Gene Expression in Escherichia coli and Purification of Recombinant Type II Pullulanase from a Hyperthermophilic Archaeon, Pyrobaculum calidifontis
Gene Expression in Escherichia coli and Purification of Recombinant Type II Pullulanase from a Hyperthermophilic Archaeon, Pyrobaculum calidifontis
Habib-ur-Rehman1, Masood Ahmed Siddiqui1,*, Abdul Qayyum1, Arifa Bano1 and Naeem Rashid2
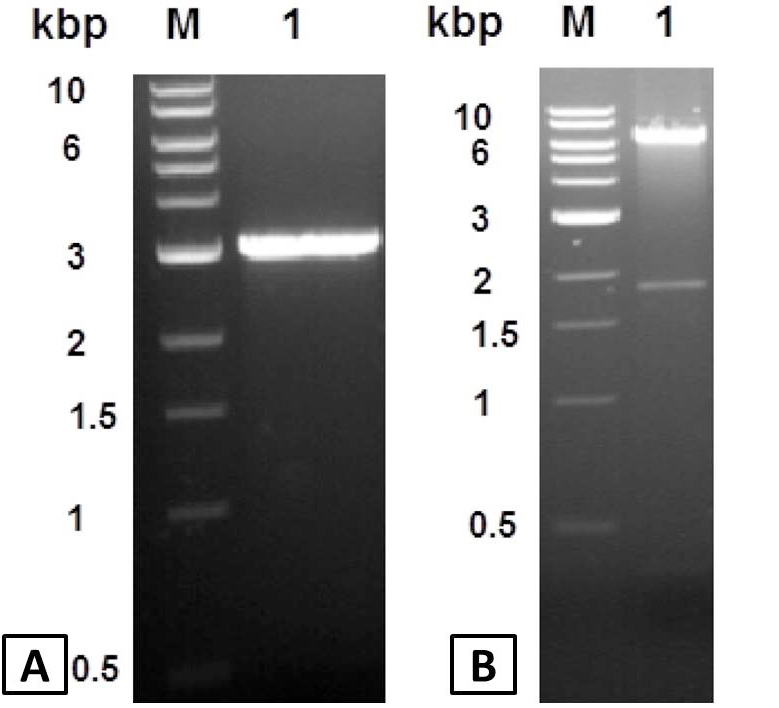
DNA fragments analysis by ethidium bromide stained 1% agarose gel. A, PCR amplified type II pullulanase gene. Lane M, Kilobase DNA marker; Lane 1, 3.1 kbp PCR amplified apu gene. B, Analysis of recombinant pET101-APU plasmid. Lane M, standard; Lane 1, pET101-APU plasmid cut with HindIII restriction enzyme.
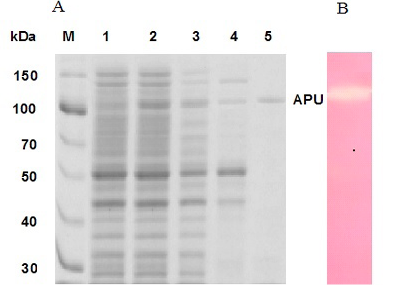
Coomassie brilliant blue stained SDS-PAGE demonstrating the production of type II pullulanase. A, Lane M, protein marker; Lane 1, cells carrying pET101 vector; Lane 2, cells carrying pET101-APU plasmid; Lane 3, soluble fraction of the sample in lane 2; Lane 4, soluble fraction after heat treatment of sample in lane 3; Lane 5, purified recombinant type II pullulanase. B, Activity of purified type II pullulanase.
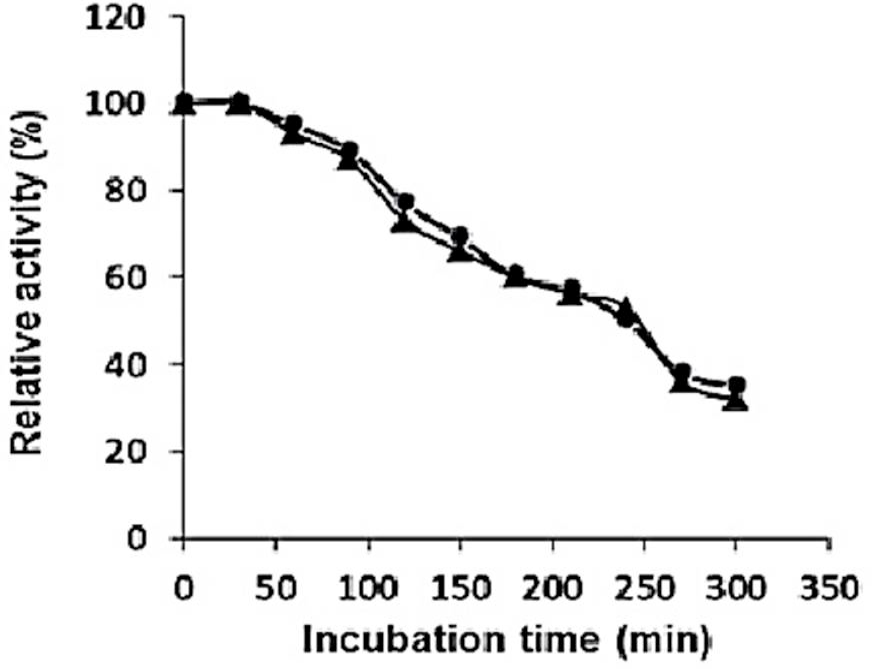
Thermostability of purified recombinant type II pullulanase in the presence (●) or absence (▲) of Ca2+.