Ginkgo Biloba Extract’s Efficacy to Mitigate the Genotoxicity that Hydroxyurea Induces in Mice
Research Article
Ginkgo Biloba Extract’s Efficacy to Mitigate the Genotoxicity that Hydroxyurea Induces in Mice
Ali I. Al-Ameedi1, Zahraa M. Ayad2*, Wed Abbas Mohammed3, Salim K. Hajwal4
1Pharmacology Department, Veterinary Medicine College, Al-Qasim Green University, Iraq; 2Nursing department, Al-Mustaqbal University College, Al-Hilla, Iraq; 3Biotechnology Department, College of Biotechnology, Al-Qasim Green University, Iraq; 4Nursing Department, Al-Mustaqbal University College, Al-Hillah, Iraq.
Abstract | Ginkgo Biloba is a potential medicinal plant used traditionally to treat various diseases. The present study aimed to evaluate the protective effect of Ginkgo Biloba against Genotoxicity Induced by Hyroxyurea in Mice. Forty male albino mice were randomly divided into 4 groups of ten animals. The first group received hydroxyurea (80mg/kg B. W) orally daily for 30 days, while second and third group received Ginkgo Biloba (100 mg/ kg.BW) and omega3 (150 mg/kg.BW) orally daily for two weeks of HU administration as protective and 4th group (C-ve) were considered as control negative group was given distilled water orally. After therapy by removing the bone marrow from the animal’s bone after anesthesia with ketamine+xylazine and slide preparation with Giemsa stain, experimental animals’ chromosomal abnormalities, mitotic index, and blast index were examined. In order to ascertain the protective activity of the plant extract, the mitotic index and blast index are also assessed. The results showed that the exposed group (T1) had a significantly lower mitotic index (the number of cells in mitosis per 1000 bone marrow cells) than the control group (which only drank distilled water). These results showed a significant decrease (p>0.05) of the mitotic index of (the T1) group that received hydroxyurea orally compared with the control group that received distilled water alone. On the other hand; (T2 and T3) groups that received Ginkgo Biloba and omega 3 respectively, revealed a significant increase (P<0.05) in the mitotic index compared with the T1 group that received hydroxyurea alone. The chromosomal aberrations results recorded a significant increase (P<0.05) in a group (T1) that was exposed to HU alone that suggested to be attributed to the genotoxic effect of the drug. On the other hand, a significant decrease (P>0.05) in the mean of chromosomal aberration in both groups (T2 and T3) that pretreated with GB extract and omega3 respectively. HU treatment caused serious CAs formations such as dicentric chromosome, Acentric, deletion, ring, and a high rate of breaks in bone marrow cells. As a results, GbE would be good candidates for administration as a supplement to decrease the Geno-toxicity side effects of a lot of chemotherapeutic agents.
Keywords | Ginkgo Biloba, Genotoxicity, Hyroxyurea, Protective effect, Phytochemical
Received | November 11, 2022; Accepted | February 04, 2023; Published | March 07, 2023
*Correspondence | Zahraa M. Ayad, Nursing department, Al-Mustaqbal University College, Iraq; Eamil: zahraa.mohammed@uomus.edu.iq
Citation | Al-Ameedi AI, Ayad ZM, Mohammed WA, Hajwal SK (2023). Ginkgo Biloba extract’s efficacy to mitigate the genotoxicity that hydroxyurea induces in mice. Adv. Anim. Vet. Sci. 11(4):552-557.
DOI | https://dx.doi.org/10.17582/journal.aavs/2023/11.4.552.557
ISSN (Online) | 2307-8316
Copyright: 2023 by the authors. Licensee ResearchersLinks Ltd, England, UK.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Introduction
An effective pharmaceutical treatment for sickle cell disease is hydroxyurea (HU) (SCD). However, it has been demonstrated that the replication stress brought on by HU inhibits premeiotic S-phase DNA, resulting in reproductive toxicity in germ cells (Fan et al., 2021). Around 4.4 million individuals worldwide suffer with sickle cell disease (SCD), which is most common in Asia and Africa. Despite the fact that SCD cannot be cured, hydroxyurea (HU) is utilized as a chemical chemotherapeutic agent to manage the condition. HU has been utilized as a clinically successful pharmacological treatment to treat adult SCD patients since the middle of the 1980s. HU was recommended for the treatment of SCD in children in the year 2002 (Lanzkron et al., 2008). It has been demonstrated that the conversion of ribonucleotides to deoxyribonucleotides caused by HU prevents S-phase DNA synthesis and repair (McGann and Ware, 2015). Although negative consequences are still a worry, HU’s short-term effects are negligible and only have a temporary negative impact on growth and development. In 2008, Lanzkron reported that HU therapy in mice reduced testis weight and sperm count and suggested that spermatogenesis disruption in male SCD patients receiving therapeutic dosages of HU may also occur. HU therapy damaged adult male mice’s testicles and promoted aberrant sperm morphology (Wyrobek and Bruce, 1975; Arlt et al., 2018). One of the oldest medicinal tree species, Ginkgo biloba L. (GB; Ginkgoaceae family), has applications in food, vitamins, and health care (Mohanta et al., 2014). Flavonoids (kaempferol, quercetin, myricetin, apigenin, isorhamnetin, luteolin, and tamarixetin), terpene trilactones, and proanthocyanidins are the key active ingredients that are assumed to be responsible for the pharmacological effects of GB (van Beek and Montoro, 2009; Liao et al., 2011). In recent years, GB leaf extracts (GBLEs) have become widely used as phytomedicines throughout the world (Tang et al., 2017). Phytochemical and pharmacological studies show that GBLE has been used in the treatment of a number of diseases, including oxidative stress (Bernatoniene et al., 2011), cerebral disorders (Saleem et al., 2008), vascular issues (Keheyan et al., 2011), and neurodegenerative disorders like Alzheimer’s disease (Ihl et al., 2011; Mohamed and Abd El-Moneim, 2017). This study work on the protective properties of the Ginkgo Biloba (GbE) extract targeted to reduce the toxicity caused by HU.
Materials and Methods
The GBE used in the present study were purchased from Maxima®,UK; HU from DIPA pharma®, Turkey; omega-3 from Bioactive®pharmaceutical company, UK. GBE was dissolved in distilled water and orally administered at 100 mg/ kg.BW) daily before two weeks of HU administration as protective was given to animals at fasting state or randomly administrated through a day). On the other hand, Omega-3 orally administered at 100 mg/kg.BW) daily before two weeks of HU administration. The H.U dissolved in D.W. and administered for 30 consecutive days at (80mg/kg B.W).
Animals
40 male albino mice (30-40g) were obtained from the animal lab of the College of Veterinary Medicine University of Baghdad, Iraq. Animals fasted for 15 hours before the study. Following the first dose of HU, food and water were provided ad libitum up to the end of the experiment. All protocols were approved by the Animal Care and Utilization Committee of the National Institute of Health Sciences, and all studies followed the guidelines for the use of laboratory animals of the National Institute of Health Sciences.
Dose preparation
The oral dosing rates of hydroxurea and G.B.E for mice were determined by adjusting the concentrations to yield a dosage volume of 0.1 mL/10 g BW of mice. All medications were dissolved in distilled water using the recommended stock 95 solution for each medication. According to (Shah et al., 2015), the human dose was 96 times the animal dose using the following formula:
Animal effective dose mg/ kg = Human does mg/ kg× K ratio
K (rabbit)=3.1 (Nair and Jacob, 2016).
Experimental design
Forty male albino mice adapted for 10 days in animal house of Al-Qasim Green University, college of veterinary medicine, Afterwards, they were randomly separated into four equal groups: 1- 1 St Group (T1) was given orally (80mg/kg B.W) of HU daily for 30 days. 2- 2nd group (T2) was administrated Ginko Biloba orally with (100 mg/ kg.BW) orally daily before two weeks of HU administration as protective 3- 3RD group (T3) was administered omega3 orally with (150 mg/kg.BW) daily before two weeks of HU administration as protective and 4th group (C-ve) were considered as control negative group was given distilled water orally. The Baghdad University, College of Veterinary Medicine approved this study in conformity with international ethical standards for laboratory animal research.
Rat bone marrow cytogenetic analysis
To do chromosomal analysis (aberrations), mitotic index, and blast index, bone marrow was extracted from the animals under ketamine+ xylazine anesthesia, and slides were stained with the Giemsa stains, as described in (Al-Ameedi et al., 2015, 2020). The following expressions represent the mitotic index and the blast index that is being used.
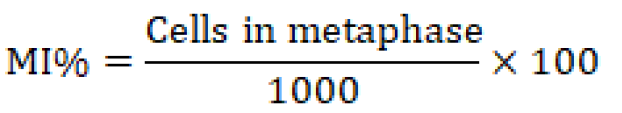
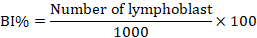
Comet assay
A adapted comet assay that targets oxidized bases in particular was carried out on mouse erythrocytes to assess the extent of oxidative damage to DNA. Using Comet 5.0 software, cells were chosen at random for the assessment of DNA breakage following ethidium bromide staining (Kinetic Imaging, Liverpool, UKIn total, 100 cells were evaluated for each sample. The percentage of DNA found in the tail was used as a reliable indicator of the severity of DNA strand breaks (Kumaravel and Jha, 2006).
Statistical analysis
The data were analyzed with SPSS, Version 9.1 using a one-way analysis of variance (ANOVA), least significant difference (LSD), and competition between means at the 0.05 significance level (SAS, 2012).
Results and Discussion
Cytogenetic analysis in rat bone marrow
The findings demonstrate that the exposed group (T1) had a much lower mitotic index (the number of cells in mitosis per 1000 bone marrow cells) than the control group (T0), which received distilled water.
These results showed a significant decrease (p>0.05) of the mitotic index of (the T1) group that received hydroxyurea orally compared with the control group that received distilled water alone. On the other hand; (T2 and T3) groups that received Ginko Biloba and omega 3, respectively, when compared to the T1 group that only received hydroxyurea, the mitotic index in the T2 and T3 groups was significantly higher (P ˂ 0.05) (Table 1).
An extremely serious biological effect of ionizing radiation and other genotoxic chemicals on humans is chromosomal aberrations (CA). The chromosomal aberrations results recorded a significant increase (P<0.05) in a group (T1) that was exposed to HU alone that suggested to be attributed to the genotoxic effect of the drug. On the other hand, a significant decrease (P>0.05) in the mean of chromosomal aberration in both groups (T2 and T3) that pretreated with GB extract and omega 3, respectively. Different types of chromosomal changes and chromosomal mutations were seen in bone marrow somatic cells, as reported in the current study. Therefore, the clastogenicity of HU in G2 may be owing to an impaired oxidative stress response and insufficient dNTPs caused by HU’s suppression of RNR. This study demonstrates that HU therapy leads to significant CAs forms in bone marrow cells, including dicentric chromosomes, Acentric chromosomes, deletions, rings, and a high rate of breakage Table 2, Figure 1.
Table 1: The effect of Ginko. Biloba was given orally before two weeks of HU administration as protective on the mitotic index of male albino rat.
|
Groups |
Mitotic index |
Blast index |
|
T1(hydroxy) |
2.39±0.13 B |
2.71±12.02C |
|
T2(Ginko) |
5.31±0.47 A |
4.28±34.02 B |
|
T3(omega3) |
5.11±0.47 A |
3.97±20.07B |
|
C-VE (D.W) |
6.24±0.25 C |
5.83±51.03 A |
|
LSD |
0.73 |
0.5 |
Significant differences (p ˂ 0.05) between groups are denoted by different capital letters.
Table 2: Chromosomal Aberrations (%) in treated and untreated groups.
|
Groups |
Chromosomal aberrations (%) |
|||||
|
Dicentric |
Acentric |
Deletion |
Ring |
Chromosome break |
Total Means ±SE |
|
|
T1(hydroxy) |
0.738 |
0.632 |
0.821 |
0.773 |
0.902 |
0.773±0.05 A |
|
T2(ginko) |
0.185 |
0.390 |
0.497 |
0.521 |
0.483 |
0.415±0.23 B |
|
T3(omega3) |
0.398 |
0.421 |
0.626 |
0.598 |
0.529 |
0.514±0.24 B |
|
C-VE (D.W) |
0.081 |
0.289 |
0.339 |
0.463 |
0.415 |
0.317±0.12 B |
|
LSD |
0.18 |
|||||
Significant differences (p ˂ 0.05) between groups are denoted by different capital letters.
The results of comet assay showed there were significant P≤0.05 differences in the DNA fragmentation of the treated group T1, T2 and T3 in comparison with control group, Table 3, the different type of comet illustrated in Figure 2.
Table 3: The results of comet assay of DNA damage in all experimental groups.
|
Groups |
DNA damage fragments % |
|
T1(hydroxy) |
39.2±1.01 A |
|
T2(ginko) |
21.8±0.6 B |
|
T3(omega3) |
24.9±0.6 B |
|
C-VE (D.W) |
15.1±0.3 C |
|
LSD |
4.53 |
Significant differences (p ˂ 0.05) between groups are denoted by different capital letters.
Depending on the dose, exposure time, and cell line, HU has been proven to induce chromosomal damage in a variety of organisms and can be cytotoxic (Singh and Xu, 2016). HU also creates oxidative stress and triggers cytokinesis arrest in mutant cells, according to research conducted on many model organisms with well-defined genetic backgrounds (Davies et al., 2009; Nakayashiki and Mori, 2013; Xu et al., 2016; Huang et al., 2016). In the current investigation, the results of a cytogenetic analysis revealed that HU had a negative effect on the T1 animals. In contrast, statistical analysis demonstrated that the administration of GB and Omega 3 possessed a protective effect. The mitotic index measures the proportion of cells that go through mitosis. Mitosis is a process in which a single somatic cell divides into two daughter cells. The cytotoxic medication called hydroxyurea was first administered for cancers. The major mechanism of action of hydroxyurea is the inhibition of the rNDP enzyme, which causes cells to terminate the cell cycle at the G1 or S phase, hence bone marrow suppression is frequently connected to its use in therapy (Agrawal et al., 2014; Luchtman-Jones et al., 2016).
After receiving HU treatment, mice showed a drop in the mitotic index’s mean values. A cell population’s proliferative state is gauged by the mitotic index. Mitotic index values in the present study may be lower than previously reported since fewer cells were found to be arrested in metaphase (Cherednichenko et al., 2022). From the results of the above experiment; the pre-exposure to Ginko Biloba at 100 mg/ kg.BW showed a protective effect against DNA damage that was represented by the significant decrease in the mitotic index in the group exposed previously G. Biloba extract. And these findings corroborate the results of another experiment when demonstrating DNA-protective effects of G. Biloba (Marques et al., 2011). Other authors also find that low doses from G. Biloba protect the cells against basal oxidative damage (Silva et al., 2019). Also, the comparison group that received omega 3 showed an approximately similar result that appeared on animals in the T2 group. So, omega 3 have also protective effects against DNA damage, and we suggest it due to the antioxidant activity of Omega 3.
Our finding is in agreements with Elelaimy et al. (2012) who discovered that male albino mice might be protected from the genotoxic effects of the anticancer medication azathioprine by giving them Omega-3s orally either before or after treatment. Recent research investigated the preventive effect of fish Omega-3 fatty acids on male rat doxorubicin-induced oxidative damage (Uygur et al., 2014). On the other hand, Manna et al. (2010) found that fish oil inhibits mammary tumorigenesis in rats by reducing HER-2/neu and c-Myc protein expression and regulating cell proliferation. It is possible that HU causes DNA damage in G2 because its metabolism generates nitric oxide, which when coupled with superoxide creates highly reactive oxygen species (ROS) that are damaging to DNA (Burkitt and Raafat, 2006).
Moreno et al. (2004) indicated that 40 mg/ml G.B has a protective effect against the plasmid DNA damage caused by 200 g/ml SnCl2. The potential production of ROS that might oxidize the stannous ion may be connected to the protection of DNA against the effects of HU. The synergistic impact of many pathways may be used to explain how G.B protects cells against hydroxyurea-induced cytotoxicity. Terpenoids, polyphenols, amino acids, organic acids, and flavonoid glycosides are all secondary metabolites detected in G.B. The most prevalent flavonoid species are quercetin, kaempferol, and isorhamnetin, whereas more than 30 flavonoids are known to be present in terpenoids, which are primarily composed of ginkgolides and bilobalides (Celik et al., 2005). characteristics such as anti-inflammatory action, suppression of lipid peroxidation, and free radical scavenging. Similar to how Celik showed that GbE protects against oxidative stress, it provides protection against chemically induced toxicity.
The results of comet assay showed there were significant P≤0.05 differences in the DNA fragmentation of the treated group T1, T2 and T3 in comparison with control group, Table 3, the different type of comet illustrated in Figure 2.
The lengthening of the comet tail, which was monitored over time, was used to gauge DNA repair. During the 20-minute incubation, comet tail length shrank with or without GBE (Figure 2). However, comet tails diminished more quickly and were undetectable within 10 minutes of incubation in GBE-treated cells. This finding supports GBE’s participation in DNA damage prevention as well as DNA repair pathways. Mutations and other physical manifestations of DNA damage can occur when free radicals are present in high enough concentrations. DNA damage in the hippocampus of rats subjected to intermittent hypoxia was found to be repaired after treatment with EGb 761. Researchers found that EGb did more than just prevent DNA damage; it also sped up the repair process (Celik et al., 2005).
Marques et al. (2011) using a modified comet assay, researchers found that EGb had a dose-dependent protective effect on yeast exposed to oxidative stress, leading to a reduction in DNA damage and a subsequent increase in viability. The extract protects DNA from oxidation in two ways, as shown by the comet assay: directly and through stimulating the DNA repair mechanism.
GbE is a massively complex compound made up of a number of components, so it is necessary to systematically analyze each component’s effects to find the compound that works best for health benefits, reducing symptoms of disease, and these bioactive components like flavonoids, which may have protective effects against DNA damage. The decoction of the GbE demonstrated the protective efficacy against Geno toxicity represented by decreasing in mitotic and blast index. Furthermore, the ameiolerative effect of GbE AGAINST genotoxicity confirmed by decrease in the mean of chromosomal aberration in both groups (T2 and T3) that pretreated with GB extract and omega 3, respectively.
Conclusions and Recommendations
GbE would be good candidates for usage as supplement to decrease the Geno-toxicity side effects of a lot of chemotherapeutic agents. Furthermore, we recommend administration of this extract as food supplement to decrease toxicity of chemotherapeutic drugs.
Acknowledgment
This work was supported by Al-Mustaqbal University College (grant number MUC-M-0222).
Novelty Statement
This research highlight the protective effects of Ginkgo biloba extracts as supplementation against genotoxicity that induced by chemotherapy.
Author’s Contribution
All authors have researched, inspected closely, reviewed the manuscript, and given their final approval for it to be published.
Conflict of interests
The authors have declared no conflict of interest.
References
Agrawal RK, Patel RK, Nainiwal L, Trivedi B (2014). Hydroxyurea in sickle cell disease: Drug review. Indian J. Hematol. Blood Transfus., 30(2): 91-96. https://doi.org/10.1007/s12288-013-0261-4
Al-Ameedi AIM, Al-Rekabi FMK, Ayad ZM (2015). Cytogenetic effects of tacrolimus after chronic oral administration in male albino rats. Kufa J. Vet. Med. Sci., 6(2).
Al-Ameedi AI, Ayad ZM, Al-Rekabi FMK (2020). Toxopathological and cytogenetic effects of commercial sweetener aspartame after chronic oral administration in rat pups. Indian J. Forensic Med. Toxicol., 14(3): 1147.
Arlt MF, Rajendran S, Holmes SN, Wang K, Bergin IL, Ahmed S, Glover TW (2018). Effects of hydroxyurea on CNV induction in the mouse germline. Environ. Mol. Mutagen., 59(8): 698-714. https://doi.org/10.1002/em.22233
Bernatoniene J, Majiene D, Peciura R, Laukeviciene A, Bernatoniene R, Mekas T, Kopustinskiene D (2011). The effect of Ginkgo biloba extract on mitochondrial oxidative phosphorylation in the normal and ischemic rat heart. Phytother. Res., 25(7): 1054-1060. https://doi.org/10.1002/ptr.3399
Burkitt MJ, Raafat A (2006). Nitric oxide generation from hydroxyurea: significance and implications for leukemogenesis in the management of myeloproliferative disorders. Blood, 107(6): 2219-2222. https://doi.org/10.1182/blood-2005-08-3429
Celik I, Cihangiroglu M, Ilhan N, Akpolat N, Akbulut HH (2005). Protective effects of different antioxidants and amrinone on vancomycin-induced nephrotoxicity. Basic Clin. Pharmacol. Toxicol., 97(5): 325-332. https://doi.org/10.1111/j.1742-7843.2005.pto_153.x
Cherednichenko, O., Pilyugina, A., and Nuraliev, S. (2022). Сytogenetical bioindication of pesticidal contamination. Pes. Nat. Environ. (pp. 227-260).
Davies, B.W., Kohanski, M.A., Simmons, L.A., Winkler, J.A., Collins, J. J., and Walker, G. C. (2009). Hydroxyurea induces hydroxyl radical-mediated cell death in Escherichia coli. Molecular cell, 36(5), 845-860.
Elelaimy IA, Elfiky SA, Hassan AM, Ibrahim HM, Elsayad RI (2012). Genotoxicity of anticancer drug Azathioprine (Imuran): role of Omega-3 (?-3) oil as protective agent. J. Appl. Pharma. Sci., (Issue), 14-23. https://doi.org/10.7324/JAPS.2012.2404
Fan X, Zhu Y, Wang N, Zhang B, Zhang C, Wang Y (2021). Therapeutic dose of hydroxyurea-induced synaptic abnormalities on the mouse spermatocyte. Front. Physiol., 12: 666339. https://doi.org/10.3389/fphys.2021.666339
Huang, M. E., Facca, C., Fatmi, Z., Baïlle, D., Bénakli, S., and Vernis, L. (2016). DNA replication inhibitor hydroxyurea alters Fe-S centers by producing reactive oxygen species in vivo. Scientific Reports, 6(1), 29361.
Ihl R, Bachinskaya N, Korczyn AD, Vakhapova V, Tribanek M, Hoerr R, Gotaday Study Group (2011). Efficacy and safety of a once-daily formulation of Ginkgo biloba extract EGb 761 in dementia with neuropsychiatric features: Arandomized controlled trial. Int. J. Geriatr. Psychiatry, 26(11): 1186-1194. https://doi.org/10.1002/gps.2662
Keheyan G, Dunn LA, Hall WL (2011). Acute effects of ginkgo biloba extract on vascular function and blood pressure. Plant Foods Hum. Nutr., 66(3): 209-211. https://doi.org/10.1007/s11130-011-0234-4
Kumaravel, T. S., and Jha, A. N. (2006). Reliable Comet assay measurements for detecting DNA damage induced by ionising radiation and chemicals. Mutation Research/Genetic Toxicology and Environmental Mutagenesis, 605(1-2), 7-16.
Lanzkron S, Strouse JJ, Wilson R, Beach MC, Haywood C, Park H, Segal JB (2008). Systematic review: Hydroxyurea for the treatment of adults with sickle cell disease. Ann. Inter. Med., 148(12): 939-955. https://doi.org/10.7326/0003-4819-148-12-200806170-00221
Liao HJ, Zheng YF, Li HY, Peng GP (2011). Two new ginkgolides from the leaves of Ginkgo Biloba. Planta Med., 77(16): 1818-1821. https://doi.org/10.1055/s-0030-1271153
Luchtman-Jones L, Pressel S, Hilliard L, Brown RC, Smith MG, Thompson AA, Ware RE (2016). Effects of hydroxyurea treatment for patients with hemoglobin SC disease. Am. J. Hematol., 91(2): 238-242. https://doi.org/10.1002/ajh.24255
Manna S, Janarthan M, Ghosh B, Rana B, Rana A, Chatterjee M (2010). Fish oil regulates cell proliferation, protect DNA damages and decrease HER-2/neu and c-Myc protein expression in rat mammary carcinogenesis. Clin. Nutr., 29(4): 531-537. https://doi.org/10.1016/j.clnu.2009.12.012
Marques F, Azevedo F, Johansson B, Oliveira R (2011). Stimulation of DNA repair in Saccharomyces cerevisiae by Ginkgo biloba leaf extract. Food Chem. Toxicol., 49(6): 1361-1366. https://doi.org/10.1016/j.fct.2011.03.020
McGann PT, Ware RE (2015). Hydroxyurea therapy for sickle cell anemia. Exp. Opin. Drug Saf., 14(11): 1749–1758. https://doi.org/10.1517/14740338.2015.1088827
Mohamed NES, Abd El-Moneim AE (2017). Ginkgo biloba extract alleviates oxidative stress and some neurotransmitters changes induced by aluminum chloride in rats. Nutrition, 35: 93-99. https://doi.org/10.1016/j.nut.2016.10.012
Mohanta TK, Tamboli Y, Zubaidha PK (2014). Phytochemical and medicinal importance of Ginkgo biloba L. Natl. Prod. Res., 28(10): 746-752. https://doi.org/10.1080/14786419.2013.879303
Moreno SRF, Freitas RS, Rocha EK, Lima-Filho GL, Bernardo-Filho AM (2004). Protection of plasmid DNA by a Ginkgo biloba extract from the effects of stannous chloride and the action on the labeling of blood elements with technetium-99m. Braz. J. Med. Biol. Res., 37: 267-271. https://doi.org/10.1590/S0100-879X2004000200015
Nair, A. B. and Jacob, S. (2016). A simple practice guide for dose conversion between animals and human. J. Basic Clin.Pharm., 7(2): 27–31.
Nakayashiki, T., and Mori, H. (2013). Genome-wide screening with hydroxyurea reveals a link between nonessential ribosomal proteins and reactive oxygen species production. Journal of bacteriology, 195(6), 1226-1235.
Saleem S, Zhuang H, Biswal S, Christen Y, Doré S (2008). Ginkgo biloba extract neuroprotective action is dependent on heme oxygenase 1 in ischemic reperfusion brain injury. Stroke, 39(12): 3389-3396. https://doi.org/10.1161/STROKEAHA.108.523480
SAS (2012). Statistical Analysis System, User’s Guide. Statistical. Version 9.1ed. SAS. Inst. Inc. Cary. N.C. USA.
Shah, M. A., Janjigian, Y. Y., Stoller, R., Shibata, S., Kemeny, M., Krishnamurthi, S., Su, Y. B., Ocean, A., Capanu, M., Mehrotra, B., Ritch, P., Henderson, C., and Kelsen, D. P. (2015). Randomized Multicenter Phase II Study of Modified Docetaxel, Cisplatin, and Fluorouracil (DCF) Versus DCF Plus Growth Factor Support in Patients With Metastatic Gastric Adenocarcinoma: A Study of the US Gastric Cancer Consortium. Journal of clinical oncology : official journal of the American Society of Clinical Oncology, 33(33), 3874–3879. https://doi.org/10.1200/JCO.2015.60.7465
Silva AM, Silva SC, Soares JP, Martins-Gomes C, Teixeira JP, Leal F, Gaivão I (2019). Ginkgo biloba L. leaf extract protects HepG2 cells against paraquat-induced oxidative DNA damage. Plants, 8(12): 556. https://doi.org/10.3390/plants8120556
Singh, A., and Xu, Y. J. (2016). The cell killing mechanisms of hydroxyurea. Genes, 7(11), 99.
Tang Y, Zhou G, Yao L, Xue P, Yu D, Xu R, Duan JA (2017). Protective effect of Ginkgo biloba leaves extract, EGb761, on myocardium injury in ischemia reperfusion rats via regulation of TLR-4/NF-κB signaling pathway. Oncotarget, 8(49): 86671. https://doi.org/10.18632/oncotarget.21372
Uygur R, Aktas C, Tulubas F, Uygur E, Kanter M, Erboga M, Ozen OA (2014). Protective effects of fish omega-3 fatty acids on doxorubicin-induced testicular apoptosis and oxidative damage in rats. Andrologia, 46(8): 917-926. https://doi.org/10.1111/and.12173
van Beek TA, Montoro P (2009). Chemical analysis and quality control of Ginkgo biloba leaves, extracts, and phytopharmaceuticals. J. Chromatogr. A, 1216(11): 2002-2032. https://doi.org/10.1016/j.chroma.2009.01.013
Wyrobek AJ, Bruce WR (1975). Chemical induction of sperm abnormalities in mice. Proc. Natl. Acad. Sci., 72(11): 4425-4429. https://doi.org/10.1073/pnas.72.11.4425
Xu, Y. J., Singh, A., and Alter, G. M. (2016). Hydroxyurea induces cytokinesis arrest in cells expressing a mutated sterol-14α-demethylase in the ergosterol biosynthesis pathway. Genetics, 204(3), 959-973.
To share on other social networks, click on any share button. What are these?






