Genotype-By-Sex Interaction Effect on Growth Traits at Different Ages in Slow-Growing Chickens
Research Article
Genotype-By-Sex Interaction Effect on Growth Traits at Different Ages in Slow-Growing Chickens
Mohamed El-Henfnawy*, Essam A. El-Gendy, Ahamed M. El-Kaiaty, Mostafa Helal
Department of Animal Production, faculty of Agriculture, Cairo University, Giza, Egypt.
Abstract | Local and indigenous chicken breeds play important roles in rural development and sustainability, and thus, evaluating and improving local breeds is essential. Genotype-by-sex interaction refers to the interaction of genetic architecture with male or female performance. The current study aimed to evaluate the genotype-by-sex (G×S) interaction on the growth performance of a selected chicken line and its genetic control line. The results revealed significant G × S interactions for body weights at 4 and 10 weeks of age. The line effects on body weight traits were significant and consistent except for 2-wk body weight. Also, significant sex effects were shown for body weights at all ages. The effect of sex on biweekly body weight gains and growth rates was significant for the traits from 0 to 4, 6 to 8, and 12 to 14 weeks of age. The line effect was significant for 0-2 and 2-4 growth rates, and 0-2 and 6-8 body-weight gains. The line × sex interaction was not significant for body weight gain and growth rate traits except for growth rate from hatch to 2 weeks of age. Growth rates in males were significantly higher than females at early ages. Although the genotype-by-sex effect on the performance of the selected line (CE2) was deceased at the 6th generation of selection, it has been retrieved at the 10th generation of selection.
Keywords | Local chicken, Body weight, Selection, Growth rate, Sustainability
Received | February 17, 2022; Accepted | March 25, 2022; Published | June 01, 2022
*Correspondence | Mohamed El-Henfnawy, Department of Animal Production, faculty of Agriculture, Cairo University, Giza, Egypt; Email: [email protected]
Citation | El-Henfnawy M, El-Gendy EA, El-Kaiaty AM, Helal M (2022). Genotype-by-sex interaction effect on growth traits at different ages in slow-growing chickens. J. Anim. Health Prod. 10(2): 226-231.
DOI | http://dx.doi.org/10.17582/journal.jahp/2022/10.2.226.231
ISSN | 2308-2801

Copyright: 2022 by the authors. Licensee ResearchersLinks Ltd, England, UK.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Introduction
The advances in the poultry industry have been associated with using long-term intensive selection programs to produce commercial meat-type chickens. However, local breeds are ubiquitous in the smallholder production system and receive little attention from poultry geneticists due to their poor productiveness, although local breeds have many advantages, such as their adaptation ability to the local environmental condition, and the richness of their genetic diversity (Dessie et al., 2011; Padhi, 2016), which is an important genetic attribute. Also, those slow-growing breeds are preferred in many countries due to their meat flavor and appearance (Sokołowicz et al., 2016; Bettridge et al., 2018; Manyelo et al., 2020).
Nowadays, the concern about sustainable production has increased, and maintaining animal genetic resources is a milestone step for sustainability. On the other hand, continuous selection of commercial parents and grandparents stocks retracted the genetic variation in such lines, and this developing alternative strategies for minting genetic variation of local and indigenous breeds as a valuable source for genetic diversity enhances suitability practices (Hoffmann, 2011; Phuong et al., 2015; Bettridge et al., 2018).
Body weight traits in males and females have been regarded as different traits in chickens and turkeys (Nestor et al., 2008; Mebratie et al., 2017). Differential effects of genes on traits at different ages in males and females are of great consideration for understanding the rate of genetic change due to selection in both sexes (Towne et al., 1997). The interaction between genotype and sex is important Effect of genotype and sex and their interaction effects have been addressed at different levels in different breeds and was not reported only on the phenotypic level, but also on the molecular level, where several QTL showed interaction with sex both autosomal and GGAZ chromosomes (Abasht et al., 2006). In Nigeria, Ajayi and Ejiofor, (2009) studied the genotype-by-sex interaction in Anak Broilers in comparison with commercial Ross chickens, and detected the existence of sexual dimorphism between both sexes, where male broilers were superior to females in all growth traits.
The current study aimed to evaluate the genotype-by-sex interaction effect on the growth performance of a local Egyptian selected chicken line selected for fast growth for 10 generations in comparison to its genetic control line.
Materials and methods
Breeding stock and genetic background of the chickens
Two chicken lines were used in the current study. The first one is the CE2 line, which was developed by selection for fast growth for 10 generations, the breeding scheme used for developing the line was detailed by El-Gendy (2009). Where the second line (CE4) is the genetic control for the CE2 line, which was maintained as a randombred flock for 10 generations.
Management and parameters
Chicks of both lines were hatched together. At the hatchery, chicks were wing-banded, vaccinated, and weighed (hatch weight). The chickens were then kept under the same environmental conditions in brooding rooms to six weeks of age, and then transferred to open floor rearing pens to 18 weeks of age. All chickens received the same routine management, including feeding, watering, lighting, and vaccination programs. Individual biweekly body weights (BW) were recorded from hatch to 18 weeks of age; body weight gains (BWG) and growth rates (GR) were calculated.
Statistical analysis
The data were analyzed using general linear model (GLM) procedure of SAS (SAS, 1999). The statistical model was:
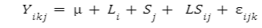
Where, Y is the given measurement, μ is the overall mean, L and S are the effects of line and sex, respectively, and ε is the random error. Significant differences were considered when p < 0.05, and were separated using Duncan multiple range test (Duncan, 1955).
Results and discussion
Levels of significance are presented in Table (1) for body weight, and in Table (2) for body weight gains and growth rates. Significant line × sex interactions were only observed for body weights at 4 and 10 weeks of age. However, the line effect on body weight traits was significant and consistent except for 2-wk body weight. Also, significant sex effects were shown for all traits. Helal and El-Gendy, (2013) reported the loss of significant sex effect of growth traits of the same selected line (CE2) during the 6th and 7th generation of selection, which was attributed to the genetic drift effect that encountered the population after four generations of selection. The results of the current study are in agreement with previously published results (Namakparvar et al., 2014; Benyi et al., 2015), as they reported significant effects for strain, sex, and their interaction on final body weight and body weight gains of broiler chickens.
Moreover, the effect of sex on biweekly body weight gains and growth rates was significant for the traits from 0 to 4, 6 to 8, and 12 to 14 weeks of age. The line effect was significant for 0-2 and 2-4 growth rates, and 0-2 and 6-8 body-weight gains. The line × sex interaction was not significant for body weight gain and growth rate traits except for growth rate from hatch to 2 weeks of age. Furthermore, the effect of the line was significant for body weight gains from 0 to 6, 6 to 12, and growth rates from 0 to 6 and 12 to 18 weeks of age. Whereas the sex effect was significant for body weight gain from 6 to 12, and growth rates from 0 to 6 and 6 to 12 weeks of age. A significant interaction was shown for the late growth rate from 12 to 18 weeks of age. Table (3) shows the means of body weights of the two lines. The selected males (CE2) had significantly higher body weights compared to males of the control line (CE4), except for body weight at 2 weeks of age. These differences are the result of the ongoing selection process in line CE2. On the other hand, the differences between the females of both lines were significant from hatch to 4 weeks of age. These differences became insignificant from 6 to 10 weeks of age and became significant again from 12 to 18 weeks of age. Ajayi and Ejiofor (2009) noticed that the difference between Anak and commercial broilers were existed at hatch and disappeared at three weeks of age. In Ghanaian chickens, Osei-Amponsah et al. (2012) reported a highly significant sex effect of body weight up to 28 weeks of age. Also, the sex effect of growth was significant for Ross, Aboaca, and Anak broiler stains (Ikusika et al., 2020).
The results of body weight gains and growth rates were convergent, as shown in Tables (4) and (5), respectively. Significant increase in body weight gains of the selected males compared to their control males at the biweekly period from 2 to 4 and from 4 to 6, and for early (0 to 6 wk) and middle (6-12 wk) body weight gains. In females,
Table 1: Level of significant of line, sex and their interaction effects on body weights
| Age (wk) |
Level of significance (p ≤ ) |
|||
| Model | Line | Sex | Interaction | |
| 1-day | 0.002 | 0.018 | 0.003 | 0.2259 |
| 2 | 0.137 | 0.868 | 0.024 | 0.5803 |
| 4 | 0.001 | 0.001 | 0.001 | 0.0300 |
| 6 | 0.001 | 0.001 | 0.702 | 0.2791 |
| 8 | 0.001 | 0.001 | 0.082 | 0.4124 |
| 10 | 0.001 | 0.001 | 0.005 | 0.0109 |
| 12 | 0.001 | 0.001 | 0.019 | 0.4337 |
| 14 | 0.001 | 0.001 | 0.001 | 0.0936 |
| 16 | 0.001 | 0.003 | 0.001 | 0.3729 |
| 18 | 0.005 | 0.006 | 0.023 |
0.1592 |
Table 2: Level of significant of line, sex and their interaction effects on body weight gains and growth rates
| Period |
Level of significance (p ≤ ) |
||||||||
|
Body weight gain |
Growth rate |
||||||||
| Model | Line | Sex | Interaction | Model | Line | Sex | Interaction | ||
| 0-2 | 0.026 | 0.656 | 0.005 | 0.325 | 0.001 | 0.057 | 0.001 | 0.021 | |
| 2-4 | 0.001 | 0.001 | 0.193 | 0.064 | 0.025 | 0.008 | 0.876 | 0.108 | |
| 4-6 | 0.187 | 0.038 | 0.523 | 0.967 | 0.426 | 0.428 | 0.166 | 0.657 | |
| 6-8 | 0.036 | 0.186 | 0.011 | 0.522 | 0.074 | 0.973 | 0.011 | 0.636 | |
| 8-10 | 0.683 | 0.655 | 0.381 | 0.499 | 0.933 | 0.740 | 0.594 | 0.948 | |
| 10-12 | 0.493 | 0.863 | 0.555 | 0.171 | 0.092 | 0.152 | 0.677 | 0.035 | |
| 12-14 | 0.057 | 0.585 | 0.009 |
0.605 |
0.168 | 0.312 | 0.046 | 0.925 | |
| 14-16 | 0.580 | 0.875 | 0.179 | 0.816 | 0.845 | 0.732 | 0.429 | 0.829 | |
| 16-18 | 0.339 | 0.963 | 0.414 | 0.109 | 0.367 | 0.868 | 0.341 | 0.144 | |
| 0-6 | 0.001 | 0.001 | 0.580 | 0.293 | 0.001 | 0.001 | 0.023 | 0.481 | |
| 6-12 | 0.013 | 0.059 | 0.007 | 0.413 | 0.110 | 0.233 | 0.044 | 0.416 | |
| 12-18 | 0.190 | 0.706 | 0.355 | 0.055 | 0.022 | 0.026 | 0.598 |
0.021 |
|
Table 3: Effect of sex and line on body weights (LSM±SE, g) of selected (CE2) and control (CE4) chicken lines
|
♂♂ |
♀♀ |
||||
| Age (wk) | Line CE2 | Line CE4 | Line CE2 | Line CE4 | |
| Hatch |
47.04a±1.70 |
38.40b±2.69 |
51.65a±1.70 |
48.30b±2.33 |
|
| 2 |
147.43a±8.36 |
158.33a±18.05 |
125.93a±8.36 |
122.83b±12.76 |
|
| 4 |
372.00a±14.24 |
242.50b±21.76 |
309.41a±15.38 |
260.37b±18.84 |
|
| 6 |
667.33a±33.70 |
412.50b±53.29 |
622.30a±36.20 |
470.00a±58.38 |
|
| 8 |
1009.25a±61.35 |
607.60b±95.05 |
845.57a±56.80 |
571.33a±86.76 |
|
| 10 |
1264.00a±64.10 |
719.33b±77.08 |
931.66a±54.50 |
705.00b±66.75 |
|
| 12 |
1324.00a±57.95 |
830.00b±89.78 |
1085.00a±57.95 |
748.00b±100.38 |
|
| 14 |
1529.09a±66.1 |
974.50b±109.61 |
1110.79a±67.53 |
839.50b±109.61 |
|
| 16 |
1601.10a±74.38 |
1146.20b±105.2 |
1150.00a±74.38 |
878.00b±135.81 |
|
| 18 |
1612.43a±134.71 |
1280.40b±106.79 |
1383.00a±97.49 |
956.00a±255.52 |
|
LSM= Least square mean
a,b, body weight of different lines, within sex, with different letters are significantly different (p < 0.05)
Table 4: Effect of sex and line on body weight gains (LSM±SE, g) of selected (CE2) and control (CE4) chicken lines
|
♂♂ |
♀♀ |
||||
| Period (wk) | Line CE2 | Line CE4 | Line CE2 | Line CE4 | |
|
Biweekly |
|||||
| Hatch -2 |
99.96a±8.33 |
126.33a±17.99 |
73.87b±8.33 |
75.26a±12.72 |
|
| 2-4 |
231.00a±15.77 |
75.00b±32.84 |
183.00a±16.42 |
116.00b±23.22 |
|
| 4-6 |
282.28a±37.56 |
170.00b±57.37 |
311.45a±42.37 |
203.4a±62.85 |
|
| 6-8 |
376.75a±54.48 |
222.60a±84.40 |
170.66a±54.48 |
112.50b±94.36 |
|
| 8-10 |
309.00a±132.36 |
150.00a±108.07 |
135.83a±76.42 |
123.00a±93.59 |
|
| 10-12 |
202.67a±25.59 |
150.66a±36.44 |
127.00a±10.82 |
73.00a±25.59 |
|
| 12-14 |
219.22a±38.52 |
168.25a±55.49 |
77.37a±39.24 |
101.00a±66.71 |
|
| 14-16 |
137.11a±35.14 |
114.00a±52.72 |
73.60a±33.34 |
72.66a±60.87 |
|
| 16-18 |
27.50a±64.90 |
134.20a±58.05 |
179.20a±58.05 |
114.00a±90.65 |
|
|
6-week body weight gains |
|||||
| Hatch -2 |
620.29a±32.67 |
374.10b±51.66 |
570.45a±35.10 |
420.74a±56.59 |
|
| 6 -12 |
645.81a±46.44 |
445.00b±68.88 |
433.10a±48.70 |
359.00a±88.92 |
|
| 12-18 |
301.00a±121.25 |
200.50a±160.40 |
336.40a±143.479 |
332.00a±112.25 |
|
LSM= Least square mean
a,b, body weight gains of different lines, within sex, with different letters are significantly different (p < 0.05)
Table 5: Effect of sex and line on growth rate (LSM±SE, %) of selected (CE2) and control (CE4) chicken lines
|
♂♂ |
♀♀ |
||||
| Period (wk) | Line CE2 | Line CE4 | Line CE2 |
Line CE4 |
|
|
Biweekly |
|||||
| Hatch -2 |
2.18a±0.21 |
3.95a±0.46 |
1.42b±0.21 |
1.63a±0.33 |
|
| 2-4 |
1.67a±0.17 |
0.47b±0.35 |
1.51a±0.17 |
1.14a±0.25 |
|
| 4-6 |
0.77a±0.15 |
0.69a±0.23 |
1.10a±0.17 |
0.83a±0.25 |
|
| 6-8 |
0.66a±0.11 |
0.71a±0.18 |
0.31a±0.11 |
0.21b±0.20 |
|
| 8-10 |
0.32a±0.15 |
0.26a±0.12 |
0.25a±0.08 |
0.21a±0.11 |
|
| 10-12 |
0.16a±0.03 |
0.21a±0.06 |
0.13a±0.02 |
0.16a±0.06 |
|
| 12-14 |
0.16a±0.04 |
0.22a±0.05 |
0.08a±0.04 |
0.12a±0.06 |
|
| 14-16 |
0.10a±0.03 |
0.12a±0.04 |
0.07a±0.03 |
0.08a±0.05 |
|
| 16-18 |
0.02a±0.05 |
0.01a±0.05 |
0.15a±0.05 |
0.12a±0.07 |
|
|
6-week growth rates |
|||||
| Hatch -2 |
13.14a±0.57 |
9.45b±0.9 |
11.12a±0.61 |
8.56b±0.99 |
|
| 6 -12 |
1.05a±0.13 |
1.35a±0.2 |
0.77a±0.14 |
0.78a±0.25 |
|
| 12-18 |
0.24a±0.06 |
0.16a±0.06 |
0.32a±0.07 |
0.40a±0.30 |
|
LSM= Least square mean
a,b, growth rates of different lines, within sex, with different letters are significantly different (p < 0.05)
although the control line had a higher body weight gain than the selected females from hatch to two weeks of age, significantly higher body weight gains were obtained for the selected line compared to its control line from 2 to 4, and from 6 to 8 weeks of age. The difference between females of both lines for early (0-6 wk), middle (6-12 wk), and late (12-18 wk) body weight gains insignificant. The differences between the two lines may be attributed to the heavier hatch weight of line CE2 compared to line CE4, where (Namakparvar et al., 2014) reported significant effects of genotype, sex, and their interaction on body weight gains of three commercial strains (Ross, Cobb, and Arian chickens), where males had significantly higher body weight gains than females. Moreover, Udeh et al. (2015) reported an insignificant effect of genotype-by-sex interaction on body weight gains from 3 to 4 weeks of age in three strains in broiler chickens. However, the interaction effect was significant in the later ages. Del Castilho et al. (2013) also reported a significant sex effect on body weight gains in all ages in six free-range chicken genotypes.
Growth rates in males were significantly higher than females at early ages as well, where males of CE2 line had higher growth rates from hatch to 6-wk, and from 2 to 4-wk. Females also showed significant increases in growth rates of the selected line compared to its control line at early ages (0 to 6 weeks of age), and also for biweekly growth rate from 6 to 8. Similar to body weight gain results, the females of the control line had higher growth rates from hatch to two weeks of age. Osei-Amponsah et al. (2012) reported significantly higher growth rates for males than females in Ghanaian chickens, especially from hatch to 10 weeks of age.
The effect of sex by genotype interaction was studied extensively in commercial chickens. For example, Udeh et al. (2015) assessed the genotype × sex interaction in Arbor Acres, Ross, and Marshal chicken strains, the results revealed that the body weights of arbor acres males were higher than males and females of the two other strains. Nevertheless, few studies addressed the interaction effect in local chickens. El-Gendy and Helal (2011), and Helal and El-Gendy (2013) used the same lines (CE2 and CE4) observed the loss of effect of sex and interaction in the late ages during the 7th and 8th generations of selection, although the effects were highly significant in the establishment of the genetic lines (El-Gendy, 2009). A similar pattern was also observed for another selected line (CE1) and its genetic control (CE3) and attributed that to the ceasing of selection due to a genetic drift effect during the sixth generation of selection this genetic drift affected the sixth generation and led to reduction in the number of selected chickens (Helal and El-Gendy, 2013). The current results indicate that the selection process has retrieved the interaction effect after 3 generations of selection. Several previous reports indicated the significant effect of sex on growth performance and superiority of chicken males (Thutwa et al., 2012; Choo et al., 2014). On the contrary, Del Castilho et al. (2013) reported insignificant genotype × sex interaction on body weight gains, feed conversion ratio, and livability.
Conclusion
The portion of genetic variance that is due to the interaction between genotype and sex is considerable. Although the genotype-by-sex effect on the performance of the selected line (CE2) was deceased at the 6th generation of selection, it has been retrieved at the 10th generation of selection.
acknowledgements
This research has received no external funding.
Conflict of interest
The authors declare no conflict of interest.
novelty statement
The obtained results indicate that the genotype-by-sex interaction is an impotent portion of genetic variance in slow-growing chicken populations. If this portion is interrupted due to disruptive circumstances, it may be retrieved after three generations.
authors contribution
All authors contributed equally to the study design, analysis and manuscript preparation.
References
Abasht B, JC.M Dekkers, SJ Lamont (2006). Review of quantitative trait loci identified in the chicken. Poult. Sci. 85:2079–2096. https://doi.org/10.1093/ps/85.12.2079
Ajayi FO, O Ejiofor (2009). Effects of Genotype X Sex Interaction on Growth and Some Development Characteristics of Ross and Anak Broiler Strains in. Asian J. Poult. Sci. 3:51–56. https://doi.org/10.3923/ajpsaj.2009.51.56
Benyi K, TS Tshilate, AJ Netshipale, KT Mahlako (2015). Effects of genotype and sex on the growth performance and carcass characteristics of broiler chickens. Trop. Anim. Health Prod. 47:1225–1231. https://doi.org/10.1007/s11250-015-0850-3
Bettridge JM., A Psifidi, ZG Terfa, TT Desta, M Lozano-Jaramillo, T Dessie, P Kaiser, P Wigley, O Hanotte, RM Christley (2018). The role of local adaptation in sustainable production of village chickens. Nat. Sustain. 1:574–582. https://doi.org/10.1038/s41893-018-0150-9
Choo YK, HJ Kwon, ST Oh, JS Um, BG Kim, CW Kang, SK Lee, BK. An. (2014). Comparison of growth performance, carcass characteristics and meat quality of Korean local chickens and silky fowl. Asian-Australasian J. Anim. Sci. 27:398–405. https://doi.org/10.5713/ajas.2013.13638
Del Castilho CC, TT Santos, CAF Rodrigues, RA Torres Filho (2013). Effects of sex and genotype on performance and yield characteristics of free range broiler chickens. Arq. Bras. Med. Veterinária e Zootec. 65:1483–1490. https://doi.org/10.1590/S0102-09352013000500029
Dessie T, T Taye, N Dana, W Ayalew, O Hanotte (2011). Current state of knowledge on phenotypic characteristics of indigenous chickens in the tropics. Worlds. Poult. Sci. J. 67:507–516. https://doi.org/10.1017/S0043933911000559
Duncan DB (1955). Multiple Range and Multiple F Tests. Biometrics 11:1. https://doi.org/10.2307/3001478
El-Gendy EA (2009). A Model for the Genetic Employment of Chickens Local to Warm Climate 1. Crossing with a Fast Growing Strain and Growth Patterns of the Crossbreds. Int. J. Poult. Sci. 8:299–306. https://doi.org/10.3923/ijps.2009.299.306
El-Gendy E, MA Helal (2011). An approach to marker-assisted selection for increased body weights in local chickens in Egypt.Page 8 in Poult. Sci. 90(E-Suppl. 1).
Helal M, EA El-Gendy. (2013). Evaluation of selection progress in two local Egyptian chicken breeds.in International Poultry Scientific Forum. Atlanta, Georgia, USA.
Hoffmann I (2011). Livestock biodiversity and sustainability. Livest. Sci. 139:69–79. https://doi.org/10.1016/j.livsci.2011.03.016
Ikusika OO, AB Falowo, TJ Zindove, AI Okoh (2020). Effect of strain, sex and slaughter weight on growth performance, carcass yield and quality of broiler meat. Open Agric. 5:607–616. https://doi.org/10.1515/opag-2020-0056
Manyelo TG, L Selaledi, ZM Hassan, M Mabelebele (2020). Local chicken breeds of Africa: their description, uses and conservation methods. Animals 10:2257. https://doi.org/10.3390/ani10122257
Mebratie W, M Shirali, P Madsen, RL Sapp, R Hawken, J Jensen (2017). The effect of selection and sex on genetic parameters of body weight at different ages in a commercial broiler chicken population. Livest. Sci. 204:78–87. https://doi.org/10.1016/j.livsci.2017.08.013
Namakparvar R, F Shariatmadari, SH Hossieni (2014). Strain and sex effects on ascites development in commercial broiler chickens. Iran. J. Vet. Res. 15:116–121.
Nestor KE, JW Anderson, RA Patterson, SG Velleman. (2008). Genetics of growth and reproduction in the turkey. 17. Changes in genetic parameters over forty generations of selection for increased sixteen-week body weight. Poult. Sci. 87:1971–1979. https://doi.org/10.3382/ps.2008-00137
Osei-Amponsah R, BB Kayang, A Naazie (2012). Age, genotype and sex effects on growth performance of local chickens kept under improved management in Ghana. Trop. Anim. Health Prod. 44:29–34. https://doi.org/10.1007/s11250-011-0010-3
Padhi MK (2016). Importance of indigenous breeds of chicken for rural economy and their improvements for higher production performance. Scientifica (Cairo). 2016. https://doi.org/10.1155/2016/2604685
Phuong TNL, KDTD Xuan, I Szalay (2015). Traditions and local use of native Vietnamese chicken breeds in sustainable rural farming. Worlds. Poult. Sci. J. 71:385–396. https://doi.org/10.1017/S0043933915000380
SAS (1999). SAS/STAT User’s Guide: Statistics. SAS Institute Inc, Cary, NC, USA. Analysis System Institute Cary.
Sokołowicz Z, J Krawczyk, S Świątkiewicz (2016). 4. Quality of Poultry Meat from Native Chicken Breeds–A Review. Ann. Anim. Sci. 16:347–368. https://doi.org/10.1515/aoas-2016-0004
Thutwa K, SJ Nsoso, PM. Kgwatalala, JC Moreki (2012). Comparative Live Weight, Growth Performance, Feed Intake, Carcass Traits and Meat Quality in Two Strains of Tswana Chickens Raised Under Intensive System in South East District of Botswana. Int. J. Appl. Poult. Res. ISSN 1:21–26.
Towne B, RM Siervogel, J Blangero. (1997). Effects of genotype by sex interaction on quantitative trait linkage analysis. Genet. Epidemiol. 14:1053–1058. https://doi.org/10.1002/(SICI)1098-2272(1997)14:6%3C1053::AID-GEPI82%3E3.0.CO;2-G
Udeh I, PN Ezebor, PO Akporahuarho (2015). Growth Performance and Carcass Yield of Three Commercial Strains of Broiler Chickens raised in a Tropical Environment. J. Biol. Agric. Healthc. 5:62–67.
To share on other social networks, click on any share button. What are these?





