Gonadal Development and Fecundity of Swimming Crab Portunus segnis (Forskal, 1775) in Coastal Waters of Balochistan, Pakistan
Gonadal Development and Fecundity of Swimming Crab Portunus segnis (Forskal, 1775) in Coastal Waters of Balochistan, Pakistan
Shazia Rasheed1,*, Ehsan Ullah Mengal1, Robina Manzoor2 and Azra Bano1
1Department of Marine Sciences, Lasbela University of Agriculture, Waters and Marine Sciences, Uthal, Balochistan
2Department of Coastal and Environmental Sciences, LUAWMS, Uthal
ABSTRACT
This paper deals with a study of sexual maturity by gonadal development, fecundity and gonado-somatic uidex (GSI) of Portunus segnis from Balochistan, Pakistan. The size at sexual maturity attained by the study of gonads, showed that the smallest mature male had 76 mm short carapace width (SCW) in size and the largest male immature crab had 90 mm SCW. In case of female smallest mature crab had 70 mm SCW, while the largest immature crab had 92 mm SCW. The minimum number of eggs estimated in the female P. segnis was 86,392 while maximum number of eggs was found to be 1,555,820 in a crab of 138 mm SCW. The average fecundity was 523,773 ± 279,204. The GSI values of male crab ranged from 0.70 to 6.20 having a mean of 3.80 ± 1.08 (Mean ± SD). In case of female, GSI values of samples ranged from 0.71 to 13.33% having a mean of 5.46 ± 3.25 (Mean ± SD).
Article Information
Received 02 March 2022
Revised 25 March 2022
Accepted 08 April 2022
Available online 20 June 2022
(early access)
Published 23 June 2023
Authors’ Contribution
SR conceived and designed the study. EM and RM conducted field and lab work. AB and EM analyzed the data. SR and EM drafted this manuscript.
Key words
Gonadal development, Fecundity, Berried crab, Portunus segnis
DOI: https://dx.doi.org/10.17582/journal.pjz/20220302060306
* Corresponding author: shaziarasheed_22@hotmail.com
0030-9923/2023/0004-1807 $ 9.00/0
Copyright 2023 by the authors. Licensee Zoological Society of Pakistan.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
INTRODUCTION
Portunus segnis (Forskal, 1775) is one of most commercially important and edible crab that belongs to the family Portunidae, found in Pakistan waters. P. segnis is native to the Indian Ocean, from Pakistan westwards to Arabian Gulf, the east coast of Africa, Madagascar and Mauritius and Red Sea (Lai et al., 2010).
Reproductive biology of P. segnis is dependent on temperature of the sea water, for example, the feeding habits and movement of these species are limited when the definite temperature increases or decreases, and this phenomenon is different between sexes. Studying the reproductive biology, particularly fecundity and season of spawning is meaningful for full comprehension of populace elements of every crustacean species. Fundamental data about inhabitants, reproductive activity and biology is indispensable for strategsts to setup guideline for the reduction of population. In this manner, an investigation of reproductive biology of P. segnis can be profitable for administration of blue crab population in this region as well as in other areas. For assessing stock reproductive potential, the estimation of fecundity might offer biological reference points (BRPs) for management of sustainable fisheries (Campbell and Robinson, 1983; Goni et al., 2003; Tallack, 2007; Cooper et al., 2013). The reproductive potential could be resulted to the biggest egg commitment by a specific number of females that spawn in the population (Kanciruk and Herrnkind, 1976; Chang et al., 2007). However, there is insignificant number of investigations about the reproductive biology of this species in this belt of the Balochistan coastline. For fishery management of this shellfish, there is likewise a complete lack of knowledge. This study was therefore undertaken for effective utilization of P. segnis asset around close shore environments of Balochistan, Pakistan. In this paper, gonad development, size of sexual maturity, fecundity and GSI of P. segnis are presented.
MATERIALS AND METHODS
Portunus segnis were collected from commercial landing site of Dam Sonmiani at (25°09’ N, 66°29’ E) Lasbela, Pakistan coast. Samples were collected monthly wise from August 2017 to May 2018. Samples of P. segnis were immediately transported to the laboratory, where crabs were sexed, weighed and measured. Crabs with intact appendages were weighted to the nearest gram on a top loading electronic balance after blotting with a paper towel. Short carapace width (SCW) was taken by the digital vernier calliper (Digimatic Caliper CD-6 ‘CSX) from the base of the ninth antero-lateral teeth (Fig. 1). To investigate the gonadal growth macroscopically, the carapace of the crabs was opened. For the study of gonadal development to establish the maturity phases, male and female crabs (Fig. 2) were dissected and macroscopically analyzed each month (Based on the reproductive staging criterion defined by Kumar et al. (2003). For the study of GSI analysis, each crab’s carapace was removed and the whole gonad was safely separated and weighted to the nearest 0.01 g (GW). Before the dissection, weight was also noted for each dissected crab. GSI was determined using the Sukumaran and Neelakantanan (1997) formula.
GSI = (Gonad weight ∕ Body weight) × 100
For the study of fecundity only those berried crabs, which had light yellow, grey and black coloured eggs, were used for the study of fecundity. Three separate egg phases were observed depending on colour: Stage I: The egg mass was light yellow to deep yellow, with no eye spots evident in the eggs. Stage II: Gray colour egg mass with eyes visible as spot in eggs. Stage III: Black colour egg mass, with visible eye spots (Fig. 3).
Pleopods of female crabs on which the eggs were attached carefully separated and then complete egg mass (pleopods with eggs) weighted and then taken to the adjacent weight of 0.01 g on the top loading electronic balance (Denver Instrument TP-214). Three samples were collected from each egg mass (from 0.01 to 0.02 g). Number of eggs were counted on a counting tray under the stereo-microscope, using hand tally counter. Average total number of eggs found in samples was calculated. To estimate the total weight of egg mass alone, egg mass was weighed with pleopods, then eggs were detached from the pleopods and pleopods washed-out with distilled water then weight of only pleopods was taken which was substracted from the weight of eggmass with pleopods. Fecundity of females was then calculated by the following formula (Kumar et al., 2003).
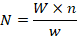
where N is cumulative amount of eggs (Fecundity), W is for whole amount of egg mass, n is amount of experimental eggs on average, w is weight of three sub sample of egg mass.
The egg mass index was calculated as fallows.
Egg mass index = (average weight of egg mass/ mean weight of crab) × 100
RESULTS
Gonadal developments
A total of 110 male crabs with a short carapace width of 48 to 135 mm (mean ±SD 94.86 ± 20.61) and 125 female crabs with a short carapace width of 38 to 140 mm (mean ±SD 91.95 ± 27.20) were measured for study of development of the gonads.
Table I shows the distribution of immature and adult crabs in multiple size categories on the basis of gonads. In the case of male P. segnis, the size groups of 38-50 mm, 51-62 mm and 63-74 mm SCW have only immature crabs, while the SCW of the size groups 75-86 and 87-98 mm have immature as well as mature crabs. 100% matured crabs were in the remaining four bigger size groups from 99-110 to 135-146 mm SCW. The first two groups of 38-50 and 51-62 mm SCW had 100 percent immature crabs in the case of female P. segnis, while the next three groups of 63-74, 75-86 and 87-98 mm had both mature and immature females. The preceding four larger size classes with a SCW of 99-110 to 135-146 mm had 100 percent mature crabs.
Male crabs
Ripe testis was found in smallest mature male (in which testis and vasa-differential was swollen and white in colour) was found in crabs acquiring 76 mm SCW, while immature testis of largest male crab found in 90 mm thick carapace (in which testis and vasa-differential was not visible by naked eye due to their pale colour and difficult to find).
Table I. Distribution of mature and immature crabs of both sexes of Portunus segnis in various size classes.
|
Size group SCW (mm) |
Male |
Female |
||||
|
N |
Immature (%) |
Mature (%) |
N |
Immature (%) |
Mature (%) |
|
|
38-50 |
4 |
100 |
0 |
7 |
100 |
0 |
|
51-62 |
5 |
100 |
0 |
8 |
100 |
0 |
|
63-74 |
10 |
100 |
0 |
15 |
80 |
20 |
|
75-86 |
32 |
37.5 |
62.5 |
38 |
39.47 |
60.52 |
|
87-98 |
31 |
14.63 |
90.24 |
30 |
10 |
90 |
|
99-110 |
10 |
0 |
100 |
12 |
0 |
100 |
|
111-122 |
8 |
0 |
100 |
8 |
0 |
100 |
|
123-134 |
6 |
0 |
100 |
4 |
0 |
100 |
|
135-146 |
4 |
0 |
100 |
3 |
0 |
100 |
Percentage of all three stages of male testis development were observed during the entire study period (Fig. 4). Highest percentage of stage I was 50% in February 2018 and 46.15% in May 2018. Lowest testis development of this stage was 12.5% in September 2017 and March 2018. Moreover, 0% was observed in August, November and December 2017. Highest percentage of testis development of second stage II was recorded in September 2017 and January 2018 which was 50% while the lowest percentage 7.69 % was observed in May 2018 and 0% in August 2017 and December 2017. The highest percentage stage III was 100% in August 2017 and November 2017 and the lowest was found 33.33% in January 2018 and February 2018.
Female crabs
The smallest mature crabs of 70 mm SCW in case of female P. segnis have large ovary, nodulated and bright yellow in colour, while off white in appearance, compressed and small ovary of 92 mm SCW was the largest immature crab. Throughout the study period, four stages of female ovarian development were described as shown in Figure 4. Stage I Immature stage were found every monthly collection and the highest percentage of occurring were found in October 2017 (62.5%) and 41.18% in April 2018. The lowest rate was observed in February 2018 (6.66%). Second stage II which is maturing stage, was recorded in highest percentage with 30% and 25% in the month of March 2018 and August 2017, respectively. However, the lowest percentage of 7.69% occurred in November 2017. Moreover, this stage was not found in October 2017, December 2017 and January 2018. Mature stage which is third stage III was also found in every month of collection. The highest percentage was 70% in the month of January 2018 while the lowest percentage was 20% in March 2018. Fourth stage of ovarian development is spent ovaries which were highly found in 35% that was observed in May 2018 and the lowest ovarian development was observed 5.88% in April 2018. Moreover, none of the spent ovaries were found from August to October 2017.
Figure 5 shows 50% maturity of male and female crabs. The male maturity of 50% is 78.5 mm and female crabs achieved scale of sexually mature at a width of short carapace of 74.5 mm.
Gonadosomatic index (GSI)
GSI mean values of male and female swimming P. segnis crabs are presented in Table II. For the study of GSI values, a total of 110 male crabs were dissected, GSI varied from 0.70 to 6.20 having an average of 3.80 ± 1.08 S.D. The lowest GSI mean value was recorded 3.27 ± 0.48 in August. After the month of August, the mean value was increased to 3.73±1.66, 3.99±1.23 in the month of October and September. However, the highest GSI mean value was observed 4.52±0.85 in the month of November but it declined in December. Again, the mean value increased 4.02±0.82 in the month of January. In the last four months the means values decreased from February to May.
Logistic model: y = a / (1+b × exp (-cx))
Coefficient data: Male; a= 9.95274370365E+001; b= 1.95578686191E+007; c= 2.12857746078E-001; Standard error: 4.7234002; Correlation Coefficient: 0.9962958.
Female; a = 1.00217211735E + 002; b= 2.01356887217E + 005; c= 1.56928940609E-001; Standard error: 1.6418724; Correlation coefficient: 0.9994943.
Table II. Gonadosomatic indices (GSI) of male and female P. segnis.
|
Month |
Male |
Female |
|
August 2017 |
3.27 ± 0.48 |
5.09 ± 1.27 |
|
September 2017 |
3.99 ± 1.23 |
6.33 ± 3.25 |
|
October 2017 |
3.73 ± 1.66 |
3.69 ± 3.76 |
|
November 2017 |
4.52 ± 0.85 |
7.35 ± 3.75 |
|
December 2017 |
3.77 ± 1.55 |
4.44 ± 2.27 |
|
January 2018 |
4.02 ± 0.82 |
5.91 ± 2.77 |
|
February 2018 |
3.87 ± 0.57 |
7.04 ± 3.80 |
|
March 2018 |
3.43 ± 0.95 |
5.71 ± 3.72 |
|
April 2018 |
3.72 ± 1.03 |
4.89 ± 3.07 |
|
May 2018 |
3.55 ± 1.07 |
4.28 ± 2.58 |
In the case of female crabs, GSI values of samples ranged from 0.71 to 13.33 having a mean of 5.46 ± 3.25 S.D. GSI mean values increased to 5.09 ± 1.27, 6.33 ± 3.25 in August and September. The lowest GSI mean value 3.69 ± 3.76 was recorded in October. The peak GSI mean value 7.35 ± 3.75 was observed in November. It was again decreased in December and January. Once again the peak GSI mean value was recorded in February in the last month the mean GSI value was decreased to 5.71 ± 3.72 in March. Moreover, the GSI mean value of females was significantly greater than that of the males.
Fecundity
Study of fecundity was carried out on 65 berried females of P. segnis having SCW from 70 to 138 mm (mean 97.47 ± 18.04 SD) and TBW from 95 to 470 g (mean 164.04 ±79.70 SD). The relationships of SCW with Fecundity (F) are shown in Figure 6 and correspondence between total weight of body TBW with F is shown in Figure 6. Smallest amount of fecundity was observed in a berried female at SCW of 70 mm and TBW was 95 g. However, the highest fecundity was recorded in 138 mm SCW and TBW was 470 g. The numbers of eggs were determined in the clutch of female P. segnis ranged from 86,392-1,555,820 eggs in various sizes of SCW from 70 to 138 mm. A few smaller crabs were also found to bear greater number of eggs than larger crabs, as approximately 119,705 eggs were found to have a smaller female measuring 72 mm SCW, while about 97,470 eggs were borne by another measuring 75 mm SCW. Likewise, some crabs bore slightly less eggs than others, while the size of the crabs remained the same.
Table III. P. segnis egg stages and fecundity of different berried crabs.
|
Egg colour |
Number (n) |
Fecundity Mean±SD (Range) |
|
Yellow |
28 |
479218 ± 380131 (130,357-1,555,820) |
|
Brown |
13 |
319,931 ± 21,3294 (86,392-813,054) |
|
Black |
24 |
332,386 ± 230389 (89030-1030142) |
Fecundity of different egg stages
The egg stages and fecundity of P. segnis are displayed in Table III. First stage (yellow colour egg mass, no eyespot was observable in the eggs) was found in 28 berried females with fecundity range 130357 to 1,555,820 (mean 479,218 ± 380,131 S.D). Thirteen berried crabs of second stage (orange to brown colour egg mass with small eyespot was visible in the eggs) were observed. This stage having maximum numbers of eggs were 813,054 and minimum numbers of eggs were 86,392 (mean 319,931 ± 213,294 S.D). Third stage (brown to black colour egg mass and big eyespot was visible in the eggs) were observed in 24 berried females. In this stage, maximum numbers of eggs were 1,030,142 and minimum numbers of eggs were 89,030 (mean 332,386 ± 230,389 S.D).
Different sizes of P. segnis were sampled and among these crabs, their size range estimated to find out the more frequent SCW size for fecundity and other biological aspects. Table IV presents the total number of eggs for different size groups. The more frequent size range 16 was observed at 90-99 mm SCW. The highest number of 1,323,466 egg, body weight average 367 ± 106.75, egg mass average weight 89.7 ± 20.68 and egg mass index 24.44 were observed at 130-139 mm SCW. However, the minimum number of 133,755 eggs, body weight average 97.09 ± 8.58, egg mass average weight 11.05 ± 4.53 and egg mass index 11.38 were observed at 70-79 mm SCW.
DISCUSSION
Fifty percent of the inhabitants of male and female crabs achieved full mature sexuality at 76.5 mm and 74.5 mm width of short carapace, respectively. Current research results agree with Sumpton et al. (1989), who established physiological maturities in both male and female P. sanguinolentus, who stated that both males and females
Table IV. Fecundity, weight of egg mass and index of egg mass in various size classes of P. segnis.
|
Size range (mm) |
Sample (no) |
Short carapace width (mm) |
Mean body weight (g) |
Mean egg mass weight (g) |
Average fecundity |
Egg mass index |
|
70-79 |
11 |
74.09 ± 2.46 |
97.09 ± 8.58 |
11.05 ± 4.53 |
133,755 |
11.38 |
|
80-89 |
14 |
85.85 ± 3.00 |
107.57 ± 11.29 |
16.70 ± 6.18 |
213,380 |
15.52 |
|
90-99 |
16 |
93.31 ± 2.98 |
141.25 ± 19.70 |
25.16 ± 9.27 |
297,403 |
17.81 |
|
100-109 |
5 |
104 ± 3.16 |
183.6 ± 30.03 |
37.8 ± 11.77 |
533,485 |
20.58 |
|
110-119 |
9 |
114.33 ± 2.5 |
201.33 ± 25.57 |
32.87 ± 3.84 |
469,081 |
16.32 |
|
120-129 |
6 |
123.5 ± 3.08 |
271.83 ± 28.93 |
52.98 ± 10.23 |
692,406 |
19.49 |
|
130-139 |
4 |
134 ± 3.65 |
367 ± 106.75 |
89.7 ± 20.68 |
1,323,466 |
24.44 |
± indicated standard deviation.
had maturities of 83 and 74 mm LCW, respectively. Reeby et al. (1990a) recorded that male P. sanguinolentus had a complete sexual maturity (both functional and physiological) of 81 to 85 mm LCW. Chu (1999) provided an estimation of gonad growth study on 50 percent gonad maturity at 42 and 36 mm carapace width for females and males of Charybdis affinis crabs, respectively. Hosseini et al. (2014) observed that 50 percent of female P. segnis had sexual maturity at 75 mm CW.
Rasheed and Mustaquim (2010) recorded that fifty percent male P. sanguinolentus matured at 60.8 mm SCW, while 50 percent female P. sanguinolentus matured at 63.5 mm SCW. Yang et al. (2014) recorded that 50 percent of males and females of P. sanguinolentus had reached maturity at 76.4 and 82.2 mm CW, respectively. Potter and De-lestang (2000) studied P. pelagicus, which showed that 50 percent of females had sexual maturity while they were 97 mm CW, while male crabs were 84 mm CW. The present findings are not comparable to those of Wardiatno and Fahrudin (2015) who reported that 50 per cent of males and females P. pelagicus sexual maturity at 98 mm and 103 mm carapace width (CW), respectively; Tureli and Yesilyurt (2017) who reported 50 per cent of females P. segnis sexual maturity at 115 and 119.99 mm CW; Safaie et al. (2013) who and recorded 50% of females sexual maturity P. segnis at size of 113 mm CW; Soundarapandian et al. (2013) who reported 50 percent of male crabs sexual maturity in P. pelagicus at the size of 106 to 110 mm of carapace width; and Wimalasiri and Dissanayake (2016) who estimated the size of P. sanguinolentus at first sexual maturity, in males with 97 mm CW, and females at 94 mm CW.
The average values of GSI for females were slightly higher than for males in this study. The present observation is close to Wardiatno and Fahrudin (2015) who recorded that the mean monthly GSI of matured female also displayed cyclic trends, although the lowest GSI mean value was 3.3 percent ± 0.5 in April and September, the highest GSI mean was 4.0 percent ± 0.3SE in December. Tureli and Yesilyurt (2017) analyzed P. segnis, which determined that the values of GSI differed from 0.13 to 13.60 percent with a mean of 2.21 ± 2.67 S.D. for the 113 female specimens. The GSI of P. sanguinolentus females were significantly higher than that of males, estimated by Wimalasiri and Dissanayake (2016). Present findings are not comparable to those of Sahoo et al. (2011) who P. pelagicus reported that in the GSI was less than 0.5 in December to February while in May to July the GSI was increased from 2.0 to 2.5. Wimalasiri and Dissanayake (2016) recorded GSI ranging from 0.02 to 3.69 for males (mean 0.75 ± 0.58 S.D) and from 0.12 to 9.44 for females (mean 3.29 ± 2.13 S.D).
Sixty five berried crabs were analyzed for the study of fecundity, sizes from 70 to 138 mm (mean 97.47 ± 18.04 SD). Number of eggs estimated in the female P. segnis clutch ranged from 86,392 to 1,555,820 eggs. Minimum fertility of 70 mm SCW and total body weight of 95 g was observed in berried females, while maximum fertility of 138 SCW mm and total body weight of 470 g was also reported. Few smaller crabs that hold more eggs than larger crabs have also been identified. The present findings are similar to Wimalasiri and Dissanayake (2016)who estimated the fecundity range of P. sanguinolentus between 112017 and1380223 number of eggs. Kamrani et al. (2010) reported that females of P. pelagicus at ranged from 32 to 173 mm CW produce 277421 to 1114348 eggs and their mean fecundity was 662978 eggs. Hermanto (2004) studied on P. pelagicus to find out their fecundity in Subang, West Java. The results show that the berried females can produces 81,000 to 1,343,000 number of eggs. Rasheed and Mustaquim (2010) recorded 272,000 to 1,395,000 eggs at 63 to 120 mm CW in P. sanguinolentus from Karachi, Pakistan. Rasheed et al. (2021) found average fecundity in P. pelagicus 523,773± 279,304 (S.D) at mean SCW 98.53±18.05 mm.
Fecundity outcomes of present study are greater than those of Yang et al. (2014), who fecundity and recorded a total of 58,600 to 565,000 berried female eggs P. sanguinolentus, with female fecundity being strongly associated with CW, with the smallest berried female being 77.1 mm CW and the largest berried female and 139.6 mm CW. The average number of egg batches produced by P. pelagicus female crabs as reported by Arshad et al. (2006) produces eggs from the 148,897 to 835,401. In south western India, Sukumaran and Neelakantan (1997) 56,000 to 1,070,000 eggs produced by berried females of P. pelagicus. Sukumaran et al. (1986) observed that P. sanguinolentus fecundity ranged in South Kanara India from 300,000 to 700,000 (83 mm to 113 mm SCW). At 79 to 126 mm SCW of P. pelagicus in Karawar India, Reeby et al. (1990b) recorded 158,608 to 712,526 eggs.
The current fecundity values are less than the results of Safaie et al. (2013) who recorded 521,027 to 6,656,599 eggs in P. segnis ovigerous crabs, and the mean fertility of 2,397,967 eggs. The fecundity values ranged from 229,468 to 2,236,355 numbers of eggs in P. pelagicus with (mean 926,638 ± 30,975 S.D) stated by Zairion and Fahrudin (2015) and the fecundity was positively and linearly associated with width of carapace. Tureli and Yesilyurt (2017) classified 44 ovigerous crabs of P. segnis ranging from 101.4 to 154 mm CW and recorded 139,379 to 2,745,236 egg development figures.
Acknowledgement
We acknowledge Dr. Safia Khanam for reviewing the article. We are also grateful to the staff of the Department of Marine Sciences, Lasbela University of Agriculture, Waters and Marine Sciences, Uthal, Balochistan, who provided technical and laboratory facilities.
Conclusions
Different sizes of P. segnis were sampled and among these their range were estimated to find out the SCW for fecundity and other biological aspects. The highest number of 1,323,466 egg, body weight average 367 ± 106.75, egg mass average weight 89.7 ± 20.68 and egg mass index 24.44 were observed at 130-139 mm SCW. However, the minimum number of 133,755 eggs, body weight average 97.09 ± 8.58, egg mass average weight 11.05 ± 4.53 and egg mass index 11.38 were observed at 70-79 mm SCW.
Statement of conflict of interest
The authors have declared no conflict of interest.
References
Arshad, A., Efrizal, K.M., and Saad, C.R., 2006. Study on fecundity, embryology and larval development of blue swimming crab (Portunus pelagicus L.) under laboratory conditions. Res. J. Fish. Hydrobiol., 1: 35-44.
Campbell, A., and Robinson, A.D., 1983. Reproductive potential of three American lobster (Homarusam ericanus) stocks in the Canadian Maritimes. Can. J. Fish aquat. Sci., 40: 1958-1967. https://doi.org/10.1139/f83-225
Chang, Y.J., Sun, C.L., Chen, Y., Yeh, S.Z., and Chiang, W.C., 2007. Reproductive biology of the spiny lobster, Panulirus penicillatus, in the south eastern coastal waters off Taiwan. J. mar. Biol., 151: 553-564. https://doi.org/10.1007/s00227-006-0488-9
Chu, K.H., 1999. Morphometric analysis and reproductive biology of the crab Charybdis affinis (Decapoda, Brachyura, Portunidae) from the Zhujiang Estuary, China. Crustaceana, 72: 647-658.
Cooper, W.T., Barbieri, L.R., Murphy, M.D., and Lowerre-Barbieri, S.K., 2013. Assessing stock reproductive potential in species with indeterminate fecundity: Effects of age truncation and size-dependent reproductive timing. Fish. Res., 138: 31-41. https://doi.org/10.1016/j.fishres.2012.05.016
Goni, R., Quetglas, A., and Renones, O., 2003. Size at maturity, fecundity and reproductive potential of a protected population of the spiny lobster (Palinuru selephas F.) from the western Mediterranean. J. mar. Biol., 143: 583-592. https://doi.org/10.1007/s00227-003-1097-5
Hermanto, D.T., 2004. Growth study and reproduction aspects of blue swimming crab (Portunus pelagicus L.) in Mayangan waters, Subang regency, West Java. Bachelor thesis, Bogor Agricultural University, Indonesia.
Hosseini, M., Pazooki, J., and Safaei, M., 2014. Size at maturity, sex ratio and variant morphometrics of blue swimming crab (Portunus segnis F.) from Boushehr Coast (Persian Gulf). J. Mar. Sci., Res. Dev., 4: 1-5.
Kamrani, E., Sabili, A.N., and Yahyavi, M., 2010. Stock assessment and reproductive biology of the blue swimming crab, Portunus pelagicus in Bandar Abbas Coastal Waters, Northern Persian Gulf. J. Persian Gulf, 1: 11-22.
Kanciruk, P., and Herrnkind, W.F., 1976. Autumnal reproduction in Panulirus argus at Bimini, Bahamas. Bull. mar. Sci., 26: 417-432.
Kumar, M.S., Xiao, Y., Venema, S., and Hooper, G., 2003. Reproductive cycle of the blue swimmer crab, Portunus pelagicus, off southern Australia. J. mar. Biol. Assoc. U.K., 83: 983-994. https://doi.org/10.1017/S0025315403008191h
Lai, J.C., Ng, P.K., and Davie, P.J., 2010. A revision of the (Portunus pelagicus L.) species complex (Crustacea: Brachyura: Portunidae), with the recognition of four species. Raf. Bull. Zool., 58: 199-237.
Potter, I.C., and De Lestang, S., 2000. Biology of the blue swimmer crab Portunus pelagicus in Leschenault Estuary and Koombana Bay, south-western Australia. J. R. Soc. West Aust., 83: 443-458.
Rasheed, S., Mustaquim, J., and Hasni, K., 2021. Size at sexual maturity and fecundity of the blue swimmer crab, Portunus pelagicus (Linnaeus, 1758) along the coast of Karachi, Pakistan. Pakistan J. Zool., 53: 295. https://doi.org/10.17582/journal.pjz/20170427100439
Rasheed, S. and Mustaquim, J., 2010. Size at sexual maturity, breeding season and fecundity of three-spot swimming crab Portunus sanguinolentus (Herbst, 1783) (Decapoda, Brachyura, Portunidae) occurring in the coastal waters of Karachi, Pakistan. Fish. Res., 103: 56-62. https://doi.org/10.1016/j.fishres.2010.02.002
Reeby, J., Prasad, P.N., and Kusuma, N., 1990a. Size at sexual maturity in the male crabs of Portunus sanguinolentus and P. pelagicus. J. Fish. Sci. Tech., 27: 115–119.
Reeby, J., Prasad, P.N., and Kusuma, N., 1990b. Fecundity of Portunus species from Karwar Waters. J. Fish. Sci. Tech., 27: 153–154.
Safaie, M., Pazooki, J., Kiabi, B., and Shokri, M.R., 2013. Reproductive biology of blue swimming crab, (Portunus segnis F.) in coastal waters of Persian Gulf and Oman Sea, Iran. Iran. J. Fish. Sci., 12: 430-444.
Sahoo, D., Panda, S., and Guru, B.C., 2011. Studies on reproductive biology and ecology of blue swimming crab Portunus pelagicus from Chilika Lagoon, Orissa, Indian J. mar. Biol. Assoc. U.K., 91: 257-264. https://doi.org/10.1017/S0025315410000354
Soundarapandian, P., Varadharajan, D., and Boopathi, A., 2013. Reproductive biology of the commercially important portunid crab, (Portunus sanguinolentus H.). J. mar. Sci., Res. Dev., 3: 1-9. https://doi.org/10.4172/2155-9910.1000124
Sukumaran, K.K., Telang, K.Y., and Thippeswamy, O., 1986. Fishery and biology of the crab Portunus sanguinolentus (Herbst) along the South Kanara coast. Indian J. Fish., 33: 188-200.
Sukumaran, K.K., and Neelakantan, B., 1997. Length-weight relationship in two marine portunid crabs, Portunus (Portunus) sanguinolentus and Portunus (Portunus) pelagicus from the Karnataka coast. Indian J. mar. Sci., 26: 39-42.
Sumpton, W.D., Smith, G.S., and Potter, M.A., 1989. Notes on the biology of the portunid crab (Portunus sanguinolentus H.), in subtropical Queensland waters. Mar. Freshw. Res., 40: 711-717. https://doi.org/10.1071/MF9890711
Tallack, S.M., 2007. Size fecundity relationships for Cancer pagurus and Necorapuber in the Shetland Islands, Scotland: how is reproductive capacity facilitated? J. mar. Biol. Assoc. U.K., 87: 507-515. https://doi.org/10.1017/S0025315407054100
Tureli, C., and Yesilyurt, I.N., 2017. Reproductive biology of blue swimming crab, (Portunus segnis F.) in Yumurtalık Cove, Northeastern Mediterranean, Turkey. Mediterr. mar. Sci., 18: 424-432. https://doi.org/10.12681/mms.13789
Wardiatno, Y., and Fahrudin, A., 2015. Sexual maturity, reproductive pattern and spawning female population of the blue swimming crab, Portunus pelagicus (Brachyura: Portunidae) in east Lampung coastal waters, Indonesia. Indian J. Sci. Tech., 8: 596-607. https://doi.org/10.17485/ijst/2015/v8i7/69368
Wimalasiri, H.B.U.G.M., and Dissanayake, D.C.T., 2016. Reproductive biology of the three-spot swimming crab (Portunus sanguinolentus) from the west coast of Sri Lanka with a novel approach to determine the maturity stage of male gonads. Invertebr. Reprod. Dev., 60: 243-253. https://doi.org/10.1080/07924259.2016.1202337
Yang, C.P., Li, H.X., Li, L., Xu, J., and Yan, Y., 2014. Population structure, morphometric analysis and reproductive biology of Portunus sanguinolentus (Decapoda: Brachyura: Portunidae) in Honghai Bay, South China Sea. J. Crust. Biol., 34: 722-730.https://doi.org/10.1163/1937240X-00002273
Zairion, W.Y., and Fahrudin, A., 2015. Sexual maturity, reproductive pattern and spawning female population of the blue swimming crab, Portunus pelagicus (Brachyura: Portunidae) in East Lampung Coastal Waters, Indonesia. Indian J. Sci. Tech., 8: 596-607. https://doi.org/10.17485/ijst/2015/v8i7/69368
To share on other social networks, click on any share button. What are these?










