temperature. Crop Sci. 39: 460-466. https://doi.org/10.2135/cropsci1999.0011183X0039000200027x
Tariq, M., M.A. Khan and S. Parveen. 2002. Response of maize to applied soil Zinc. Asian J. Plant Sci. 1:476-477. https://doi.org/10.3923/ajps.2002.476.477
Ullah, N., A. Ditta, A. Khalid, S. Mehmood, M.S. Rizwan, M. Ashraf, F. Mubeen, M. Imtiaz and M.M. Iqbal. 2020. Integrated effect of algal biochar and plant growth promoting rhizobacteria on physiology and growth of maize under water deficit irrigations. J. Soil Sci. Plant Nutr. 20: 346–356. https://doi.org/10.1007/s42729-019-00112-0
Viswanathan, C. and R.K. Chopra. 2001. Effect of heat stress on grain growth, starch synthesis and protein synthesis in grains of wheat varieties differing in grain weight stability. J. Agron. Crop Sci. 186: 1-7. https://doi.org/10.1046/j.1439-037x.2001.00432.x
To share on other social networks, click on any share button. What are these?







