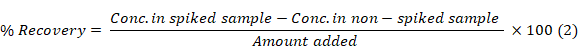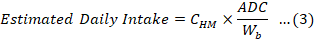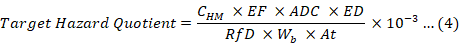Heavy Metals Content in Broiler Chicken Tissues and Health Risk Assessment Posed to Consumers in Jazan Region, Saudi Arabia
Heavy Metals Content in Broiler Chicken Tissues and Health Risk Assessment Posed to Consumers in Jazan Region, Saudi Arabia
Sadique A. Javed1*, Mohammed Al Bratty1, Abdul Jabbar Al-Rajab2,3, Hassan A. Alhazmi1,4, Asim Najmi1, Mohammad Firoz Alam5, Hafiz A. Makeen6 and Waquar Ahsan1
1Department of Pharmaceutical Chemistry, College of Pharmacy, Jazan University, Jazan, Saudi Arabia
2Centre for Environmental Research and Studies, Jazan University, Jazan, Saudi Arabia
3Etcetera Publications, Chesterville, ON, K0C1H0, Canada
4Substance Abuse and Toxicology Research Center, Jazan University, Jazan, Saudi Arabia
5Department of Pharmacology and Toxicology, College of Pharmacy, Jazan University, Jazan, Saudi Arabia
6Pharmacy Practice Research Unit, Department of Clinical Pharmacy, College of Pharmacy, Jazan University, Jazan, Saudi Arabia
ABSTRACT
Heavy metals can enter into food chain including chicken meat; indeed, several studies have reported their notable concentration in chicken parts. There is enormous consumption trend of broiler chicken in Saudi Arabia, which indicated the requirement of regular screening for possible toxic contaminants. In this investigation, level of seven trace elements in broiler chicken parts collected from Jazan region was measured by ICP-MS. The level of heavy metals were in the range of 0.01-0.04, 0.12-0.59, 1.15-6.32, 12.21-76.41, 0.13-1.63, 0.26-0.60 and 11.28-103.67 mg/kg for Cd, Cr, Cu, Fe, Ni, Pb and Zn, respectively. Results showed that the essential elements such as Fe and Zn detected in higher concentrations as compared to non-essential and toxic elements including Pb and Cd. The results of health-risk assessment showed that intake of heavy metals through consumption of broiler chicken in the tested region is unlikely to pose obvious adverse effects to the consumer population (THQ˂1).
Article Information
Received 24 February 2022
Revised 18 June 2022
Accepted 20 July 2022
Available online 04 September 2023
(early access)
Published 15 January 2025
Authors’ Contribution
MA conceived, designed and supervised the work and participated in data interpretation. AJAR and AN performed the experimental work, collected and interpreted the data. WA performed statistical analysis. SAJ, HAA and MFA collected samples and prepared and revised the manuscript.
Key words
Heavy metals, ICP-MS, Broiler chicken, Health risk assessment, Saudi Arabia
DOI: https://dx.doi.org/10.17582/journal.pjz/20220224120205
* Corresponding author: [email protected], [email protected]
0030-9923/2025/0001-0395 $ 9.00/00
Copyright 2025 by the authors. Licensee Zoological Society of Pakistan.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
INTRODUCTION
Rapid economic growth, unorganized urbanization and injudicious use of the natural resources have resulted in detrimental impact on the environment. To fulfill the high food demands of exponentially growing human population, production of food stuffs has extensively been enhanced, which has greatly influenced its quality. Heavy metals are considered to be one of the most widely spread pollutants of the environment. The ecosystem is increasingly exposed to these pollutants due to fast industrial development and economic growth. Heavy metals are considered to be serious pollutants too; because of being persistent, accumulating and have the ability to be incorporated into the food chain (Bodin et al., 2013; Ke et al., 2017; Xiao et al., 2015). Heavy metal intake through food chain has been reported world-wide and if accumulated beyond the permissible limits, they severely affect the vital organs such as bones, heart, kidneys, brain, liver and blood (Sharma et al., 2016). Trace elements are toxic because of their water solubility and based on their exposure time the toxicity may be acute or chronic (Dorne et al., 2011; Järup, 2003). According to European Union (2002), the heavy metals produced neurotoxicity, teratogenicity, carcinogenicity and mutagenicity in humans.
Toxic elements are highly unsafe and can produce toxic effects even at trace levels, if ingested over a long period of time, while the essential metals can be harmful when exposed at excessively higher concentrations (Celik and Oehlenschlager, 2007; Tuzen, 2003). For example, excessive intake of zinc (Zn) for several months may induce abdominal cramps, anemia, pancreatic damage, nausea and vomiting and reduction of HDL level. On the other hand, Zn deficiency may decrease the body immunity, hinder the development of sex organs and overall growth in young men and induce birth defects in pregnant women. Furthermore, Zn deficiency can also cause reduction in taste and smell sensations, loss appetite and reduce HDL level (Oteef et al., 2015). Lead (Pb) exposure beyond the permissible limits can harm body systems including neurological, hematological, and cardiovascular. The neurological symptoms include peripheral neuropathy, fatigue, headache, encephalopathy, severe convulsions and impaired intellectual and behavioral development in children (Ciobanu et al., 2012; Javed et al., 2019). Prolonged exposure of cadmium (Cd) may produce damaging effects on bone and renal systems and can induce chronic renal failure as the final endpoint. In addition to that, excess Cd intake may cause prostate and lung cancers (Ciobanu et al., 2012; Fraser et al., 2013).
The consumption of contaminated food stuffs constitutes the principal route of heavy metal intake in the humans. Although, most of the heavy metals at certain levels are naturally present in the food stuffs, further contamination occurs from the surrounding environment during their production and processing. The food products are regularly investigated for trace metals to evaluate their nutritional as well as toxicological associations and it was observed that the concentration of individual metal significantly varies among the variety of food products. Regular monitoring of trace elements in food stuffs is critical for setting up their dietary requirements and to assure the food safety (Berg and Licht, 2002; Ismail and Albolghait, 2013).
Broiler chicken (Gallus gallus domesticus) and chicken products are one of the major components of human platter because of high nutritional values, especially protein. There is a huge non-vegetarian trend in Saudi Arabia and consumption of broiler chicken is very high across the country; indeed, it is the highest among all available meat types, possibly due to lower price than other meat products. The main source of heavy metals exposure to chicken tissues is the poultry feeds. Several investigations conducted on poultry feeds have reported significantly high concentrations of heavy metals and few studies showing direct correlation between the heavy metal contents in the tissue samples and animal feeds (Kim and Koo, 2007; Sedki et al., 2003). Several studies across the globe have reported remarkable levels of trace elements in various chicken parts and related products (Abduljaleel et al., 2012; Bortey-Sam et al., 2015; Ismail and Albolghait, 2013; Uluozlu et al., 2009; Ogwok et al., 2014). However, there is scarcity of data on heavy metal contents in chicken samples in Saudi Arabia and to the level of our apprehension, up till now no study performed for determining the concentration of toxic metals in broiler chicken available in the markets of Jazan region. However, with reference to the public health concerns, it is essential to monitor the heavy metal concentrations in food stuffs including chicken. Therefore, this investigation was aimed, to estimate the concentrations of Cd, Cr, Cu, Fe, Ni, Pb and Zn in different tissues of broiler chicken collected from Jazan city using ICP-MS, to evaluate the accumulation behavior of metals in different organs of chicken and possible adverse health effects posed by the exposure of tested heavy metals to the local inhabitants.
MATERIALS AND METHODS
Chemicals and instruments
Nitric acid (70% v/v) analytical grade, hydrogen peroxide (30% v/v) and metal standards were purchased from Sigma Aldrich, Germany. ETHOS 1 advanced microwave digestive system (Milestone Inc., USA) was used for the digestion of chicken samples. All the tested metals were analyzed by ICP-MS 7500 (Agilent, Germany). The instrument was monitored by ChemStation Software (Agilent, Germany). All the glassware and plastic wares used in this work were washed with high quality soap with tap water and then de-ionized water and soaked in dilute nitric acid (10%) overnight. Then rinsed again with deionized water and dried. All solutions and dilutions were made using Milli-Q water produced in-house using Millipore water purification system (Millipore, France).
Sample collection
Broiler chicken samples of different available brands were collected from major supermarkets of Jazan city, Saudi Arabia. The skin, meat, bone and cartilages were separated. Other chicken parts including liver (n= 20), heart (n= 10) and gizzard (n= 19) were separately collected along with chicken samples from same markets (supplied in separate packs from the companies). The chicken products from leading brands such as Alyoum, Al-Watania, Osoul and Rawda were included in this study. The samples were collected during October-November 2021, transported directly to the Research Laboratory, Faculty of Pharmacy, Jazan University. The collected samples were kept in clean food grade plastic bags and stored in freezer until further processing.
Sample digestion and preparation
The chicken samples were digested by using the procedure used by our group with slight modification (Al-Bratty et al., 2019). Each separated chicken part was properly homogenized and approximately 1.5 g of each sample was dried at 40°C in an oven and digested in a closed vessel microwave digestion system. Dried samples were transferred to clean Teflon digestion vessels and a mixture of nitric acid (10 ml, 70%) and hydrogen peroxide (2 ml, 30%) was added to each vessel. The sample were predigested for 12h at room temperatures. Thereafter, the samples were digested by applying the following ramped temperature program: from ambient to 160°C (hold time 5 min) and then increased to 190°C (25 min hold), followed by ventilation for 10 min. After cooling the digested samples diluted to 25 ml with deionised water. A blank digest was prepared using the same procedure without tissue sample.
Method validation and determination of heavy metal concentration
The ICP-MS method used in this analysis was evaluated according to ICH Q2 (R1) guidelines (ICH, 2005) for parameters including linearity, sensitivity, precision and accuracy. To determine linearity, the calibration standard solutions of 5-100 ppb concentration (7 points) for each metal ion were prepared and analysed by ICP-MS. Calibration curves were constructed using signal against the corresponding concentration and linear regression analysis was performed to find out the correlation coefficient (R2). Limits of detection (LOD) were calculated form calibration curve using standard deviation and slope of the calibration line. Four quality control samples of concentrations 5, 20, 60 and 90 ppb for each metal ion were analysed in triplicate to determine precision and accuracy of the method. The % relative standard deviation (RSD) of the repeated results were considered as precision; while the accuracy of the method was determined by using the Equation 1 (Al-Bratty et al., 2019).
The applicability of the developed method was established by recovery experiment for each heavy metal through addition of fixed amount of metal standards in the chicken meat sample. The spiked and non-spiked chicken meat samples were analysed by the developed method. The percentage recovery of metals were calculated by using the Equation 2 (Al Bratty et al., 2019).
Quantitative analysis of seven heavy metals including cadmium, chromium, copper, iron, nickel, lead and zinc was performed by using ICP-MS instrument. The operating conditions for the instrument were set as follows- nebulizer type: concentric, carrier gas: argon, pump rate: 50 rpm, nebulizer gas flow: 0.6 L/min, coolant gas flow: 12 L/min, auxiliary gas flow: 1 L/min, RF forward power: 1350 W and integration time: 5 – 15 s. Detection wavelengths used for Cd, Cr, Cu, Fe, Ni, Pb and Zn were 226.502, 205.559, 324.754, 238.204, 221.647, 220.353 and 213.856 nm, respectively. The sample solutions were analysed in triplicate and the mean heavy metal contents were calculated in mg/kg of the chicken tissue on fresh weight basis.
Health risk assessment
Non-carcinogenic risks on adult and children population posed by intake of multiple toxic elements through consumption of edible parts of broiler chicken in Jazan region of Saudi Arabia were assessed according to the method recommended by United State Environmental Protection Agency (USEPA IRIS, 2007; USEPA, 1989). Estimated daily intake (EDI, mg/kg/day) of heavy metals has been calculated by using the Equation 3 (Bortey-Sam et al., 2015).
Where, CHM (mg/Kg) is the observed mean concentration of heavy metal in chicken; ADC (kg/person/day) is average daily per capita chicken consumption in Saudi Arabia and Wb (kg) is average body weight of consumer population. In this calculation average body weight of 70 kg and 30 kg were considered for adult and children (Bortey-Sam et al., 2015). According to United States Department of Agriculture (USDA), Global Agricultural Information Network, US Embassy, Riyadh, the per capita consumption of poultry meat in Saudi Arabia (GAIN, 2020) is approximately 40 kg (109.6 g/person/day). The average daily intake of chicken products for children was assumed to be about 75% of that consumed by adult human being on daily basis (82.2 g/person/day).
The non-carcinogenic health risks posed by tested heavy metals through consumption of chicken parts were estimated based on the target hazard quotient (THQ), which is a ratio of EDI of a pollutant to a reference dose (RfD) of that pollutant. RfD is a reference oral daily dose of a contaminant such as heavy metal to which a human population can be exposed for lifetime without appreciable adverse effect. The exposed contaminant to the human population is unlikely to produce obvious adverse effects, if the value of the calculated THQ is less than 1. The health risks based on THQ was determined by following the method described by USEPA (USEPA IRIS, 2007; Wang et al., 2005). The following Equation 4 was used (Bortey-Sam et al., 2015).
Where, EF, exposure frequency (365 days/ year); ADC, average daily chicken consumption in Saudi Arabia (109.6 and 82.2 g/person/day for adults and children respectively); ED, exposure duration (70 years); Wb, average body weight of the population and At, average time of exposure (365 days/year x ED). The values of RfD considered in the present investigation were 3.5E-03, 2.0E-02, 1.0E-03, 4.0E-02, 3.0E-03 and 3.0E-01 for Pb, Ni, Cd, Cu, Cr and Zn, respectively (De Miguel et al., 2007) whereas, the RfD value for Fe (7.0E-01) was obtained from Harb et al. (2015).
Statistical analysis
Statistical analysis was performed using NCSS statistical software 2020 version after calculating the average measured heavy metal concentrations in each chicken tissue. Principal component analysis (PCA) was applied to the heavy metal data to find out the distribution pattern of trace elements in different chicken parts. The components with eigenvalues greater than 1 were retained. The data were subjected to correlation analysis to determine the correlation between each pair of variables and estimate the commonness in the sources of metals. Component loading of each metal was performed to distribute the variables into principle components. The Hierarchical cluster analysis (HCA) was also performed, which distributes the variables into clusters of different characteristics.
RESULTS AND DISCUSSION
Method validation
The validation parameters such as linearity, sensitivity, precision and accuracy were accessed as per ICH tripartite guideline Q2 (R1) (ICH, 2005). Multi-element calibration curve exhibited excellent linearity for all the tested elements, as the correlation coefficient (R2) ˃0.99 was observed. The % RSD values were in the range of 0.56-1.79; percent accuracy was between 96.2 – 114.6% and the % recovery values obtained to be in the range of 91.3 – 108.1%. The method detection limit was less than 0.23 ppb indicating that the method is suitable for determination of heavy metal concentration in the chicken samples.
Heavy metal concentrations in different chicken tissues
The chicken meat is one of the major protein sources and its consumption in Saudi Arabia is quite high as compared to other alternate protein sources. According to report published by USDA, Global Agricultural Information Network, US Embassy, Riyadh, chicken meat consumption in Saudi Arabia was 1.48 million MT in 2020, which has been estimated to be 1.58 million MT in 2021. In 2020, production of chicken meat was estimated at 930,000 MT, which has been expected to be 950,000 MT in 2021 (GAIN, 2020). Owing to the huge consumption rate by the Saudi Arabian population and to warrant the government food safety guidelines, it was very important to determine the trace elements levels in consumable chicken parts. Consequently, to evaluate the effects on human health posed by these trace elements, the current study was commenced to estimate the concentrations of seven heavy metal in different parts of the broiler chicken and the results recorded are summarized in Table I. The levels of trace elements were significantly varied from one chicken part to another; however, there was relatively lower level of difference in the heavy metal concentrations observed in same chicken parts from different suppliers. Among the tested elements, Zn was detected at highest level; while Cd was recorded at minimum concentration in the chicken samples. In the present investigation, average concentrations of trace elements in all the chicken parts were in the range of 0.01-0.04, 0.12-0.59, 1.15-6.32, 12.21-76.41, 0.13-1.63, 0.26-0.60 and 11.28-103.67 mg/kg fresh weight basis for Cd, Cr, Cu, Fe, Ni, Pb and Zn, respectively. The main source of heavy metal intake in the broiler chicken is the feed provide to them, however, proper correlation can only be made, if the metal concentrations in the chicken feed is also measured. Some of the detected metals are essential for biological system and present naturally in different part of chicken tissues and detected in relatively higher concentrations as compared to toxic metals. Lower level of toxic elements detected in this study indicated that the proper care has been taken in the manufacturing of the chicken feed and the level of toxic elements in the raw materials were taken into consideration. Apart from feed, the surrounding soil may be another source of heavy metal intake, especially if the chickens were reared in open farm. To avoid excess concentration of heavy metal in broiler chicken and other meat product, the level of these metal should be properly monitored in the feed provide to them.
Cadmium
Cadmium is a toxic element and as per FAO/WHO, the maximum permissible intake of cadmium is 0.5 mg per week (FAO/WHO, 1976). Maximum cadmium concentration in this study was reported as 0.04 mg/kg in liver and heart samples, whereas the minimum concentration (0.01 mg/kg) was observed in bone samples. The Cd concentration in liver and heart samples were widely variable and recorded between 0.01-0.11 mg/kg in liver and 0.01-0.10 mg/kg in heart. The mean concentration of Cd was recorded as 0.03 mg/kg. A study conducted on chicken meats in Finland reported Cd concentration below
Table I. Concentration of trace elements in the broiler chicken tissues collected from markets of Jazan region, Saudi Arabia.
|
Samples source |
Heavy metal concentration (mg/kg)± SD (range) |
||||||
|
Cd |
Cr |
Cu |
Fe |
Ni |
Pb |
Zn |
|
|
Liver (n = 20) |
0.04±0.03 (0.01-0.11) |
0.19±0.06 (0.12-0.32) |
6.32±2.05 (2.92-9.77) |
59.03±12.27 (31.68-91.68) |
1.04±0.57 (0.40-2.97) |
0.29±0.11 (0.16-0.55) |
37.45±10.87 (24.18-67.18 |
|
Heart (n = 10) |
0.04±0.03 (0.01-0.10) |
0.59±0.40 (0.16-1.11) |
5.62±2.11 (3.22-8.78) |
48.13±6.28 (37.34-59.98) |
1.63±0.87 (0.77-3.14) |
0.34±0.11 (0.17-0.49) |
29.16±2.10 (25.66-31.48) |
|
Gizzard (n = 19) |
0.03±0.02 (0.01-0.09) |
0.18±0.12 (0.07-0.49) |
2.08±0.89 (0.88-4.70) |
28.38±8.70 (18.54-45.83) |
0.29±0.56 (nd-1.82) |
0.60±0.53 (0.04-1.35) |
37.43±9.63 (26.87-59.59) |
|
Breast (n = 12) |
0.02±0.01 (0.01-0.06) |
0.12±0.04 (0.05-0.19) |
3.23±2.21 (1.03-8.01) |
13.59±6.80 (5.94-29.02) |
0.39±0.56 (0.02-1.67) |
0.34±0.19 (0.10-0.74) |
11.28±4.88 (6.58-23.87) |
|
Bone (n = 12) |
0.01±0.01 (nd-0.04) |
0.12±0.04 (0.06-0.17) |
1.15±0.63 (0.44-2.37) |
76.41±16.85 (43.62-101.49) |
0.38±0.41 (0.14-1.44) |
0.57±0.40 (0.16-1.19) |
103.67±19.11 (65.56-131.06 |
|
Skin (n = 10) |
0.03±0.02 (0.01-0.07) |
0.15±0.05 (0.07-0.25) |
2.06±0.91 (1.02-3.53) |
18.84±13.41 (9.23-54.92) |
0.37±0.35 (0.01-1.01) |
0.39±0.31 (0.08-1.07) |
21.23±22.48 (6.67-79.07) |
|
Cartilage (n= 5) |
0.02±0.00 (0.02-0.02) |
0.14±0.10 (0.06-0.31) |
1.72±0.92 (1.04-3.21) |
12.21±6.81 (6.75-23.51) |
0.13±0.11 (0.01-0.29) |
0.26±0.19 (0.07-0.57) |
31.24±25.73 (9.00-68.49) |
|
Meat (n = 10) |
0.03±0.01 (0.02-0.06) |
0.17±0.06 (0.09-0.26) |
4.25±1.14 (2.67-5.86) |
34.40±8.96 (25.52-51.49) |
0.45±0.29 (0.21-1.19) |
0.26±0.07 (0.17-0.38) |
26.05±5.97 (15.07-34.31) |
detection limit (Tahvonen and Kumpulainen, 1994), 0.00168 ppm in Tenerife Island, Spain (González-Weller et al., 2006). On the other hand, the studies from Nigeria and Turkey have reported Cd concentration of 0.05-0.9 mg/kg and 0.25-6.09 μg/kg, respectively in chicken samples, which were higher than that recorded in the present investigation (Onianwa et al., 2000; Uluozlu et al., 2009).
Lead
Lead and cadmium are potentially toxic elements and are of great concern in relation to FAO/WHO standards for toxic elements, if present in higher concentrations in food stuffs. The maximum permissible limit for lead is 3 mg per week, however, the recommended doses of lead and cadmium are five time lower than their maximum permissible doses (FAO/WHO, 1976). According to WHO, no amount of Pb exposure can be considered as safe. The highest and lowest lead concentration in the chicken samples analyzed in the present study were 0.60 mg/kg in gizzard and 0.26 mg/kg in meat and cartilage samples. The mean lead concentration in all tested chicken parts was 0.40 mg/kg. According to FAO/WHO Codex Alimentarius and European Union, the highest permitted concentration of lead in poultry meat is 0.1 mg/kg which is significantly lower than the level of lead observed in any of the tested chicken part in this investigation (Zhuang et al., 2014). In the literature, lead concentrations in chicken parts were reported in the range of 0.13-0.38 mg/kg (Ghana) (Bortey-Sam et al., 2015) and 0.01-0.40 µg/g (Turkey) (Uluozlu et al., 2009), which are lesser than the observed level in this study. On the other hand, higher lead concentrations in chicken tissues was reported in Egypt (0.3186-0.8762 mg/kg) and Uganda (0.04-1.1 mg/kg) (Ismail and Abolghait, 2013; Ogwok et al., 2014).
Chromium
Average chromium level observed in this study was 0.21 mg/kg and the maximum and minimum chromium levels were 0.59 mg/kg (0.16-1.11 mg/kg) in heart and 0.12 (0.05-0.19 mg/kg) in breast meat and bone samples. Overall, the average concentration of chromium detected in this investigation was greater than its concentration (0.1 mg/kg) found in most of the food stuffs (Kumpulainen, 1992). Chromium has been considered as essential element and play important role in insulin function and lipid metabolism; as a result the amount of chromium in food stuffs is extremely important (Anderson, 1997; Bratakos et al., 2002). The US National Academy of Science has recommended that the daily dietary intake of chromium should be in the range of 50-200 µg/day (NAP, 1989).
Copper
Copper is one of the essential elements playing vital role in the biological system, however, it is toxic at higher concentrations. In the present study, maximum and minimum copper concentration was measured in liver (6.32 mg/kg) and bone (1.15 mg/kg) samples, respectively, with an average concentration including all the tested chicken parts of 3.17 mg/kg. The concentrations of copper detected in various chicken tissues in this investigation were greater than maximum permissible copper concentration (1.0 mg/kg) in poultry meat prescribed by European commission and FAO/WHO Codex Alimentarius (Zhuang et al., 2014). In previous investigations, Cu concentrations in various chicken products were in the range of 0.1–114.0 mg/kg in Turkey (Uluozlu et al., 2009), 0.34-3.67 mg/kg in Ghana (Bortey-Sam et al., 2015), and 1.00-1.13 mg/kg in Nigeria (Onianwa et al., 2001).
Iron
Iron is an essential element for biological system and the diets with its adequate amount is important for humans. Presence of certain elements may interfere with the absorption of iron leading to reduced bioavailability and iron deficiency (Lynch and Baynes, 1996; Ashraf and Mian, 2008). Iron is one of the most naturally abundant metals and accordingly in the studied chicken samples its level is highest among the tested elements. In this study, the iron concentration in different chicken tissues were recorded in the range of 12.21-76.41 mg/kg with an average content of 36.66 mg/kg. Highest iron content was detected in bone followed by liver (59.03 mg/kg) and the lowest concentration was observed in cartilage samples. There was no information regarding the maximum permissible limit of iron could be obtained. The iron levels observed in this study were slightly lower than our previous investigation, where we reported an average iron content of 39.53 mg/kg in various parts of baladi chicken (domestically grown) in the range of 9.61-119.0 mg/kg (Al-Bratty et al., 2018).
Nickel
Minimum and maximum nickel concentrations in the chicken samples were recorded in heart (1.63 mg/kg) and cartilage (0.13 mg/kg), respectively. The average nickel content in all the tested chicken tissues was 0.60 mg/kg. The nickel concentration in heart (1.63 mg/kg) and liver (1.04 mg/kg) samples exceeded the maximum permissible nickel content (0.5 mg/kg) in food (WHO, 2000). Furthermore, the overall average Ni level also exceeded its permissible limit. Nickel is one of the naturally abundant elements and also enter the environment through combustion and incineration of waste, petroleum products and coal. It can contaminate food products from stainless steel kitchen utensils (Cempel and Nikel, 2006). It is not among the potentially hazardous heavy metals; however, its long-term exposure may lead to respiratory complication and may be carcinogenic when accumulated in higher concentrations (Bortey-Sam et al., 2015). In the literature, nickel concentration in the chicken samples reported in the range of 0.01-2.08 mg/kg in Turkey (Uluozlu et al., 2009), 0.01-0.55 mg/kg in Ghana (Bortey-Sam et al., 2015), an average content of 1.67 mg/kg and 0.027 µg/g in Nigeria (Onianwa et al., 2000). In this study, higher nickel concentrations were detected than that of our previous investigation (0.16 mg/kg) on domestically grown chicken samples in the same region (Al-Bratty et al., 2018)
Zinc
Zinc is one of the naturally abundant elements and its widespread presence in the living system is due to its significant functional roles in most of the metabolic pathways. The prescribed daily intake of Zn is 15 mg and 12 mg for adult males and females, respectively (Uluozlu et al., 2009). Zn is considered to be relatively non-toxic (according to National Library of Medicine USA, the oral LD50 for zinc is about 3 g/kg of body weight) and is an essential metal, but, its prolonged exposure may lead to respiratory complications, copper deficiency, gastro-intestinal disorders, and increased probability of prostate cancer (Plum et al., 2010). In the present investigation, highest and lowest zinc concentrations were recorded as 103.67 mg/kg in bone and 11.28 mg/kg in breast meat samples, respectively. The average zinc concentration was found to be 38.78 mg/kg. In previous similar studies, zinc concentrations reported as 7.1-22.5 mg/kg in Turkey (Uluozlu et al., 2009), 15.92-104.63 mg/kg (dry weight) in Malaysia (Abduljaleel et al., 2012), 2.3245-1.5701 mg/kg in Nigeria (Oforka et al., 2012) and 2.87 mg/kg in Nigeria (Onianwa et al., 2001). Furthermore, lower zinc levels (8.63-67.58 mg/kg) were also recorded in our previous study conducted on domestically raised chicken parts collected from the same region (Al-Bratty et al., 2018).
Non-carcinogenic health risk assessment
According to European Environment and Health Information system (ENHIS, 2009), the children are more prone to the effects of ingesting chemical pollutants because their food consumption per unit of body weight is greater as compared to adults. Furthermore, the developing tissues and organs are more vulnerable to toxic effects of certain contaminants. Accordingly, in this study, the EDIs of these toxic elements though consumption of chicken meat were higher than the adults. The calculated EDIs in this study have been given in Table II. The highest EDI was calculated for Fe (5.60E-02 and 8.40E-02 mg/kg bw/day) followed by Zn (4.06E-02 and 7.59E-02 mg/kg bw/day for adults and children, respectively) which contributed 48.5% and 43.8% of the total tested heavy metal intake through the consumption broiler chicken tissues.
Table II. Estimated daily intake (EDIs, mg/kg bw/day) and risk assessment results posed by tested elements through consumption of broiler chicken tissues in Jazan region Saudi Arabia.
|
Metal |
Cm |
RfD |
EDI |
THQ |
||
|
Adult |
Children |
Adult |
Children |
|||
|
Cd |
0.04 |
1.00E-03 |
5.48E-05 |
8.22E-05 |
5.48E-02 |
8.22E-02 |
|
Cr |
0.22 |
3.00E-03 |
4.02E-04 |
6.03E-04 |
1.34E-01 |
2.01E-01 |
|
Cu |
3.61 |
4.00E-02 |
6.59E-03 |
9.89E-03 |
1.65E-01 |
2.47E-01 |
|
Fe |
30.66 |
7.00E-01 |
5.60E-02 |
8.40E-02 |
8.00E-02 |
1.20E-01 |
|
Ni |
0.61 |
2.00E-02 |
1.11E-03 |
1.67E-03 |
5.57E-02 |
8.36E-02 |
|
Pb |
0.36 |
3.50E-03 |
6.58E-04 |
9.86E-04 |
1.88E-01 |
2.82E-01 |
|
Zn |
27.69 |
3.00E-01 |
5.06E-02 |
7.59E-02 |
1.69E-01 |
2.53E-01 |
Both of these elements are relatively non-toxic metals, moreover, the EDIs of these metals are less than their tolerated reference daily intakes (RfDs) prescribed by US EPA. The lowest EDIs was recorded for Cd (5.48E-05 and 8.22E-05 mg/kg bw/day for adults and children, respectively) which is approximately 0.05% of the total metal intake. The calculated EDIs for Cr, Cu, Ni and Pb through chicken consumption were significantly lower than their RfDs. The calculated percent contributions of the analyzed heavy metals are represented in Figure 1A.
Highest THQ values in this study was recorded for Pb (1.88E-01 and 2.82E-01 in adults and children, respectively), indicating that broiler chicken consumption in the studied region may cause highest health risks due to Pb exposure. The Pb exposure contribute about 22.2% of the cumulative risk caused by all the tested heavy metals. The next element which showed highest THQ values after Pb was Zn (1.69E-01 and 2.53E-01), contributing about 19.9% of the all the risk associated with heavy metal exposure through chicken consumption by both adults and children population. Meanwhile, the THQ values for Cd (5.48E-02 and 8.22E-02), Fe (8.00E-02 and 1.20E-01) and Ni (5.57E-02 and 8.36E-02 for adults and children, respectively) were extremely low among the tested heavy metals, contributing in the range of 6.5-9.5% of the health risks caused by the consumption of broiler chicken in the tested region. The THQs of heavy metals from broiler chicken consumption have been shown in Table II and the percent contribution of the individual elements in the total non-carcinogenic health risks are shown in Figure 1. The THQ values for individual elements were far less than 1.0, indicating that none of the elements supposed to produce obvious adverse effect to the exposed adults and children population. However, these toxic elements may tend to accumulate in the body and produce toxic effects. Therefore, there is a clear requirement of regular monitoring of toxic contaminants in the food products for the interest of consumer populations. Overall, the broiler chicken consumption in Jazan region of Saudi Arabia is considered to be safe with respect to the exposure of tested heavy metals. None of the heavy metal found at a level that can produce obvious adverse health effects; however, exposure of these metals through chicken consumption are in addition to the exposures through other sources such as other food chain, processed food products, water, inhalation of domestic and street dusts etc.
Statistical analysis
The correlation analysis was used to determine the correlations between each pair of variables. The correlation values (r) obtained after analysis are summarized in Table III. As evident from the table, the r values were observed in the range of -0.547 to 0.884 which shows significant correlations between several variables. Highest correlation was obtained in case of Ni and Cr metal ion (r = 0.884), followed by Ni and Cu (r = 0.821). Nickel showed high positive correlation values with three metal ions Cd, Cr and Cu. Cadmium also showed significantly high r values with all the metal ions except Fe and Pb. The Gleason-Staelin redundancy measure, phi was obtained to be 0.521 which is moderately high. The phi value is used to measure the extent to which the variables are inter-related to each other. Lesser phi values denote lesser correlation and higher values (above 0.5) show good correlation between the variables. The Log (Det|R|) value is the log (base e) value of the determinant of the corresponding correlation matrix. The Bartlett’s specificity test showed the probability value to be 0.004 which shows that the test is valid.
Table III. Correlation matrix of variables showing correlation between metal ions.
|
Variables* |
Variables |
||||||
|
Cd |
Cr |
Cu |
Fe |
Ni |
Pb |
Zn |
|
|
Cd |
1 |
|
|
|
|
|
|
|
Cr |
0.612** |
1 |
|
|
|
|
|
|
Cu |
0.816 |
0.574 |
1 |
|
|
|
|
|
Fe |
0.008 |
0.218 |
0.256 |
1 |
|
|
|
|
Ni |
0.709 |
0.884 |
0.821 |
0.450 |
1 |
|
|
|
Pb |
-0.360 |
-0.143 |
-0.547 |
0.334 |
-0.237 |
1 |
|
|
Zn |
-0.533 |
-0.158 |
-0.373 |
0.791 |
-0.094 |
0.604 |
1 |
*Phi, 0.521280; Log (Det|R|), -10.942132; Bartlett Test, 41.94; DF, 21; Prob, 0.004275; **values in bold shows significant correlation at 0.05 level (2-tailed).
In order to visualize the correlations between the variables, heat map was generated using the absolute correlation between the metal ions (Fig. 2). As visible in the picture more the color is red, more is the correlation between metal ions. The rows and columns of the heat map were sorted in the order as suggested by the hierarchical clustering of the correlation matrix. This heat map plot helps to identify the subsets of variables that are highly correlated with each other. As seen in the figure, Fe and Zn are highly correlated; Ni is highly correlated with Cr, Cu and Cd; and Cu and Cd are highly correlated with each other. Similarly, Fe and Cd did not show any correlation with each other and Ni and Zn showed very less correlation.
Eigenvalues
When the principle component analysis (PCA) was run on the samples, they were divided into seven components with different eigenvalues. Components, their eigenvalues along with individual and cumulative percent are given in Table IV. The eigenvalues represent the total amount of variance which can be explained by a particular principle component. As evident in the table, the first component is found to retain 3.6 of the original seven variables (51.44% of the total variation) followed by the second component which retained 2.19 out of six making these two the major components corresponding to most of the variations in the data (82.77%). Generally, when the PCA is run, the eigenvalues above 1 is considered and the number of components with eigenvalues greater than 1 is retained.
Table IV. Eigenvalues obtained for each component.
|
Number |
Eigenvalue |
Individual percent |
Cumulative percent |
|
1 |
3.60115 |
51.44 |
51.44 |
|
2 |
2.192402 |
31.32 |
82.77 |
|
3 |
0.680479 |
9.72 |
92.49 |
|
4 |
0.406428 |
5.81 |
98.29 |
|
5 |
0.096138 |
1.37 |
99.67 |
|
6 |
0.018955 |
0.27 |
99.94 |
|
7 |
0.004447 |
0.06 |
100 |
Component loadings
The component loading for each variable was determined which represent the correlations between variables and the principle components. It shows the extent to which a variable lies in any component and values are shown in Table V. As seen clearly in the table, the variables, Cd, Cr, Cu and Ni had major loadings in component 1 and Fe, Pb and Zn showed major loadings in component 2. The distribution of variables in their respective two principle components are shown in Figure 3 which gives a clear picture of the similarity and position of variables.
Hierarchical cluster analysis (HCA)
The Hierarchical cluster analysis (HCA) utilizes the agglomerative clustering algorithms and is employed to develop a cluster-hierarchy shown as a tree diagram or a dendrogram. Each variable is placed in a separate cluster based on their properties. The two most similar clusters are then joined further into a new cluster. As shown in Figure 4, all the variables were divided into two clusters; the cluster 2 was the largest one containing Cr, Cu, Ni and Pb metals, whereas the cluster 1 consisted of Fe and Zn metal ions. Interestingly, Cd could not be placed in any cluster and showed different characteristics and was placed in none. The horizontal axis of the dendrogram tree represents the distance or the dissimilarities between the variables. More is the distance between two variables; larger is the dissimilarity between them. Generally, the distance values less than 1 are considered similar and are supposed to be originated from the same source. Similarly, the horizontal line joining two clusters at some distance shows the similarity between the two clusters and the distance value represents the extent of similarity between the two.
Table V. Component Loadings obtained for each variable in the two principle components.
|
Variables |
Components |
|
|
PC1 |
PC2 |
|
|
Cd |
0.892847 |
-0.07053 |
|
Cr |
0.783127 |
0.310804 |
|
Cu |
0.926666 |
0.079253 |
|
Fe |
0.09244 |
0.945329 |
|
Ni |
0.885272 |
0.423138 |
|
Pb |
-0.544173 |
0.553919 |
|
Zn |
-0.493559 |
0.83966 |
The first cluster showed presence of four out of seven variables and was the largest cluster. It consisted of Cu, Pb, Ni and Cr metals and Pb and Ni was separated by a distance of 0.316 which showed good similarity between them. These two metals again showed similarity with the third metal Cr which was separated at a distance of 0.361 from the previous two metal ions (variables). Another metal in this cluster was Cu which showed lesser similarities with all other metals and were separated by a value of 0.876. The second cluster consisted of two metal ions Zn and Fe which were separated at a distance of 0.169 which showed high similarity between the two metal ions and may have originated from the single source.
CONCLUSION
Heavy metals are one of the most potential environmental contaminants and few are extremely toxic even at trace levels. In this study, the concentration of seven trace elements was determined in different tissues of broiler chicken collected form Jazan region of Saudi Arabia. The results obtained has suggested that different parts have different capacity to accumulate the metal contaminants. Furthermore, essential elements such as Fe and Zn were detected at higher levels as compared non-essential metals such as Cd and Pb. The chicken parts included in this study were not associated with increased levels of toxic elements, except Pb, which was recorded at higher concentration (0.26-0.60 mg/kg) than its highest permissible level in poultry meat (0.1 mg/kg) prescribed by WHO. The heavy metal concentrations observed in this investigation was less than reported by most of the studied in other part of the world. This may be due to low pollution level in the region and strict government policies related to food safety in Saudi Arabia. The chicken in the tested region is acceptable for human consumption because THQ value for none of the trace elements exceed one and hence, the consumer population is unlikely to experience obvious adverse effects due to heavy metal intake through the consumption of broiler chicken. Moreover, the estimated daily intake of all the tested elements were less than their reference daily dose (RfD) set by US EPA, which further, minimizes the possibility of adverse effects due to consumption of chicken parts.
Acknowledgment
The authors extend their appreciation to the Deputyship for Research & Innovation, Ministry of Education in Saudi Arabia for funding this research work through the Project Number ISP22-2.
Statement of conflict of interest
The authors have declared no conflict of interest.
REFERENCES
Abduljaleel, S.A., Shuhaimi-othman, M., and Babji, A., 2012. Assessment of trace metals contents in chicken (Gallus gallus domesticus) and quail (Coturnix coturnix japonica) tissues from Selangor (Malaysia). J. environ. Sci. Technol., 5: 441-451 https://doi.org/10.3923/jest.2012.441.451.
Al-Bratty, M., Ahsan, W., Alhazmi, H.A., Attafi, I. M., Khardali, I.A., and Abdelwahab, S.I., 2019. Determination of trace metal concentrations in different parts of the khat varieties (Catha edulis) using inductively coupled plasma-mass spectroscopy technique and their human exposure assessment. Phcog. Mag., 15: 449-458. https://doi.org/10.4103/pm.pm_658_18
Al-Bratty, M., Alhazmi, H.A., Ogdi, S.J., Otaif, J.A., Al-Rajab, A.J., Alam, M.F., and Javed, S.A., 2018. Determination of heavy metals in various tissues of locally reared (Baladi) chicken in Jazan Region of Saudi Arabia: Assessment of potential health risks. Pakistan J. Zool., 50: 1509-1517. https://doi.org/10.17582/journal.pjz/2018.50.4.1509.1517
Anderson, R.A., 1997. Chromium as essential nutrient for humans. Regul. Toxicol. Pharmacol., 26: S35-41. https://doi.org/10.1006/rtph.1997.1136
Ashraf, W., and Mian, A.A., 2008. Levels of selected heavy metals in black tea varieties consumed in Saudi Arabia. Bull. Environ. Contam. Toxicol., 81: 101–104. https://doi.org/10.1007/s00128-008-9402-0
Berg, T., and Licht, D., 2002. International legislation on trace elements as contaminants in food: A review. Fd. Addit. Contam., 19: 916–927. https://doi.org/10.1080/02652030210156359
Bodin, N., N’Gom-Ka, R., Ka, S., Thiaw, O.T., Morais, L.T.D., Loc’H, F.L., Rozuel-Chartierd, E., Auger, D., and Chiffoleau, J.F., 2013. Assessment of trace metal contamination in mangrove ecosystems from Senegal, West Africa. Chemosphere, 90: 150-157. https://doi.org/10.1016/j.chemosphere.2012.06.019
Bortey-Sam, N., Nakayama, S.M.M., Ikenaka, Y., Akoto, O., Baidoo, E., Yohannes, Y.B., Mizukawa, H., and Ishizuka, M., 2015. Human health risks from metals and metalloid via consumption of food animals near gold mines in Tarkwa, Ghana: Estimation of the daily intakes and target hazard quotients (THQs). Ecotoxicol. environ. Safe., 111: 160–167. https://doi.org/10.1016/j.ecoenv.2014.09.008
Bratakos, M. S., Lazos, E. S., and Bratakos, S.M., 2002. Chromium content of selected Greek foods. Sci. Total Environ., 290: 47-58. https://doi.org/10.1016/S0048-9697(01)01057-9
Celik, U., and Oehlenschlager, J., 2007. High contents of cadmium, lead, zinc and copper in popular fishery products sold in Turkish supermarkets. Fd. Contr., 18: 258–261. https://doi.org/10.1016/j.foodcont.2005.10.004
Cempel, M., and Nikel, G., 2006. Nickel: A review of its sources and environmental toxicology. Pol. J. environ. Stud., 15: 375-382.
Ciobanu, C., Slencu, B.G., and Cuciureanu R., 2012. Estimation of dietary intake of cadmium and lead through food consumption. Rev. medico-chirurg. Soc. Med. Natural. Din Iasi., 116: 617-623.
De Miguel, E., Iribarren, I., Chacón, E., Ordoñez, A., and Charlesworth, S., 2007. Risk-based evaluation of the exposure of children to trace elements in playgrounds in Madrid (Spain). Chemosphere, 66: 505-513. https://doi.org/10.1016/j.chemosphere.2006.05.065
Dorne, J.L.C.M., Kass, G.E.N., Bordajandi, L.R., Amzal, B., Bertelsen, U., Castoldi, A.F., Heppner. C., Eskola, M., Fabiansson, S., Ferrari, P., Scaravelli, E., Dogliotti, E., Fuerst, P., Boobis, A.R., and Verger P., 2011. Human risk assessment of heavy metals: principles and applications. Met. Ions Life Sci., 8: 27-60. https://doi.org/10.1039/9781849732116-00027
ENHIS, 2009. European Environment and Health Information System. Exposure of children to chemical hazards in food. Fact Sheet No. 4.4, December 2009, CODE: RPG4_Food_Ex1. World Health Organization. http://www.euro.who.int/__data/assets/pdf_file/0004/97042/4.4.-Exposure-of-children-to-chemical-hazards-in-food-EDITED_layouted.pdf
European Union, 2002. Heavy metals in wastes, European Commission on Environment. http://ec.europa.eu/environment/waste/studies/pdf/heavymetalsreport.pdf
FAO/WHO, 1976. List of maximum levels recommended for contaminants by the Joint FAO/WHO Codex Alimentarius Commission, Second Series, CAC/FAL, Rome. 3: 1–8
Fraser, M., Surette, C., and Vaillancourt, C., 2013. Fish and seafood availability in markets in the Baie des Chaleurs region, New Brunswick, Canada: a heavy metal contamination baseline study. Environ. Sci. Pollut. Res. Int., 20: 761-770. https://doi.org/10.1007/s11356-012-1134-3
GAIN Report (number: SA2020-0013). 2020. Saudi Arabia: Poultry and products annual, (2020). USDA Foreign Agricultural Service, US Embassy, Riyadh. Available online on: https://apps.fas.usda.gov/newgainapi/api/Report/DownloadReportByFileName?fileName=Poultry%20and%20Products%20Annual_Riyadh_Saudi%20Arabia_09-01-2020.
González-Weller, D., Karlsson, L., Caballero, A., Hernández, F., Gutiérrez, A., González-Iglesias, T., Marino, M., and Hardisson, A., 2006. Lead and cadmium in meat and meat products consumed by the population in Tenerife Island, Spain. Fd. Addit. Contam., 23: 757-763. https://doi.org/10.1080/02652030600758142
Harb, M.K., Ebqa’ai, M., Al-Rashidi, A., Alazigi, B.H., Al-Rashidi, M.S., and Ibrahim B., 2015. Investigation of selected heavy metals in street and house dust from Al-Qunfudah, Kingdom of Saudi Arabia. Environ. Earth Sci., 74: 1755–1763. https://doi.org/10.1007/s12665-015-4184-2
International Conference on Harmonization (ICH), 2005. Validation of analytical procedures: Text and methodology, Q2 (R1), November, 2005. Available online: http://www.ipqpubs.com/wp-content/uploads/2011/09/Q2_R1__Guideline.pdf.
Ismail, S.A., and Abolghait, S.K., 2013. Estimation of lead and cadmium residual levels in chicken giblets at retail markets in Ismailia city, Egypt. Int. J. Vet. Sci. Med., 1: 109-112. https://doi.org/10.1016/j.ijvsm.2013.10.003
Järup, L., 2003. Hazards of heavy metal contamination. Br. med. Bull., 68: 167-182. https://doi.org/10.1093/bmb/ldg032
Javed, S.A., Al-Bratty, M., Al-Rajab, A.J., Alhazmi, H.A., Ahsan, W., Abdelwahab, S.I., and Thangavel, N., 2019. Risk-based exposure assessment for multiple toxic elements encountered by children in school playgrounds and parks in the southwest region of Saudi Arabia. Environ. Monit. Assess., 191: 549. https://doi.org/10.1007/s10661-019-7640-8
Ke, X., Gui, S., Huang, H., Zhang, H., Wang, C., and Guo, W., 2017. Ecological risk assessment and source identification for heavy metals in surface sediment from the Liaohe River protected area, China. Chemosphere, 175: 473-481. https://doi.org/10.1016/j.chemosphere.2017.02.029
Kim, J., and Koo, T.H., 2007. Heavy metal concentrations in diet and livers of black-crowned night heron Nycticorax nycticorax and grey heron Ardea cinerea chicks from Pyeongtaek, Korea. Ecotoxicology, 16: 411-416. https://doi.org/10.1007/s10646-007-0143-3
Kumpulainen, J.T., 1992. Chromium content of foods and diets. Biol. Trace Elem. Res., 32: 9-18. https://doi.org/10.1007/BF02784582
Lynch, S.R., and Baynes, R.D., 1996. Deliberations and evaluations of the approaches, endpoints and paradigms for iron dietary recommendations. J. Nutr., 126(9 Suppl): 2404S-2409S. https://doi.org/10.1093/jn/126.suppl_9.2404S
NAP, 1989. National research council recommended dietary allowances, 10th ed. National Academy Press, Washington DC.
Oforka, N.C., Osuji, L.C., and Onwuachu, U.I., 2012. Assessment of heavy metal pollution in muscles and internal organs of chickens raised in rivers state, Nigeria. J. Emerg. Trends Eng. appl. Sci., 3: 406-411.
Ogwok, P., Bamuwamye, M., Apili, G., and Musalima, J.H., 2014. Health risk posed by lead, copper and iron via consumption of organ meats in Kampala City (Uganda). J. Environ. Pollut. Hum. Hlth., 2: 69-73.
Onianwa, P.C., Adeyemo, A.O., Idowu, O.E., and Ogabiela, E.E., 2001. Copper and zinc contents of Nigerian foods and estimates of the adult dietary intakes. Fd. Chem., 72: 89-95. https://doi.org/10.1016/S0308-8146(00)00214-4
Onianwa, P.C., Lawal, J.A., Ogunkeye, A.A., and Orejimi, B.M., 2000. Cadmium and nickel composition of Nigerian foods. J. Fd. Compost. Anal., 13: 961-969. https://doi.org/10.1006/jfca.2000.0944
Oteef, M.D.Y., Fawy, K.F., Abd-Rabboh H.M., and Idris, A.M., 2015. Levels of zinc, copper, cadmium, and lead in fruits and vegetables grown and consumed in Aseer Region, Saudi Arabia. Environ. Monit. Assess., 187: 676. https://doi.org/10.1007/s10661-015-4905-8
Plum, L.M., Rink, L. and Haase, H., 2010. The Essential toxin: Impact of zinc on human health. Int. J. environ. Res. Publ. Hlth., 7: 1342-1365. https://doi.org/10.3390/ijerph7041342
Sedki, A., Lekouch, N., Gamon, S., and Pineau, A., 2003. Toxic and essential trace metals in muscle, liver and kidney of bovines from a polluted area of Morocco. Sci. Total Environ., 317: 201-205. https://doi.org/10.1016/S0048-9697(03)00050-0
Sharma, A., Katnoria, J.K., Nagpal, A.K., 2016. Heavy metals in vegetables: screening health risks involved in cultivation along wastewater drain and irrigating with wastewater. Springerplus, 5: 488. https://doi.org/10.1186/s40064-016-2129-1
Tahvonen, R., and Kumpulainen, J., 1994. Lead and cadmium contents in pork, beef and chicken, and in pig and cow liver in Finland during 1991. Fd. Addit. Contam., 11: 415–526. https://doi.org/10.1080/02652039409374243
Tuzen, M., 2003. Determination of heavy metals in fish samples of the middle Black Sea (Turkey) by graphite furnace atomic absorption spectrometry. Fd. Chem., 80: 119–123. https://doi.org/10.1016/S0308-8146(02)00264-9
Uluozlua, O.D., Tuzena, M., Mendila, D., and Soylak, M., 2009. Assessment of trace element contents of chicken products from Turkey. J. Hazard. Mater., 163: 982–987. https://doi.org/10.1016/j.jhazmat.2008.07.050
USEPA, 1989. Human health evaluation manual, EPA/540/1-89/002, Vol. I. Office of Solid Waste and Emergency Response. US Environmental Protection Agency. Washington, DC. Available from: http://www.epa.gov/superfund/programs/risk/ragsa/index.htm
USEPA, 2007. Integrated Risk Information System-Database (IRIS). Philadelphia PA; Washington, DC.
Wang, X., Sato, T., Xing, B., and Tao S., 2005. Health risk of heavy metals to the general public in Tianjin, China via consumption of vegetables and fish. Sci. Total Environ., 350: 28–37. https://doi.org/10.1016/j.scitotenv.2004.09.044
WHO, 2000. Report of the Fifty-Third of the joint FAO/WHO expert committee on food additives. Technical Report Series No. 896, Geneva.
Xiao, R., Bai, J.H., Lu, Q.Q., Zhao, Q.Q., Gao, Z.Q., Wen, X.J., and Liu, X.H., 2015. Fractionation, transfer, and ecological risks of heavy metals in riparian and ditch wetlands across a 100-year Chrono sequence of reclamation in an estuary of China. Sci. Total Environ., 517c: 66-75. https://doi.org/10.1016/j.scitotenv.2015.02.052
Zhuang, P., Zou, B., Lu, H., and Li Z., 2014. Heavy metal concentrations in five tissues of chickens from a mining area. Pol. J. environ. Stud., 23: 2375-2379.
To share on other social networks, click on any share button. What are these?