Impacts of Lactobacillus acidophilus Fermentation Product on Digestibility, Immune System Response, Blood Parameters, Milk Production and Milk Composition in Mid-Lactating Buffaloes (Bubalus bubalis)
Impacts of Lactobacillus acidophilus Fermentation Product on Digestibility, Immune System Response, Blood Parameters, Milk Production and Milk Composition in Mid-Lactating Buffaloes (Bubalus bubalis)
Wafaa M.A. Ghoneem*, Muhammad M. Shaklouf, Ali M. Ali and Mohammed H. Bakr
Department of animal production, Faculty of Agriculture, Cairo University, 12613, Giza, Egypt
ABSTRACT
Thirty lactating buffaloes (650 ±50 kg average body weight, in the first to the fifth seasons of lactation, 127±4 Days in milking and average of 12.32±1.13 kg daily milk yield) were divided into three similar groups to evaluate the effect Lactobacillus acidophilus fermentation product (LAFP) commercially named (Culbac®) on nutrients digestibility, milk yield and composition, blood biochemistry and immune system response of the animals. Culbac® was added at 0, 10 and 20g/h/d in G1 (control), G2 and G3, respectively. Digestion coefficients of the most nutrients and nutritive value did not significantly (P<0.05) differ between G2 and G1. However, G3 recorded lower values. WBCs, RBCs, Ht, Hb, lymphocytes, monocytes, basophils, eosinophils and neutrophils were not affected by treatment. Culbac® significantly (P<0.05) decreased blood concentration of ALT, AST, cholesterol, triglyceride and urea compared with control. While, lymphocyte transformation and phagocytic index were increased by (18 and 23%) and (20 and 62 %), respectively for G2 and G3 compared with G1. Average daily milk yield was increased by 2.23 kg/d and 0.9 kg/d in G2 and G3, respectively compared with G1. It could be concluded that addition of 10 g LAFP/h/d seems to be profitable whereas, it increased milk yield, improved feed efficiency and enhanced immune system response without significant effect on digestibility and milk composition. While, overdose of LAFP might have a negative effect on digestibility.
Article Information
Received 21 July 2021
Revised 25 August 2021
Accepted 02 September 2021
Available online 04 November 2021
(early access)
Published 02 June 2022
Authors’ Contribution
WAAG discussed the data. MMS and WAAG wrote the manuscript. MMS performed trails, analysed the lab work. AMA designed the experiments and performed statistical analysis. MHB following up the trial and lab analysis.
Key words
Fermentation product, Lactating buffaloes, Immune system response, Milk yield, Digestibility
DOI: https://dx.doi.org/10.17582/journal.pjz/20210721120754
* Corresponding author: wafaaghoneem@agr.cu.edu.eg
0030-9923/2022/0005-2167 $ 9.00/0
Copyright 2022 by the authors. Licensee Zoological Society of Pakistan.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
INTRODUCTION
For decades, farmers used some antibiotics (AB) as a growth promoter, the misuse and overexploitation of AB has contributed to the development of bacteria and other microbes resistant to AB in animal and human consequently (Tang et al., 2017). So, European Union banned the use of antibiotics as growth promoters in animal feed (EU, 2006). While, there have been many diseases and epidemics in recent times, providing high-quality food attracted the concern of many researchers over the world to meet this challenge. Therefore, researchers try to find safe and natural alternative of AB in animal feed like microbial feed supplements (MFS) such as probiotic, prebiotic and synbiotic (Radzikowski, 2017; Mingmongkolchai and Panbangred, 2018). The definition of MFS is unlimited and may include specific and nonspecific probiotic (yeast, fungi or bacteria), prebiotic (cell fragments, fermentation products and filtrates) as mentioned by Azzaz et al. (2016). Probiotic is defined as a live microbial food supplements, which beneficially affect the host by enhancing the balance of intestinal microbiota (Yirga, 2015). Prebiotics are defined as “non-digestible food ingredient which are not metabolized in the small intestine and fermented in large intestine” (Patel et al., 2020) and Synbiotic are considered as products which comprise prebiotics and probiotics together, so extend the effect of prebiotic and probiotic (Malik et al., 2019).
It was observed that using MFS as natural growth promoter might act a role to enhance animal health and performance through decreasing pathogens and increasing nutrients digestibility, which resulted from activation of desirable microbiota and pH stabilization of the ruminal environment (Acharya et al., 2017). Also, MFS could reduce stress through enhancing the response of immune system (Al-Qaisi et al., 2020). However, the variable effect of MFS on animal performance may be attributed to many factors such as strain, dose or viability (Ghoneem and Mahmoud, 2014).
Lactobacillus acidophilus is one of lactic acid bacteria that are used as probiotics in animal feed. It can convert the dietary carbohydrate into lactic acid as a primarily end product (Doyle et al., 2019), which activate the growth of lactate utilizers bacteria in the rumen and prevent pH drop (Chiquette, 2009). Also, L. acidophilus showed an ability to reduce the number of E. coli and Salmonella spp., the most two frequently pathogens in animals, (Puniya et al., 2015) either by producing hydrogen peroxide which acts as bactericidal (Doyle et al., 2019) or by producing bactericins, which are antimicrobial peptide (Cotter et al., 2013). These effects may reflect on improving the immune system response of the Holstein cows (Roodposhti and Dabiri 2012; Frizzo et al., 2018; Vieco-Saiz et al., 2019), hence increasing milk production (Chen et al., 2013; Mostafa et al., 2014).
Recently, the world demand of high-quality food increased, so buffalo is one of the most important farm animals which world can depend on to meet the current era requirements of high-quality food due to the high content of milk protein (4.57 vs 3.36%) and fat (7.34 vs 4.13%), respectively compared with cows (Ménard et al., 2010), also the water buffalo (Bubalus bubalis) considered the second most important animal in the world for milk production, after dairy cattle and considered first source of milk in Egypt (Borghese, 2010; Arefaine and Kashwa, 2015). The population of buffaloes in Egypt is about 3.48 million head (FAOSTAT, 2019). They are known by good adaptability capacity to Egyptian climate, have a high efficiency in utilization of low-quality roughage and resistance to many parasites (such as Schistosoma japonicum) and diseases (Fahim et al., 2018), these characteristics made buffaloes a unique productive and improvable animals.
A little number of studies is available about the effect of bacterial fermentation products in animal nutrition compared with yeast and fungi fermentation products, so we have a novelty to assess the effect Lactobacillus acidophilus fermentation product on the performance of lactating buffaloes.
MATERIALS AND METHODS
The present study was carried out at a private farm (TAMA farms- Dr. Tarek Helmy) 275-kilometer Al-Dabaa, Al-Dabaa corridor, Egypt and lasted 13 weeks, from January to March 2020 (3 weeks for adaptation and 10 weeks as experimental period). The chemical analysis of feeds, feces, milk and blood samples were conducted at laboratories of the animal nutrition (Animal Production Department), Faculty Of Agriculture, Cairo University, Egypt.
Ethical approval
The protocol of this study was approved by the Institutional Animal Care and Use Committee, Cairo University (IACUC), Giza, Egypt (Approval No. CU/II/F/11/21).
Experimental animals
Thirty mid-lactating buffaloes (650 ±50 kg average body weight, in the first to the fifth seasons of lactation, 127±4 Days in milking (DIM) and average of 12.32±1.13 kg daily milk yield) were divided into three similar groups according to its weight, parity milk production (ten of each). Animals were housed during the experimental period in open house system. The experimental concentrate feed mixture (CFM) and corn silage were offered together for buffaloes 3 times per day at 5.00 am, 1.00 pm and 9.00 pm (after milking times).
Culbac® (Abiotic)
Lactobacillus acidophilus fermentation product (Culbac®, TransAgra Company, USA) was supplemented to the expermintal groups. Every 1 kg Culbac® contains (195 g Lactobacillus acidophilus fermentation product, 85 g Lactic acid (88%), count of non-viable Lactobacillus acidophilus 1×108 cfu/ml at least and milled corn cob up to 1 kg as a carrier milled to 1440 grit).
Ration and feeding procedures
The experimental groups were fed the same ration consists of concentrate to roughage ratio approximately 63.51:36.49%, respectively on dry matter (DM) basis. Formulation of the experimental CFM is presented in Table I. Corn silage was used as a roughage in formulation of the experimental total mixed ration (TMR).
Data of chemical composition on DM basis and cell wall constituents (%) of experimental CFM, silage and the experimental ration depending on roughage to concentrate ratio (36.49:63.51), are shown in Table II.
The control group (G1) received CFM without Culbac® while, Animals in the second (G2) and third (G3) groups were fed CFM supplemented with 10 and 20 g/h/d of Culbac®, respectively. Adaptation period lasted for three weeks. In the 1st week, the two experimental groups (G2 and G3) were fed CFM containing 5 g/h/d of Culbac®. In the 2nd week, Culbac® was added to G2 and G3 by 5 and 10 g/h/d, respectively. However, the maximum addition level of Culbac® (10 and 20 g/h/d for G2 and G3, respectively) was achieved in the 3rd week.
Table I. Formulation of the experimental concentrate feed mixture (CFM).
|
Ingredients |
Percentage |
|
Yellow Corn |
40 |
|
Chocolate by-product1 |
12.5 |
|
Wheat bran |
8 |
|
Medical and aromatic herbs meal2 |
5 |
|
Soybean meal |
19 |
|
Undecorticated cotton seed meal |
2.5 |
|
Egyptian clover seed meal |
8 |
|
Limestone |
1.95 |
|
Sodium chloride |
1 |
|
Tri-buffer3 |
1.25 |
|
Dicalcium phosphate |
0.5 |
|
Vit. andMin. Mix.4 |
0.3 |
|
Total % |
100 |
1Chocolate products that are not identical to specifications and less quality to human (8.5% CP). 2Mixture of black seed, fenugreek, arugula seed, jojoba and safflower seed meals (33% CP). 3Each 1 kg tri-buffer contains: sodium carbonate 300 g; sodium bicarbonate 300 g; magnesium oxide 400 g. (produced by united brothers for feed additives, Badr city, Cairo, Dec.2019). 4Each 3 kg vitamins and minerals mixture contain: Vit A 7000000 IU; Vit D3 1500000 IU; Vit E 30000 mg; Zinc 60000 mg; Manganese 60000 mg; Iron 50000 mg; Copper 20000 mg; Iodine 1000 mg; Cobalt 250 mg; Selenium 300 mg; Calcium bicarbonate up to 3 kg. (Produced by dakahlia Group, Sadat City, Egypt, Jan. 2020).
Table II. Chemical composition and cell wall constituents of concentrate feed mixture (CFM), corn silage and the experimental ration (on DM basis).
|
Items |
Feed stuffs |
Experimental ration* |
|
|
CFM |
Silage |
||
|
DM |
92.33 |
24.40 |
|
|
Chemical composition, % (on DM basis) |
|||
|
OM |
91.24 |
92.91 |
91.85 |
|
CP |
19.06 |
7.56 |
14.86 |
|
CF |
6.58 |
22.33 |
12.33 |
|
EE |
5.70 |
1.41 |
4.13 |
|
NFE |
59.90 |
61.61 |
60.53 |
|
Ash |
8.76 |
7.09 |
8.15 |
|
Cell wall constituents % |
|||
|
NDF |
17.26 |
46.85 |
28.06 |
|
ADF |
8.35 |
31.29 |
16.72 |
|
ADL |
2.27 |
5.37 |
3.40 |
|
Hemicelluloses |
8.91 |
15.56 |
11.34 |
|
Cellulose |
6.08 |
25.92 |
13.32 |
* calculated: DM, dry matter; OM, organic matter; CP, crude protein; CF, crude fiber; EE, ether extract; NFE, nitrogen free extract; NDF, neutral detergent fiber; ADF, acid detergent fiber; ADL, acid detergent lignin; Hemicellulose= NDF – ADF; Cellulose = ADF- ADL.
The quantities of daily feed per day per buffalo were 11.5 kg CFM + 25 kg corn silage. Free drinking water was available all the time during the day. The offered feeds were assessed to cover the nutrient requirements for each lactating buffalo according to Ghoneim (1964).
Milk production trial, milk sampling and analysis
The milk production trial lasted for 13 weeks (3 weeks for adaptation plus 10 weeks as an experimental period). Lactating buffaloes were milked 3 times per day by machine milking system (DeLaval Parlour) at 4.00 am, 12.00 pm and 8.00 pm. The daily milk yield was recorded daily (10 animals/ group) three successive milking on day.
Milk samples were collected at the last three days of milk production trial from 5 animals in each group, three successive samples per animal per day were taken and mixed together as a proportion from milk produced (Xu et al., 2017). Milk samples were transported from farm to lab in ice box and stored by deep freezing before the analysis. Actual milk yield was corrected to 4% FCM according to the formula of Gaines (1928) as follow:
4% FCM (Kg) = 0.4(Kg milk yield) + 15 (Kg fat yield).
Digestion trial and feces sampling
The digestion trial was conducted at the last week of the experiment. Feces samples were taken individually from five animals from each group at the end of milk production trial. Acid insoluble ash (AIA) as an internal marker was applied to calculate the nutrients digestibility according to the equations of Van-Keulen and Young (1977) as follow:
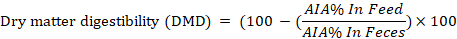
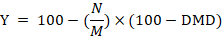
Whereas; N= % nutrient in feces; M =% nutrient in feed; Y= % nutrient digestibility
Feces samples were collected from animal rectum at 10.00 am and 4.00 pm for three successive days from 3 animals in each group and mixed together (six samples for each animal) then stored in deep freezing (-18 °C) before the analysis. Samples were dried at 70 °C for 24 h, and then kept individually in polyethylene bags for chemical analysis.
Blood sampling
Blood samples were taken at the end of the digestion trial from 5 animals per group from the jugular vein in glass tubes containing heparin at 4 hr. after morning feeding (9.00 am) as described by Mahmoud and Ghoneem (2014). Two blood samples were taken from each animal to obtain whole blood and blood plasma samples.
Analytical methods
Feeds and feces analysis
CFM, corn silage and feces were analyzed for DM, ash, EE, CP and CF according to the methods of AOAC (2000). NFE and OM were calculated as DM basis as follows:
NFE % = 100 – (%CP + % CF + % EE +% Ash)
OM= 100 - % Ash
NDF, ADF and ADL were analyzed according to Van Soest et al. (1991). Cellulose percentage was calculated by the difference between ADF and ADL, and hemicelluloses percentage was calculated by the difference between NDF and ADF.
Milk analysis
Milk samples were prepared to determine fat, protein, lactose, total solid (TS), solid not fat (SNF), ash, and moisture by Milko-Scan analyzer (Milkotester Ltd. 49 Hristo Botev Str., 4470 Belovo, Bulgaria.) at laboratories of the animal nutrition (animal production department), faculty of agriculture, Cairo university, Egypt.
Whole blood and blood plasma analysis
Hemoglobin (Hb) was determined by colorimetric method (Spectrophotometer Jenway 6300 U.K) according to Wintrobe (1956). Hematocrit (Ht, %), Red Blood Cells (RBCs) and White Blood Cells (WBCs) were determined by hemocytometer device according to Pushkar and Bhatta (2013). Lymphocyte transformation test was determined by colorimetric method (Rai-EL-Balhaa et al., 1985), Phagocytic index was determined according to (Kawahara et al., 1991) and the percentage for each type of differential leukocytic count were calculated according to Schalm and Jain (1986).
Blood plasma was taken after centrifuging blood samples at 5000 rpm for 15 min in a clean dried glass vial and stored at -20°C to determine other blood constituents. Blood plasma parameters were done calorimetrically using Jenway 6300 Spectrophotometer U.K: plasma alanine transaminase (ALT) and aspartate transaminase (AST) concentrations (RFU/ml) were measured according to Reitman and Frankel (1957). Creatinine (mg/dl) was determined according to Bartels et al. (1972). Cholesterol and triglyceride (mg/dl) were determined as described by (Eisemann et al., 1986). Urea (mg/dl) was measured as described by Fawcett and Scott (1960).
Statistical analysis
The experimental data obtained from the present study were statistically analyzed using one-way analysis of variance according to SPSS (version 15) using the following model:
Yij=μ+Ti+eij
Where: Yij= experimental observation; μ= general mean of treatments; Ti= effect of treatment; eij= experimental error
Values were given as mean ± standard error mean (SEM), differences among means were compared by Duncan’s multiple range (Duncan, 1955).
RESULTS AND DISCUSSION
Digestibility and nutritive value
Digestion coefficients and nutritive values (TDN and DCP) for the experimental ration containing different levels (0, 10 and 20 g/h/d in G1, G2 and G3, respectively) of Lactobacillus acidophilus fermentation product (LAFP) are illustrated in Table III.
Table III. Effect of Lactobacillus acidophilus fermentation product on nutrients digestibility and nutritive value of the experimental ration.
|
Items |
Experimental groups |
±SEM |
p value |
||
|
G1 |
G2 |
G3 |
|||
|
Digestion coefficient, % |
|||||
|
DM |
69.06a |
67.39ab |
64.30b |
1.6 |
0.067 |
|
OM |
72.97a |
71.80ab |
68.63b |
1.6 |
0.097 |
|
CP |
72.07a |
69.34ab |
64.70b |
2.33 |
0.070 |
|
CF |
59.07a |
52.37b |
48.61b |
1.9 |
0.013 |
|
EE |
85.61 |
77.56 |
80.36 |
3.19 |
0.339 |
|
NFE |
75.86 |
76.47 |
74.26 |
1.80 |
0.545 |
|
NDF |
46.95a |
38.66ab |
30.88b |
3.89 |
0.008 |
|
ADF |
44.10a |
39.17a |
30.32b |
2.69 |
0.098 |
|
Hemicelluloses |
51.17a |
37.91b |
31.70b |
3.16 |
0.022 |
|
Cellulose |
47.59 |
43.46 |
36.22 |
3.83 |
0.182 |
|
Nutritive values, % |
|||||
|
TDN |
71.86a |
70.25ab |
68.02b |
1.6 |
0.079 |
|
DCP |
10.71a |
10.30ab |
9.61b |
0.35 |
0.070 |
a,b, Means in the same row with various superscripts are different at (P<0.05). TDN: total digestible nutrients, DCP: digestible crude protein. G1 (control): 0 g LAFP /h/d; G2 and G3: 10 and 20 g LAFP /h/d, respectively.
Current study showed that digestion coefficients of DM, OM, CP, NDF and ADF did not significantly (P<0.05) differ when LAFP added at 10 g/h/d (G2) compared with control (G1), while there were significant decreases (P<0.05) with 20 g LAFP /h/d (G3). There were significant (P<0.05) decreases in the digestions of CF and hemicellulose by (11.3 and 26%) and (17.7 and 38 %), respectively for G2 and G3 compared with G1. But there were no significant (P<0.05) differences among group in the digestion of EE, NFE and cellulose.
In the same trend, Dias et al. (2018) indicated that the total-tract digestibility of DM, OM, NDF and ADF were not affected by addition of S. cerevisiae at level of 15 g/d to dairy cows. Also, supplementation of L. acidophilus had no significant effect on in vitro DM and NDF degradability with both rice straw and maize stover as fermentation substrates (Chen et al., 2017). However, Deters and Hansen (2019) observed significant decreases (P ≤ 0.03) in NDF and ADF digestibility with addition of 12 and 18 g/d S. cerevisiae fermentation product to beef steers rations, without change in digestibility of DM, OM, or CP.
A significant decrease (P<0.05) was recorded in crude fiber digestibility with addition either 10 or 20 g of LAFP (G2 and G3) being 52.37 and 48.61%, respectively compared to 59.07% in control. This was unexpected as many studies have found an improvement in microbiota profile and activity of cellulolytic bacteria, which improve the ruminal digestion of fiber due to addition of yeast and fungi fermentation products (Wiedmeier et al., 1987; Callaway and Martin, 1997; McCann et al., 2017; Shen et al., 2018).
Lactic acid bacteria, such as L. acidophilus, are the bacteria that convert carbohydrate into lactic acid as a primarily end product (Doyle et al., 2019). Jouany and Morgavi (2007) indicated that addition of lactate producers such as Lactobacillus sp. as a probiotic should be at the level that permit a constant and low release of lactic acid, which activate the growth of lactate utilizers bacteria that prevent accumulation of lactic acid in the rumen and pH drop (Nocek et al., 2002; Chiquette, 2009). Decreases in the number and activity of protozoa and cellulolytic bacteria were noted when ruminal pH was decreased (Mosoni et al., 2007; Chung et al., 2011; Retta, 2016). In current study, the addition of ready fermentation product of L. acidophilus (LAFP) especially at the high level (20 g LAFP /h/d) to corn silage-based diet may cause a decrease in rumen pH without rapid compensation by rumen micro-organisms, which reflects on nutrients digestibility.
Also, the adverse effect of LAFP overdose (>15 g/h/d) on digestibility maybe due to the effect of either L. acidophilus or their fermentation substrate on microbiota profile and activity in rumen which may be reflected in increasing NH3-N concentration and decreasing the efficiency of fiber decomposition in the rumen (Chen et al., 2017).
In the same context, Kung (2006) showed that supplementation with high level of L. acidophilus (more than 107 cfu/h/d) reduced the nutrients absorption. While, it was reported that the action site of L. acidophilus is the lower gut, while their effect on rumen fermentation is little (Doyle et al., 2019). On the other side, Chen et al. (2017) noted that dead cells of L. acidophilus were less effective than live cells.
In the same trend of the digestibility, there were no significant (P<0.05) differences in nutritive value as TDN and DCP between G1 and G2, with significant (P<0.05) decreases with G3.
Blood parameters and immune system response
In agreement with many previous studies (Tuohy et al., 2003; Dicks and Botes, 2010; Roodposhti and Dabiri 2012; Frizzo et al., 2018), current study revealed that adding LAFP to the rations of lactating buffaloes had a positive effect on the immune system response as showed in Table IV. It was noticed that LAFP significantly (P<0.05) increased lymphocyte transformation in G2 and G3 by 18 and 20%, respectively compared with control group (G1). Also, phagocytic index which reflects the ability of immune system to identify pathogens, was significantly (P<0.05) higher with LAFP addition than control by 23% in G2 and 62% in G3.
It was recorded that L. acidophilus had the ability to reduce the number of, the most two frequently pathogens in animals, E. coli (Peterson et al., 2007; Chaucheyras-Durand et al., 2010; Puniya et al., 2015) and Salmonella spp. (Puniya et al., 2015). Different mechanisms were suggested to explain the lactic acid bacteria (LAB) mode of action as immune enhancer, one of them that LAB had the ability to inhibit pathogens either by producing some of organic acids such as lactic and acetic acids which reduce the pH in intestine, or by producing hydrogen peroxide which acts as bactericidal (Holzapfel et al., 1995; Nousiainen and Setälä, 1998; Doyle et al., 2019). Also, it was indicated that LAB can produce bactericins, which are antimicrobial peptide (Fuller, 1992; De Vuyst et al., 1996; Dicks and Botes, 2010; Cotter et al., 2013).
LAFP significantly decreased (P<0.05) blood concentration of ALT, AST, cholesterol, triglyceride and urea compared with control. It was reported by Pettersson et al. (2008) that decreasing ALT and AST levels is an indicator for healthier liver. In parallel to our results, Noori et al. (2016) and Dar et al. (2018) indicated lower blood cholesterol and triglyceride levels with probiotic addition. Also, the reduction of blood urea concentration was an indicator of higher protein utilization according to Bruno et al. (2009).
Comparing among different groups, LAFP insignificantly (P<0.05) decreased the concentration of creatinine similarly to Sallam et al. (2019). On the other hand, blood concentrations of WBCs, RBCs, Ht, Hb, lymphocytes, monocytes, basophils, eosinophils and neutrophils were not significantly affected by LAFP addition. These results agree with those conducted by Jiang et al. (2017) and Al-Qaisi et al. (2020) when S. cerevisiae fermentation product was added to diets of dairy cows.
Table IV. Effect of Lactobacillus acidophilus fermentation product on blood parameters and immune system response of lactating buffaloes.
|
Item |
Experimental groups |
±SEM |
p value |
||
|
G1 |
G2 |
G3 |
|||
|
WBCs (×103) |
5.41 |
5.79 |
5.63 |
0.19 |
0.663 |
|
RBCs (×106) |
7.19 |
6.64 |
6.97 |
0.28 |
0.342 |
|
Ht (%) |
30.16 |
28.62 |
29.98 |
078 |
0.590 |
|
Hb (g/dl) |
10.64 |
9.50 |
9.68 |
0.58 |
0.368 |
|
ALT (IU/ml) |
70.60a |
33.00b |
45.60b |
6.18 |
0.002 |
|
AST (IU/ml) |
109.40a |
67.20b |
61.40b |
6.86 |
0.001 |
|
Cholesterol (mg/dl) |
107.00a |
50.20b |
63.60b |
7.03 |
0.000 |
|
Triglyceride (mg/dl) |
10.40a |
5.80b |
6.80b |
0.60 |
0.000 |
|
Urea (mg/dl) |
53.60a |
30.80b |
32.40b |
3.07 |
0.000 |
|
Creatinine (mg/dl) |
2.03 |
1.34 |
1.32 |
0.36 |
0.132 |
|
Lymphocytes (%) |
50.80 |
51.00 |
49.00 |
1.58 |
0.870 |
|
Monocytes (%) |
4.00 |
3.00 |
3.00 |
0.57 |
0.230 |
|
Basophils (%) |
2.40 |
2.40 |
1.80 |
0.31 |
0.148 |
|
Eosinophils (%) |
3.00 |
3.40 |
2.80 |
031 |
0.516 |
|
Neutrophils (%) |
39.80 |
40.20 |
43.60 |
1.95 |
0.686 |
|
Lymphocyte transformation |
30.00b |
35.40a |
36.00a |
1.18 |
0.060 |
|
Phagocytic index |
1.74c |
2.14b |
2.82a |
0.13 |
0.000 |
a,b, Means in the same row with various superscripts are different at (P<0.05). WBCs, white blood cells; RBCs, red blood cells; Ht, Hematocrit; Hb, Hemoglobin; ALT, Alanine Transaminase; AST, Aspartate Transaminase. G1 (control): 0 g LAFP/h/d; G2 and G3: 10 and 20 g LAFP/h/d, respectively.
Feed intake, feed efficiency, milk yield and milk composition
Data in Table V showed that lactating buffaloes fed ration supplemented with 10 g L. acidophilus fermentation product (LAFP)/h/d had significantly (P<0.05) the highest average daily milk yield by 2.23 kg/d compared with those fed control ration. Also, G3 recorded insignificant higher value by 0.9 kg/d than control.
In parallel to the previous results, it was observed that dietary supplementation of lactic acid bacteria increased milk production (Jiang et al., 2008; Chen et al., 2013; Mostafa et al., 2014), and also reduced the occurrence of mastitis (Beecher et al., 2009). In the same context, dairy cows fed rations added with S. cerevisiae fermentation product recorded higher milk production than those fed control (Zhu et al., 2016, 2017). The increase in average daily milk yield may have resulted from improving blood biochemistry or stimulating the immunity response of animals (McAllister et al., 2011).
Table V. Effect of Lactobacillus acidophilus fermentation product on Dry matter intake (DMI), milk yield and milk composition of lactating buffaloes during the experimental period.
|
Item |
Experimental groups |
SEM |
p value |
||
|
G1 |
G2 |
G3 |
|||
|
DMI kg/d |
16.71 |
16.72 |
16.58 |
- |
- |
|
Milk yield kg/d |
8.68b |
10.91a |
9.58ab |
0.67 |
0.029 |
|
4% FCM kg/d |
13.47 |
15.82 |
14.18 |
1.18 |
0.121 |
|
feed efficiency1 kg/kg |
0.52b |
0.65a |
0.58ab |
0.04 |
0.031 |
|
Milk composition % |
|||||
|
Fat |
7.68 |
7.00 |
7.2 |
0.22 |
0.437 |
|
Protein |
3.86 |
3.88 |
3.77 |
0.06 |
0.669 |
|
Lactose |
5.82 |
5.88 |
5.73 |
0.08 |
0.658 |
|
Total solids |
18.38 |
17.68 |
17.60 |
0.39 |
0.487 |
|
SNF |
10.70 |
10.68 |
10.40 |
0.16 |
0.626 |
|
Ash |
1.02 |
0.92 |
0.90 |
0.53 |
0.142 |
a,b, Means in the same row with various superscripts are different at (P<0.05). G1 (control): 0 g LAFP/h/d; G2 and G3: 10 and 20 g LAFP /h/d, respectively. FCM, fat corrected milk; SNF, solids non fat; 1Feed efficiency= milk production (kg)/ DMI (kg).
In the same trend, 4% FCM yield tended to increase with both G2 and G3 compared with control, with no significant difference among them. Also, no significant differences were recorded among different groups in milk composition. The insignificant differences in 4% FCM yield may be attributed to the decrease in milk fat content in G2 (7.00%) and G3 (7.20%) compared to 7.68% in control.
In agreement with the current results, Acharya et al. (2017) reported an insignificant decrease in milk fat content by 5.52% when 14 g/d S. cerevisiae fermentation products (SCFP) was added to rations of lactating cows. However, SCFP had no significant effect on all milk components according to Zhu et al. (2017). Also, milk contents of protein, lactose and total solids were not significantly affected by addition of 28 g/cow/d yeast culture plus enzymatically hydrolyzed yeast (Faccio-Demarco et al., 2019).
In the present study, DMI was almost similar among groups being, 16.71, 16.72 and 16.58 kg/h./d., respectively for G1, G2 and G3. This result agree with findings of Zhu et al. (2017) that DMI was similar among Holstein cows fed SCFP rations compared with control. On the other hand, feed efficiency improved by 25 and 11.54 % in G2 and G3, respectively compared with G1 due to the contribution of LAFP supplementation to increase the milk production. Also, Chen et al. (2013) indicated improvement in feed efficiency with adding lactic acid bacteria to rations of dairy cows.
CONCLUSION
Addition of Lactobacillus acidophilus fermentation product (LAFP) to rations of lactating buffaloes specially at level of 10 g/h/d, increased milk production and feed efficiency as a result of enhancing immune system response and blood biochemistry that could be reflected on increasing the profitability. Although overdose of LAFP (20 g/h/d) decreased digestibility, but it was not reflected on animal production compared with control due to enhancement of animals immunity.
ACKNOWLEDGMENTS
The authors wish to thank PROMOVET Egypt Trade Company for supplying with Culbac®. Also, acknowledgment is due to Dr. Tarek Helmy for the permission to apply the experiment in his farm “TAMA Farm”.
Statement of conflict of interest
The authors have declared no conflict of interests.
REFERENCES
Acharya, S., Pretz, J.P., Yoon, I., Scott, M.F. and Casper, D.P., 2017. Effects of Saccharomyces cerevisiae fermentation products on the lactational performance of mid-lactation dairy cows. Anim. Sci., 1: 221–228. https://doi.org/10.2527/tas2017.0028
Al-Qaisi, M., Horst, E.A., Mayorga, E.J., Goetz, B.M., Abeyta, M.A., Yoon, I., Timms, L.L., Appuhamy, J.A. and Baumgard, L.H., 2020. Effects of a Saccharomyces cerevisiae fermentation product on heat-stressed dairy cows. J. Dairy Sci., 103: 9634–9645. https://doi.org/10.3168/jds.2020-18721
AOAC, 2000. Official methods of analysis, 14th ed. Association of Official Analytical Chemists, Washington, DC., USA. pp. 684.
Arefaine, H. and Kashwa, M., 2015. A review on strategies for sustainable buffalo milk production in Egypt. J. Biol. Agric. Hlthc., 5: 63-67. https://www.iiste.org/Journals/index.php/JBAH/article/view/22314
Azzaz, H.H., Morsy, T.A. and Murad, H.A., 2016. Microbial feed supplements for ruminant’s performance enhancement. Asian J. agric. Res., 10: 1-14. https://doi.org/10.3923/ajar.2016.1.14
Bartels, H., Böhmer, M. and Heierli, C., 1972. Serum creatinine without interference. Clin. Chem. Acta, 37: 193-197. https://doi.org/10.1016/0009-8981(72)90432-9
Beecher, C., Daly, M., Berry, D.P., Klostermann, K. and Flynn, J., 2009. Administration of a live culture of Lactococcus lactis DPC3147 into the bovine mammary gland stimulates the local host immune response, particularly IL-1 and IL-8 gene expression. J. Dairy Res., 76: 340–348. https://doi.org/10.1017/S0022029909004154
Borghese, A., 2010. Development and perspective of buffalo and buffalo market in Europe and Near East. In Proc. 9th World Buffalo Congress, Buenos Aires, pp. 25-28.
Bruno, R.G.S., Rutigliano, H.M., Cerri, R.L., Robinson, P.H. and Santos, J.E.P., 2009. Effect of feeding Saccharomyces cerevisiae on performance of dairy cows during summer heat stress. Anim. Feed Sci. Techn., 150: 175-186. https://doi.org/10.1016/j.anifeedsci.2008.09.001
Callaway, E.S. and Martin, S.A., 1997. Effects of Saccharomyces cerevisiae culture on ruminal bacteria that utilize lactate and digest cellulose. J. Dairy Sci., 80: 2035-2044. https://doi.org/10.3168/jds.S0022-0302(97)76148-4
Chaucheyras-Durand, F., Faqir, F., Ameilbonne, A., Rozand, C. and Martin, C., 2010. Fates of acid-resistant and non-acid-resistant shiga toxin-producing Escherichia coli strains in ruminant digestive contents in the absence and presence of probiotics. Appl. environ. Microbiol., 76: 640-647. https://doi.org/10.1128/AEM.02054-09
Chen, L., Ren, A., Zhou, C. and Tan, Z., 2017. Effects of Lactobacillus acidophilus supplementation for improving in vitro rumen fermentation characteristics of cereal straws. Ital. J. Anim. Sci., 16: 52-60. https://doi.org/10.1080/1828051X.2016.1262753
Chen, L., Zhou, C.S., Liu, G., Jiang, H.M. and Lu, Q., 2013. Application of lactic acid bacteria, yeast and bacillus as feed additive in dairy cattle. J. Fd. Agric. Environ., 11: 626–629.
Chiquette, J., 2009. The role of probiotics in promoting dairy production. WCDS Adv. Dairy Tec
hnol., 21: 143-157. http://performanceprobiotics.com/Downloads/Articles/Chiquette%202009%201.pdf
Chung, Y.H., Walker, N.D., McGinn, S.M. and Beauchemin, K.A., 2011. Differing effects of 2 active dried yeast (Saccharomyces cerevisiae) strains on ruminal acidosis and methane production in non-lactating dairy cows. J. Dairy Sci., 94: 2431-2439. https://doi.org/10.3168/jds.2010-3277
Cotter, D., Ross, P. and Hill, C., 2013. Bacteriocins a viable alternative to antibiotics? Nat. Rev. Microbiol., 11: 95–102. https://doi.org/10.1038/nrmicro2937
Dar, H.Y., Shukla, P., Mishra, P.K., Anupam, R., Mondal, R.K., Tomar, G.B., Sharma, V. and Srivastava, R.K., 2018. Lactobacillus acidophilus inhibits bone loss and increases bone heterogeneity in osteoporotic mice via modulating Treg-Th17 cell balance. Bone Rep., 8: 46–56. https://doi.org/10.1016/j.bonr.2018.02.001
De Vuyst, L., Callewart, R. and Pot, B., 1996. Characterization of the antagonistic activity of Lactobacillus amylovorus DCE 471 and large-scale isolation of its bacteriocin amylovorin L471. Syst. appl. Microbiol., 19: 9-20. https://agris.fao.org/agris-search/search.do?recordID=US201301530429 https://doi.org/10.1016/S0723-2020(96)80003-8
Deters, E.L. and Hansen, S.L., 2019. Effect of supplementing a Saccharomyces cerevisiae fermentation product during a preconditioning period prior to transit on receiving period performance, nutrient digestibility, and antioxidant defense by beef steers. Anim. Sci., 3: 1-11. https://doi.org/10.1093/tas/txz140
Dias, A.L.G., Freitas, J.A., Micai, B., Azevedo, R.A., Greco, L.F. and Santos J.E.P., 2018. Effect of supplemental yeast culture and dietary starch content on rumen fermentation and digestion in dairy cows. J. Dairy Sci., 101: 1–21. https://doi.org/10.3168/jds.2017-13241
Dicks, l.M.T. and Botes, M., 2010. Probiotic lactic acid bacteria in the gastro-intestinal tract: health benefits, safety and mode of action. Beneficial Microbes, 1: 11-29. https://doi.org/10.3920/BM2009.0012
Doyle, N., Mbandlwa, P., Kelly, W.J., Attwood, G., Li, Y., Ross, R.P., Stanton1, C. and Leahy, S., 2019. Use of lactic acid bacteria to reduce methane production in ruminants, a critical review. Front. Microbiol., 10: 1-13. https://doi.org/10.3389/fmicb.2019.02207
Duncan, D.B., 1955. Multiple range and multiple F-test. Biometrics, 11: 1-42. https://doi.org/10.2307/3001478
Eisemann, J.H., Hammond, A.C., Bauman, D.E., Renolds, P.J., Maculchoen, S.N., Tyrrel, H.F. and Haaland, G.L., 1986. Effect of bovine growth hormone administration on metabolism of growing Hereford heifers: Protein and lipid metabolism and plasma concentrations of metabolites and hormones. J. Nutr., 116: 2504-2515. https://doi.org/10.1093/jn/116.12.2504
EU, 2006. Ban on antibiotics as growth promoters in animal feed enters into effect. European union. https://ec.europa.eu/commission/presscorner/detail/en/IP_05_1687
Faccio-Demarco, C., Mumbach, T., Oliveira-de-Freitas, V., Silva-Raimondo, R.F., Medeiros-Gonçalves, F., Nunes-Corrêa, M., Pino, F.A.B., Mendonça, H., Filho, N. and Cassal-Brauner, C., 2019. Effect of yeast products supplementation during transition period on metabolic profile and milk production in dairy cows. Trop. Anim. Hlth. Prod., 51: 2193–2201. https://doi.org/10.1007/s11250-019-01933-y
Fahim, N.H., Abdel-Salam, S.A.M., Mekkawy, W., Ismael, A., Abou-Bakr, S., El Sayed, M. and Ibrahim, M.A.M., 2018. Delta and Upper Egypt buffalo farming systems: a survey comparison. Egypt. J. Anim. Prod., 55: 95-106. https://ejap.journals.ekb.eg/cle_93242_67c80ea242bf88110b890abdb88f307e.pdf
FAOSTAT, 2019. Statistical database. Food and Agriculture Organization. http://www.fao.org/faostat/en/#data/QA
Fawcett, J.K. and Scott, J.E., 1960. A rapid and precise method for the determination of urea. J. clin. Pathol., 13: 156-159. https://doi.org/10.1136/jcp.13.2.156
Frizzo, L.S., Signorini, M.L. and Rosmini, M.R., 2018. Probiotics and prebiotics for the health of cattle. In: Probiotics and prebiotics in animal health and food safety (eds. D. Di Gioia and B. Biavati). Springer International Publishing, Switzerland. pp. 269-273 https://doi.org/10.1007/978-3-319-71950-4_11
Fuller, R., 1992. History and development of probiotics. In: Probiotics: the scientific basis. (ed. R. Fuller). Chapman and Hall, London, pp. 1–8. https://doi.org/10.1007/978-94-011-2364-8_1
Gaines, W.L., 1928. The energy basis of measuring energy milk in dairy cows. Univ., Illinois Agric. Experiment Station. Bulletin No. 308.
Effect of in-activated and dried yeast on productive performance of Barki lambs. Asian J. Anim. Vet. Adv., 9: 664-673. https://doi.org/10.3923/ajava.2014.664.673
Ghoneim, A., 1964. Animal nutrition. 6th Ed, Egyptian library, Cairo. pp. 258.
Holzapfel, W.H., Geisen, R. and Schillinger, U., 1995. Biological preservation of foods with reference to protective cultures, bacteriocins and food grade enzymes. Int. J. Fd. Microbiol., 24: 343-362. https://www.researchgate.net/publication/267380581_Developmentr_East https://doi.org/10.1016/0168-1605(94)00036-6
Jiang, X.Y., Yang, D.P., Liu, Z.L., Mei, C. and Luo, Y.F., 2008. Effects of different lactic acid bacterium preparations on milk production and milk quality in dairy cows. China Dairy Ind., 36: 41–43. https://en.cnki.com.cn/Article_en/CJFDTotal-RPGY200804011.htm
Jiang, Y., Ogunade, I.M., Kim, D.H., Li, X., Pech-Cervantes, A.A., Arriola, K.G., Oliveira, A.S., Driver, J.P., Ferraretto, L.F., Staples, C.R., Vyas, D. and Adesogan, A.T., 2017. Effect of adding clay with or without a Saccharomyces cerevisiae fermentation product on the health and performance of lactating dairy cows challenged with dietary aflatoxin B1. J. Dairy Sci., 101: 3008–3020. https://doi.org/10.3168/jds.2017-13678
Jouany, J.P. and Morgavi, D.P., 2007. Use of ‘natural’ products as alternatives to antibiotic feed additives in ruminant production. Animal, 10: 1443–1466. https://doi.org/10.1017/S1751731107000742
Kawahara E., Ueda T. and Nomura S., 1991. In vitro phagocytic activity of white spotted shark cells after injection with Aeromonas salmoncida extracellular products. Gyobyo Kenyu. Japan, 26: 213-214. https://doi.org/10.3147/jsfp.26.213
Kung, L., Jr., 2006. Direct-fed microbial and enzyme feed additives. In: Direct-fed
microbial, enzyme and forage additive compendium. Miller Publishing. Minnetonka, MN. http://d2vsp3qmody48p.cloudfront.net/wp-content/uploads/2014/02/DirectFedMicrobialsandEnzymes.pdf
Mahmoud, A.E.M. and Ghoneem, W.M.A., 2014. Effect of partial substituation of dietary protein by Nigella sativa meal and sesame seed meal on performance of Egyptian lactating buffalo. Asian J. Anim. Vet. Adv., 9: 489-498. https://doi.org/10.3923/ajava.2014.489.498
Malik, J.K., Prakash, A., Srivastava, A.K. and Srivastava, A.K., 2019. Synbiotics in animal health and production. In: Nutraceuticals in veterinary medicine (eds. R.C. Gupta et al.). Springer Nature Switzerland AG. pp. 287-290. https://doi.org/10.1007/978-3-030-04624-8_20
McAllister, T.A., Beauchemin, K.A., Alazzeh, A.Y., Baah, J., Teather, R.M. and Stanford, K., 2011. Review: The use of direct fed mi- crobials to mitigate pathogens and enhance production in cattle. Can. J. Anim. Sci., 91: 193–211. https://doi.org/10.4141/cjas10047
McCann, J.C., Elolimy, A.A. and Loor, J.J., 2017. Rumen microbiome, probiotics and fermentation additives. Vet. Clin. Fd. Anim., 33: 539-553. https://doi.org/10.1016/j.cvfa.2017.06.009
Ménard, O., Ahmad, S., Rousseau, F., Briard-Bion, V., Gaucheron, F. and Lopez, C., 2010. Buffalo vs. cow milk fat globules: Size distribution, zeta-potential, compositions in total fatty acids and in polar lipids from the milk fat globule membrane. Fd. Chem., 120: 544-551. https://doi.org/10.1016/j.foodchem.2009.10.053
Mingmongkolchai, S. and Panbangred, W., 2018. Bacillus probiotics: An alternative to antibiotics for livestock production. J. appl. Microbiol., 124: 1334-1346. https://doi.org/10.1111/jam.13690
Mosoni, P., Chaucheyras-Durand, F., Béra-Maillet, C. and Forano, E., 2007. Quantification by real-time PCR of cellulolytic bacteria in the rumen of sheep after supplementation of a forage diet with readily fermentable carbohydrates: Effect of a yeast additive. J. appl. Microbiol., 103: 2676-2685. https://doi.org/10.1111/j.1365-2672.2007.03517.x
Mostafa, T.H., Elsayed, F.A., Ahmed, M.A. and Elkholany, M.A., 2014. Effect of using some feed additives (tw- probiotics) in dairy cow rations on production and reproductive performance. Egypt. J. Anim. Prod., 51(1): 1-11. https://journals.ekb.eg/cle_93661_878eb8be71d9ce65c6846ef6d96b37c8.pdf, https://doi.org/10.21608/ejap.2014.93661
Nocek, J.E., Kautz, W.P., Leedle, J.A.Z. and Allman, J.G., 2002. Ruminal supplementation of direct-fed microbials on diurnal pH variation and in situ digestion in dairy cattle. J. Dairy Sci., 85: 429-433. https://doi.org/10.3168/jds.S0022-0302(02)74091-5
Noori, M., Alikhani, M. and Jahanian, R., 2016. Effect of partial substitution of milk with probiotic yogurt of different pH on performance, body conformation and blood biochemical parameters of Holstein calves. J. appl. Anim. Res., 44: 221–229. https://doi.org/10.1080/09712119.2015.1031772
Nousiainen, J. and Setälä, J., 1998. Lactic acid bacteria as animal probiotics. In: Lactic acid bacteria: microbiology and functional aspects (eds. S. Salminem and A.Von Wright). Marcel Dekker, New York, pp. 437–473.
Patel, D., Lakhani, G.P., Jain, A., Baghel, R.P.S., Saini, K.P.S. and Kumar, N., 2020. Effect of probiotic (Saccharomyces cerevisiae) and prebiotic (Mannan oligosaccharide) on cell mediated immune response and economics of rearing of murrah buffalo calves. J. Ent. Zool. Stud., 8: 5-8. https://www.entomoljournal.year=2020andvol=8andissue=3andArticleId=6723
Peterson, R.E., Klopfenstein, T.J., Erickson, G.E., Folmer, J., Hinkley, S., 2007. Strain NP51 on E. coli O157:H7 fecal shedding and finishing performance of beef feedlot cattle. J. Fd. Prot., 70: 287-291. https://doi.org/10.4315/0362-028X-70.2.287
Pettersson, J., Hindorf, U. and Persson, P., 2008. Muscular exercise can cause highly pathological liver function tests in healthy men. Br. J. clin. Pharmacol., 65: 253–259. https://doi.org/10.1111/j.1365-2125.2007.03001.x
Puniya, A.K., Salem, A.Z.M., Kumar, S., Dagar, S.S., Griffith, G.W., Puniya, M., Ravella, S.R., Kumar, N., Dhewa, T. and Kumar R., 2015. Live microbial feed supplements with reference to anaerobic fungi in ruminant productivity: A review. J. Integ. Agric., 14: 550–560. https://doi.org/10.1016/S2095-3119(14)60837-6
Pushkar, P. and Bhatta, R., 2013. Determination of blood metabolites in cross HF cattle at pre-parturient stage: Reference value. Int. J. Pharm. Med. Biol. Sci., 2: 54-57. http://www.ijpmbs.com/uploadfile/2015/0412/20150412031700962.pdf
Radzikowski, D., 2017. Effect of probiotics, prebiotics and synbiotics on the productivity and health of dairy cows and calves. World Sci. News, 78: 193-198. http://www.worldscientificnews.com/wp-content/uploads/2017/05/WSN-78-2017-193-198.pdf
Rai-El-Balhaa, G., Pellerin, J.L., Bodin, G., Abdullah, A. and Hiron, H., 1985. Lymphoblastic transformation assay of sheep peripheral blood lymphocytes: A new rapid and easy-to-read technique. Comp. Immunol. Microbiol. Infect. Dis., 8: 311-318. https://doi.org/10.1016/0147-9571(85)90010-4
Reitman, A. and Frankel, S., 1957. A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am. J. clin. Pathol., 28: 56-63. https://doi.org/10.1093/ajcp/28.1.56
Retta, K.S., 2016. Role of probiotics in rumen fermentation and animal performance: A review. Int. J. Livest. Prod., 7: 24-32. https://doi.org/10.5897/IJLP2016.0285
Roodposhti, P.M. and Dabiri, N., 2012. Effects of probiotic and prebiotic on average daily gain, fecal shedding of Escherichia coli, and immune system status in newborn female calves. Asian-Aust. J. Anim. Sci., 25: 1255-1261. https://doi.org/10.5713/ajas.2011.11312
Sallam, S.M.A., Abdelmalek, M.L.R., Kholif, A.E., Zahran, S.M., Ahmed, M.H., Zeweil, H.S., Attia, M.F.A., Matloup, O.H. and Olafadehan, O.A., 2019. The effect of Saccharomyces cerevisiae live cells and Aspergillus oryzae fermentation extract on the lactational performance of dairy cows. Anim. Biotechnol., 31: 491-497. https://doi.org/10.1080/10495398.2019.1625783
Schalm, O.W. and Jain, N.C., 1986. Schalmʼs veterinary hematology. 4th ed. Lea and febiger, Philadelphia. pp. 1221.
Shen, Y., Wang, H., Ran, T., Yoon, I., Saleem, A.M. and Yang, W., 2018. Influence of yeast culture and feed antibiotics on ruminal fermentation and site and extent of digestion in beef heifers fed high grain rations. J. Anim. Sci., 96: 3916–3927. https://doi.org/10.1093/jas/sky249
Tang, K.L., Caffrey, N.P., Nóbrega, D.B., Cork, S.C., Ronksley, P.E., Barkema, H.W., Polachek, A.J., Ganshorn, H., Sharma, N., Kellner, J.D. and Ghali, W.A., 2017. Restricting the use of antibiotics in food-producing animals and its associations with antibiotic resistance in food-producing animals and human beings: A systematic review and meta-analysis. Lancet Planet Hlth., 1: 316-327. https://doi.org/10.1016/S2542-5196(17)30141-9
Tuohy, K.M., Probert, H.M., Smejkal, C.W. and Gibson, G.R., 2003. Using probiotics and prebiotics to improve gut health. Drug Discov.Today, 8: 692-700. https://doi.org/10.1016/S1359-6446(03)02746-6
Van Soest, P.J., Robertson, J.B. and Lewis, B.A., 1991. Methods for dietary fiber, neutral detergent fiber, and non-starch polysaccharides in relation to animal nutrition. J. Dairy Sci., 74: 3583– 3597. https://doi.org/10.3168/jds.S0022-0302(91)78551-2
Van-keulen, J.V. and Young, B.A., 1977. Evaluation of acid insoluble ash as a natural marker in ruminant digestibility studies. J. Anim. Sci., 44: 282-287. https://doi.org/10.2527/jas1977.442282x
Vieco-Saiz, N., Belguesmia, Y., Raspoet, R., Auclair, E., Gancel, F., Kempf, I. and Drider, D., 2019. Benefits and inputs from lactic acid bacteria and their bacteriocins as alternatives to antibiotic growth promoters during food-animal production. Front. Microbiol., 10: 1-17. https://doi.org/10.3389/fmicb.2019.00057
Wiedmeier, R.D. Arambel, M.J. and Walters, J.L., 1987. Effect of yeast culture and Aspergillus oryzae fermentation extract on ruminal characteristics and nutrient digestibility. J. Dairy Sci., 70: 2063–2068. https://doi.org/10.3168/jds.S0022-0302(87)80254-0
Wintrobe, M.M., 1956. Clinical hematology. 4th ed. Lea and Fibger, Philadelphia, USA, pp. 1148.
Xu, H., Huang, W., Hou, Q., Kwok, L., Sun, Z., Ma, H., Zhao, F., Lee, Y. and Zhang, H., 2017. The effects of probiotics administration on the milk production, milk components and fecal bacteria microbiota of dairy cows. Sci. Bull., 62: 767-774. https://doi.org/10.1016/j.scib.2017.04.019
Yirga, H., 2015. The Use of Probiotics in animal nutrition. J. Prob. Hth., 3: 132 142. https://doi.org/10.4172/2329-8901.1000132
Zhu, W., Wei, Z., Xu N., Yang, F., Yoon, I., Chung, Y., Liu, J. and Wang, J., 2017. Effects of Saccharomyces cerevisiae fermentation products on performance and rumen fermentation and microbiota in dairy cows fed a diet containing low quality forage. J. Anim. Sci. Biotech., 8: 36-45. https://doi.org/10.1186/s40104-017-0167-3
Zhu, W., Zhang, B.X., Yao, K.Y., Yoon, I., Chung, Y.H., Wang, J.K. and Liu, J.X., 2016. Effects of supplemental levels of Saccharomyces cerevisiae fermentation product on lactation performance in dairy cows under heat stress. Asian Austral. J. Anim. Sci., 29: 801-806. https://doi.org/10.5713/ajas.15.0440
To share on other social networks, click on any share button. What are these?










