Exploring the Genetic Diversity of Citrullus vulgaris L. Against Salinity Stress
Research Article
Exploring the Genetic Diversity of Citrullus vulgaris L. Against Salinity Stress
Muhammad Zeeshan Siddiqui1, Mujahid Ali2*, Shoaib ur Rehman3, Shahid Iqbal4, Malik Abdur Rehman5, Hafiz M. Tayyab Khan4, Muhammad Ali6, Muhammad Azher Nawaz4 and Saqib Ayyub1
1Institute of Horticultural Sciences, University of Agriculture, Faisalabad, Pakistan; 2Water Management Research Farm, Renala Khurd, Okara, Pakistan; 3Institute of Horticultural Sciences, University of Agriculture, Faisalabad, (Subcampus Depalpur, Okara); 4Department of Horticulture, College of Agriculture, University of Sargodha, Pakistan; 5Citrus Research Institute, Sargodha, Pakistan; 6Department of Horticulture, Bahauddin Zakariya University, Multan, Pakistan.
Abstract | Salinity is a major issue for tinda gourd (Citrullus vulgaris L.) production throughout the world. In a pot experiment, five selected tinda gourd genotypes i.e., Green ball, Dilpasand, Durga, Round gold, and Indian desi were sown. One month after emergence, the growing medium was supplied with the lowest NaCl level 2, two medium levels 4, 6, and the highest salinity level was 8 dS m-1 and was compared with the control (1.5 dS m-1 considered as normal). The Hoagland solution was applied every week as a nutrient solution. High sodium contents lead to sodicity. Finally, selected genotypes displayed significantly dissimilar responses toward the concentration of sodium ions according to their genetic potential. Various agronomic traits and physiological traits along with seedlings’ ionic content of sodium, phosphorous, potassium, and sodium in leaves revealed that ‘Round ball’ has better NaCl tolerance ability compared with other genotypes used in this study. The highest ionic sodium concentration (10.34 µg g-1 DW) was found in Green ball, whereas Round gold (8.92 µg g-1 DW) showed the minimum. NaCl-induced salinity leads to chlorophyll damage in Round gold (58.44 SPAD index) and ‘Green ball’ (50.55 SPAD index) accordingly. On an overall basis, ‘Round gold’ (12.1 µg g-1) had maximum followed by Indian desi (11.7 µg g-1) at the highest level of salinity (8 dS/m-1) with respect to control (6.9 µg g-1). It was revealed that the lowest ionic sodium concentration was observed in the Green ball (6.3 µg g-1). Considering the result of this study, the Round gold tinda gourd genotype may be used for cultivation in salt-affected soils.
Received | December 11, 2021; Accepted | May 02, 2023; Published | May 17, 2023
*Correspondence | Mujahid Ali, Water Management Research Farm, Renala Khurd, Okara, Pakistan; Email: mujahidali2263@gmail.com
Citation | Siddiqui, M.Z., Ali, M., Rehman, S., Iqbal, S., Rehman, M.A., Khan, H.M.T., Ali, M., Nawaz, M.A., and Ayyub, S., 2023. Exploring the genetic diversity of Citrullus vulgaris L. against salinity stress. Journal of Innovative Sciences, 9(1): 95-105.
DOI | https://dx.doi.org/10.17582/journal.jis/2023/9.1.95.105
Keywords | Tinda gourd, NaCl stress, Genotypes, Physiology, Na toxicity
Copyright: 2023 by the authors. Licensee ResearchersLinks Ltd, England, UK.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
1. Introduction
Tinda gourd (Citrullus vulgaris) is a nutritious vegetable that belongs to the family Cucurbitaceae. In Pakistan, total land covered by tinda gourd is 10,588 ha with an annual production of 101,734 tons. Punjab is the leading province for its cultivation (6,170 hectares) and production (61,929 tons), followed by Sindh (2,221 hectares, 13,990 tons), KPK (1,299 ha, 19,928 tons), and Baluchistan (898 ha, 5,887 tons) (GOP, 2019).
It has been reported that about 6% of agricultural land is under salinity stress by different chemicals in the world (Parihar et al., 2015). Overall, 900 MH of land is affected in the world is under cultivation for agronomic and horticultural crops, including vegetables (Velmurugan et al., 2020). For sustainable agriculture development to secure the globe from salinity, defensive mechanisms are vital to protect plants against reactive oxygen species and cellular damage (Arif et al., 2020).
Salt stress damages plant organs by producing reactive oxygen species that affect plant growth and development and water potential (Kamran et al., 2020; Ferreira et al., 2022). The major abiotic factor restricts crop growth and development and reduces crop yield (Roy et al., 2014). H+-ATPase factors are necessary to produce energy to cope with the upcoming, and entrance of NaCl in the tonoplast (Munns et al., 2020). The most recent developments in nanotechnologies are used nowadays to deal with salinity (Fu et al., 2023).
Various soil chemical characteristics, soil particle disintegration, soil moisture, removal of organic matter, acidity, hardpan, immobilization of soil nutrients to plants, and addition of calcium and magnesium salts reduce crop growth and production (Jung et al., 2011). Sodium and chloride particles cause salt stress. Soils having unnecessary sodium substances have a sodicity issue and are known as sodic soils, and soils with exorbitant solvent salts have a saltiness issue and are called saline soils. Saltiness is an overall rural issue; as half of the flooded grounds and 20% of developed regions are saline (Edelstein et al., 2011). Further, this fact was supported by Moldakimova et al. (2012) who depicted that between 2001 and 2011 saline areas became two times gradually. Various techniques such as biological, chemical, and physiological have been adopted to cope with salinity (Yang et al., 2013). In addition to these, grafting is also a suitable technique to cope with salinity stress (Elsheery et al., 2020; Singh et al., 2020).
The Cucrbitaceae family contains a morphologically diverse group of plants that have META genes for salinity, heavy metals, and drug-tolerant plants (Shah et al., 2022). Among these family members’ pumpkin and squashes have ability to save salts in stems could work as rootstocks in saline soils. Plant development and growth are greatly influenced by a variety of soil elements, including the physical and chemical properties of the soil (Munns and Tester, 2008). The total area under salinity in Pakistan is about 6.3 million hectares which were forecasted to increase by up to 14% and 64% yield losses were expected (Afzal et al., 2005).
Salinity is a major abiotic factor for vegetable production (Toscano et al., 2023). Salt stress hinders the uptake of essential ions (K+, Ca+2, and Mn+2) and causes imbalance (Hasegawa et al., 2000). There is a need to check the response of plants under salt stress conditions (İbrahimova et al., 2021). The plant has adopted interaction strategies that influence plants grown under salt stress to modify them biologically (Acosta-Motos et al., 2017). Keeping in view the above-mentioned review, the study aimed to evaluate the genetic potential of genotypes of tinda gourd against salt stress.
2. Materials and Methods
At the research area of Horticultural, University of Agriculture, Faisalabad (31°30’N, 73°10’E), experimental plastic pots were kept underneath the greenhouse. The Green ball, Dilpasand, Durga, round Gold, and Indian desi genotypes genetic material was acquired from a reputable breeding research facility in Faisalabad, Pakistan. Data were compared with the control (EC of the growth medium was kept at 1.5 dS m-1) after seedlings were treated with 2, 4, 6, and 8 dS m-1 of sodium chloride. These levels were induced by adding sodium chloride in the growing medium and were maintained by daily confirming with an EC meter (DEC-2-4340). EC was induced by following a formula.
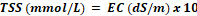
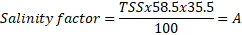
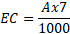
Where EC is electrical conductivity, TSS is total soluble salts, 35.5% is the saturation percentage of salts in the sand, and 58.5 is the molar mass of sodium chloride. 7 is the weight (kg) of sand in one pot.
In each pot (plastic pot with 25 cm x 10 cm dimension) four seedlings were maintained in the sand as a growing medium. NaCl concentrations were applied and EC was established after thirty days of seed sowing and was replicated thrice. The salinity was produced gradually so that it may not injure the plants. We measured morphological characteristics such length of seedlings, root length, the total length of the seedlings, total seedlings’ fresh biomass (SFB), and total seedling s’ dry biomass (SDB). Similar to this, SPAD measuring device (CCM-200plus Bio- Scientific USA). SPAD values were used to directly evaluate physiological traits including chlorophyll concentration. Additionally, the ionic contents of nitrogen, phosphorous, potassium, calcium, and sodium were determined.
2.1 Chemical analysis
The most influential ionic content in seedlings i.e., nitrogen, phosphorus, potassium, sodium, and calcium concentrations were determined using the Kjeldahl and wet digestion procedures, as per Kjeldahl’s (1883). Furthermore, N+, P, K+, Na+, and Ca2+ ions were measured by Flame Photometer (Sherwood Flame Photometer Model-410), and actual values of nutrients were calculated from the standard curve.
2.2 Hoagland solution
Hoagland solution (Table 1) was prepared by adding various chemicals already present in the stake solutions according to Hoagland and Arnon (1950) and to avoid its negative influence its pH value was kept between 6 and 6.5 by measuring with a pH meter (Model Genway, 3510 USA).
Table 1: Macro and micronutrients composition of Hoagland solution applied during the experiment.
|
Reagent |
Stock (g/L) |
ml of stock soln. for 10L ½ conc. |
ml of stock soln. for 200L ½ conc. |
|
Macro nutrients |
|||
|
KH2PO4 |
136 |
5 |
100 |
|
KNO3 |
101 |
25 |
500 |
|
Ca(NO3)2.4H2O |
236 |
25 |
500 |
|
MgSO4.7H2O |
246 |
10 |
200 |
|
Micro nutrients |
|||
|
H3BO3 |
2.86 |
5 |
100 |
|
MnCl2.4H2O |
1.81 |
5 |
100 |
|
ZnSO4.7H2O |
0.22 |
5 |
100 |
|
CuSO4.5H2O |
0.08 |
5 |
100 |
|
H2MoO4.H2O |
0.02 |
5 |
100 |
|
Fe-EDTA |
37.33 |
5 |
100 |
2.3 Statistical analysis
The research plan was executed according to Complete Randomized Design (CRD) with 2-factor factorial studies with three replications. Statistical significance among the treatment means was evaluated by ANOVA (LSD) techniques at P ≤ 0.05.
2.4 Morphological attributes
The plants that were grown under control conditions got maximum seedling height (SH) (3.12 cm), followed by the plants grown at 2 dS m-1 (2.82 cm), 4 dS m-1 (2.47 cm), and 6 dS m-1 (2.17 cm), while minimum plant height was observed at 8 dS m-1 (1.90 cm). It was observed that the ‘Round gold’ genotype exhibited the longest SH (3.18 cm), followed by ‘Dilpasand’ (2.56 cm), ‘Indian desi’ (2.51 cm), ‘Durga’ (2.29 cm), and its maximum value of SH was seen in plants of ‘Green ball’ (2.90 cm). Combined data analysis of EC levels in the growing medium and genotypes was also significant. On an overall basis, the longest SH was observed at the control and 4 dS m-1 (3.54 cm) in ‘Round gold’, and the least SH in ‘Green ball’ (1.71 cm) at 8 dS m-1, respectively regarding sodium chloride-induced salinity (Figure 1).
Control (no salt application) revealed a significantly higher root length (RL) (5.77 cm), while the minimum RL was found at the highest salt level 8 dS m-1 (3.93 cm). Whereas, 2 dS m-1 (5.28 cm), 4 dS m-1 (4.59 cm), 6 dS m-1 (4.16 cm) decreased RL with increasing concentration, respectively. The genotype ‘Round gold’ exhibited the longest RL (5.58 cm), followed by ‘Indian desi’ (5.38 cm), ‘Durga’ (4.69 cm), ‘Dilpasand’ (4.69 cm), and the shortest RL was observed for ‘Green ball’ (3.44 cm). Combined data analysis of EC levels in the growing medium and genotypes was also significant. On an overall basis, the longest RL was observed at control (8.33 cm), followed by 2 dS m-1 (6.47 cm), 4 dS m-1 (5.33 cm), 6 dS m-1 (4.50 cm), and 8 dS m-1 (4.63 cm), respectively. The least value of RL was observed in ‘Green ball’ at 8 dS m-1 (2.74 cm), while ‘Round gold’ showed a significantly higher value at control (8.33 cm) regarding NaCl-induced salinity (Figure 2).
Plant fresh biomass was influenced by salt stress. The maximum seedling fresh biomass (SFB) was observed under control conditions (1.37 g) and the minimum SFB at 2 dS m-1 (1.21 g), followed by 4, 6, and 8 dS m-1, respectively. ‘Round gold’ genotype showed greater seedling fresh biomass (1.42 g) as compared to other genotypes. ‘Round gold’ showed the maximum SFB at the highest salinity application. Combined data analysis of EC levels in the growing medium and genotypes was also significant (Figure 3).
The minimum value of seedling dry mass (SDB) ‘Green ball’ (0.33 g), followed by ‘Indian desi’ (0.47 g) and ‘Durga’ (0.47 g), respectively. The maximum SDB was found in control (0.67 g) and minimum SDB at 8 dS m-1 (0.34 g), respectively. Thus, the influence of the highest salinity level 8 dS m-1 was more prominent. Combined data analysis of EC levels in the growing medium and genotypes was also significant. The maximum SDB (0.83 g) was observed in ‘Round gold’ at 2 dS m-1 NaCl. ‘Indian desi’ genotype at the highest salinity level 8 dS m-1 (0.34 g). ‘Round gold’ was highly influenced by all treatments than other genotypes (Figure 4).
2.5 Physiological attributes
The highest chlorophyll contents index (CCI) was found at the control (61.56 SPAD index) and the lowest at 8 dS m-1 (46.43 SPAD index). ‘Round gold’ showed the highest CCI (58.44 SPAD index) under salinity stress, after that ‘Durga’, ‘Dilpasand’, and ‘Indian desi’ (53.38 SPAD index), respectively. Whereas, the minimum CCI were exhibited by ‘Green ball’ (50.55 SPAD index). Combined data analysis of EC levels in the growing medium and genotypes was also significant. The maximum CCI were observed at control (without salt application), and the minimum at the highest level of sodium chloride application in the growing medium, while it was recorded that the minimum SPAD value was observed in ‘Green ball’ (46.43 SPAD index). At all salinity levels, all CCI were reduced in all genotypes but the maximum reduction of CCI was observed in ‘Green ball’ at 8 dS m-1 (46.43 SPAD index) (Table 1).
2.6 Ionic attributes
A significant difference was observed among the means of genotypes regarding NaCl-induced salinity. ‘Round gold’ (0.50 mg g-1) revealed the highest, whereas, ‘Green ball’ (0.43 mg g-1) least.
Table 2: Effect of various levels of salt stress on chlorophyll contents (SPAD index) of selected tinda gourd cultivars.
|
Treatments |
Green ball |
Dilpasand |
Durga |
Round gold |
Indian desi |
Mean |
|
Control |
53.34±2.0 |
59.88±2.1 |
58.87±2.1 |
61.57±2.7 |
57.66±2.2 |
58.26 a |
|
2 dS m-1 NaCl |
52.22±2.3 |
55.28±2.4 |
56.88±2.1 |
60.55±2.1 |
55.56±2.1 |
56.09 b |
|
4 dS m-1 NaCl |
50.32±2.1 |
53.33±2.4 |
55.14±2.1 |
58.68±1.9 |
53.35±2.1 |
54.16 b |
|
6 dS m-1 NaCl |
48.44±2.1 |
52.23±2.3 |
54.67±2.5 |
56.77±1.8 |
51.02±2.9 |
52.63 bc |
|
8 dS m-1 NaCl |
46.43±2.2 |
51.33±2.0 |
50.34±2.7 |
54.66±2.1 |
49.35±2.4 |
50.81 c |
|
Mean |
50.55 c |
54.41 b |
55.18 b |
58.44 a |
53.39 bc |
In a row or column, same lettering depicts non-significant difference, while different lettering depicts significant difference (Tukey HSD Test at 0.05)
Table 3: Effect of various levels of salt stress on nitrogen contents of selected tinda gourd cultivars.
|
Treatments |
Green ball |
Dilpasand |
Durga |
Round gold |
Indian desi |
Mean |
|
Control |
0.45±0.02 |
0.50±0.01 |
0.50±0.01 |
0.50±0.01 |
0.45±0.02 |
0.48 a |
|
2 dS m-1 NaCl |
0.45±0.01 |
0.40±0.01 |
0.45±0.02 |
0.50±0.02 |
0.45±0.01 |
0.45 ab |
|
4 dS m-1 NaCl |
0.45±0.02 |
0.45±0.04 |
0.45±0.03 |
0.50±0.01 |
0.40±0.01 |
0.45 ab |
|
6 dS m-1 NaCl |
0.40±0.01 |
0.45±0.02 |
0.45±0.03 |
0.45±0.02 |
0.40±0.05 |
0.43 b |
|
8 dS m-1 NaCl |
0.40±0.03 |
0.40±0.02 |
0.40±0.03 |
0.40±0.02 |
0.40±0.03 |
0.400 c |
|
Mean |
0.43 |
0.44 ab |
0.4500 b |
0.47a |
0.42 ab |
In a row or column, same lettering depicts non-significant difference, while different lettering depicts significant difference (Tukey HSD Test at 0.05).
Combined data analysis of EC levels in the growing medium and genotypes was also significant. NaCl-induced salt stress negatively influenced the nitrogen concentration so the lowest nitrogen contents were noted in the ‘Green ball’ (0.43 mg g-1) at 8 dS/m-1. The highest concentration of nitrogen was verified in the ‘Round gold’ genotype (0.50 mg g-1) at control regarding NaCl-induced salinity (Table 2).
Round gold exhibited the maximum phosphorous concentration in plants, while the lowest concentration was found in ‘Green ball’ under NaCl-induced salt stress. The highest value of phosphorous was noted at 6 dS/m-1 (0.952 mg g-1), while the lowest value was observed at 8 dS/m-1 (0.590 mg g1). Combined data analysis of EC levels in the growing medium and genotypes was also significant. The lowest phosphorous concentration was noted in the ‘Green ball’ (0.041 mg g-1) at 8 dS/m-1. The highest concentration of phosphorous was present in ‘Round gold’. (1.01 mg g-1) at control comparing NaCl-induced salinity (Table 3).
Under NaCl-induced salinity the maximum potassium concentration in plants was seen in ‘Round gold’ (24.1 mg g-1), and lowest in ‘Green ball’ (10.2 mg g-1), however, potassium concentration in ‘Indian desi’ and ‘Dilpasand’ was observed as non-significant. Combined data analysis of EC levels in the growing medium (owing to the different levels of uptake in genotypes) and genotypes was also significant. The highest potassium concentration in the ‘Round gold’ genotype (16.2 mg g-1) was maximum at control regarding NaCl-induced salt stress (Table 4).
The ionic concentration of calcium in plants declines with an increase under NaCl-induced salinity. The maximum decline in Ca+ concentration was seen in ‘Green ball’ (0.037 m eqv L-1) at 8 dS/m-1 as compared to its respective control (0.09 m eqv L-1) and as compared with other genotypes. Combined data analysis of EC levels in the growing medium and genotypes was also significant (Table 5).
It was depicted that sodium ion concentrations were maximum at the highest level of salinity (8 dS/m-1) and decreased with a decrease in the level of sodium ion concentration i.e., 6 dS/m-1 (11 µg g-1), 4 dS/m-1 (10.08 µg g-1), 2 dS/m-1 (9.54 µg g-1), and control (6.66 µg g-1). Regarding genotypes, its concentration was highest in ‘Round gold’ (10.34 µg g-1). Combined data analysis of EC levels in the growing medium and genotypes was also significant. On an overall basis, ‘Round gold’ (12.1 µg g-1) got maximum followed by ‘Indian desi’ (11.7 µg g-1) at the highest level of salinity (8 dS/m-1) with respect to control (6.9 µg g-1). It was revealed that the lowest ionic sodium concentration was observed in the ‘Green ball’ (6.3 µg g-1) at the same salinity level as compared to other genotypes under NaCl-induced salinity (Table 6).
Table 4: Effect of various levels of salt stress on phosphorous contents of selected tinda gourd cultivars.
|
Treatments |
Green ball |
Dilpasand |
Durga |
Round gold |
Indian desi |
Mean |
|
Control |
0.089±0.004 |
0.096±0.002 |
0.094±0.007 |
0.101±0.002 |
0.096±0.002 |
0.095 a |
|
2 dS m-1 NaCl |
0.072±0.002 |
0.079±0.004 |
0.081±0.002 |
0.099±0.005 |
0.087±0.004 |
0.083 ab |
|
4 dS m-1 NaCl |
0.640±0.003 |
0.067±0.005 |
0.076±0.002 |
0.090±0.003 |
0.078±0.001 |
0.075 b |
|
6 dS m-1 NaCl |
0.059±0.003 |
0.064±0.005 |
0.071±0.002 |
0.081±0.004 |
0.065±0.005 |
0.068 bc |
|
8 dS m-1 NaCl |
0.041±0.005 |
0.058±0.004 |
0.066±0.004 |
0.074±0.002 |
0.056±0.003 |
0.059 c |
|
Mean |
0.065 c |
0.0782 b |
0.0776 b |
0.089 a |
0.076 b |
In a row or column, same lettering depicts non-significant difference, while different lettering depicts significant difference (Tukey HSD Test at 0.05).
Table 5: Effect of various levels of salt stress on potassium contents of selected tinda gourd cultivars.
|
Treatments |
Green ball |
Dilpasand |
Durga |
Round gold |
Indian desi |
Mean |
|
Control |
18.2±0.91 |
21.9±0.89 |
21.4±0.91 |
24.1±0.92 |
22.7±0.84 |
21.66 a |
|
2 dS m-1 NaCl |
17.7±0.72 |
19.7±0.99 |
19.1±0.94 |
22.6±0.93 |
20.7±0.90 |
19.96 b |
|
4 dS m-1 NaCl |
16.6±0.94 |
17.9±0.97 |
18.8±0.88 |
20.0±0.72 |
19.3±0.96 |
18.52 b |
|
6 dS m-1 NaCl |
13.7±0.67 |
17.1±0.78 |
15.1±0.87 |
18.3±0.59 |
18.8±0.92 |
16.6 bc |
|
8 dS m-1 NaCl |
10.2±0.98 |
16.1±0.93 |
14.1±0.99 |
16.2±0.59 |
14.3±0.58 |
14.18 c |
|
Mean |
15.28 c |
18.54 bc |
17.7 bc |
20.24 a |
19.16 b |
In a row or column, same lettering depicts non-significant difference, while different lettering depicts significant difference (Tukey HSD Test at 0.05).
Table 6: Effect of various levels of salt stress on calcium contents of selected tinda gourd cultivars.
|
Treatments |
Green ball |
Dilpasand |
Durga |
Round gold |
Indian desi |
Mean |
|
Control |
0.080±0.004 |
0.090±0.002 |
0.100±0.002 |
0.098±0.001 |
0.088±0.003 |
0.091 a |
|
2 dS m-1 NaCl |
0.059±0.002 |
0.066±0.002 |
0.090±0.003 |
0.086±0.002 |
0.062±0.005 |
0.07 ab |
|
4 dS m-1 NaCl |
0.049±0.001 |
0.059±0.005 |
0.077±0.004 |
0.083±0.003 |
0.058±0.003 |
0.065 b |
|
6 dS m-1 NaCl |
0.040±0.005 |
0.050±0.008 |
0.056±0.006 |
0.078±0.002 |
0.050±0.001 |
0.054 bc |
|
8 dS m-1 NaCl |
0.036±0.003 |
0.037±0.003 |
0.047±0.003 |
0.067±0.005 |
0.049±0.002 |
0.047 c |
|
Mean |
0.053 c |
0.060 b |
0.074 ab |
0.082 a |
0.061 b |
In a row or column, same lettering depicts non-significant difference, while different lettering depicts significant difference (Tukey HSD Test at 0.05).
Table 7: Effect of various levels of salt stress on sodium contents (µg g-1) of selected tinda gourd cultivars.
|
Treatments |
Green ball |
Dilpasand |
Durga |
Round gold |
Indian desi |
Mean |
|
Control |
6.9±0.41 |
6.7±0.54 |
6.6±0.67 |
6.3±0.45 |
6.8±0.47 |
6.66 e |
|
2 dS m-1 |
10.2±0.51 |
9.2±0.76 |
9.6±0.68 |
9.2±0.56 |
9.5±0.51 |
9.54 d |
|
4 dS m-1 |
10.6±0.55 |
9.8±0.59 |
10.4±0.59 |
9.4±0.45 |
10.2±0.53 |
10.08 c |
|
6 dS m-1 |
11.9±0.29 |
11.7±0.59 |
11.4±0.58 |
9.6±0.34 |
10.4±0.40 |
11.0 b |
|
8 dS m-1 |
12.1±0.36 |
11.7±0.66 |
11.8±0.72 |
10.1±0.50 |
11.8±0.43 |
11.5 a |
|
Mean |
10.34 a |
9.82 b |
9.96 b |
8.92 c |
9.74 b |
In a row or column, same lettering depicts non-significant difference, while different lettering depicts significant difference (Tukey HSD Test at 0.05).
3. Results and Discussion
Genetic diversity exploration is a dynamic tool for the tolerance of salts in the soil, and plant adopts various strategies to cope with this (Shahbaz et al., 2012). The main threat of sodium chloride is sodium ions which affect the growth and productivity of Cucurbitaceae vegetables because of loss of water balance (Ghani et al., 2018). In the present research, different genotypes of tinda gourd responded differently to various salt levels applied under consideration due to different genetic behavior. HKT, a Na+/K+ symporter that controls the movement of Na+ and K+ through the membrane of plant cells, may be adopted by genotypes that are tolerant to it. The HKT transporter in rice eliminates excess Na+ from the xylem in heat-tolerant genotypes, shielding the photosynthetic leaf tissues from Na+ harmful effects (Schroeder et al., 2013). Outcomes of peas under saline conditions by Ahmad and Riffat (2005) support current results as high sodium chloride concentrations reduced fresh and dry mass of leaves and roots which might be due to hindrance of K+ uptake. Similarly, plant height and root length were affected by salinity (Qu et al., 2012).
Tolerant genotypes have more salinity tolerance linked gene networks (Soda et al., 2013). Salinity is becoming most dangerous for plant growth and development (Kumar, 2020). Similar results were observed in the Basil plant under salinity with the lessening of chlorophyll content along with a decrease in root growth and development (Heidari, 2012). It was also revealed previously that chlorophyll content (both a and b) is destroyed with the enhancement of salt stress in the cucumber plant (Shu et al., 2012). Tolerant genotypes keep their chlorophyll a/b ratio under salt-stress conditions (Senguttuvel et al., 2014). When under salt stress, mitogen-activated protein kinase (MAPK) is stimulated, which results in the generation of reactive oxygen species (ROS) (Jammes et al., 2009).
With the enhancement in the salt stress situation, a significant difference was explored in heat-sensitive and heat-tolerant genotypes as described by Maiti et al. (2010). This might be due to fact of reduction of plant height with the enhancement of toxic salts due to shrinkage of cell walls. Abid et al. (2002) supported this statement by observing the reduction of plant height in okra genotypes against salinity stress. Current research also strengthens the finding of Maiti et al. (2008), who reported that vegetables are affected by salt stress.
These findings of chlorophyll contents are in association with the literature of Heidari et al. (2004) who stated that NaCl has adverse effects on photosynthesis, chlorophyll, fluorescence, and their components. Plant growth of tinda gourd ceased due to the non-availability of carbohydrates as photosynthesis is affected because K+ is impaired and replaced by toxic nutrients as the opening and closing of stomata are affected. Every species of plant has a different mechanism of salt tolerance owing to K+ uptake (Pakniyat and Armion, 2007). Negrão et al. (2017) justify these ionic and physiologically negative responses.
A few plants can tolerate salt stress due to the selective nature of plasma membranes to uptake potassium ions. Ultimately uptake of potassium and phosphorous was higher in salt-tolerant plants. So according to data, ‘Round gold’ had a higher amount of phosphorous and potassium than others (Shaheen et al., 2013). Sodium ions are a major source of osmotic stress (Tavakkoli et al., 2011). Results depicted the screening would help alleviate the salt stress along with various techniques adopted by previous researchers (Acosta-Motos et al., 2017). Salinity disturbs the osmotic balance of tinda gourd genotypes and causes stunted growth and development. Expression of effectors and regulatory genes can improve plant tolerance to various abiotic stresses including salinity (Fita et al., 2015).
Conclusions and Recommendations
Based on the above results, five genotypes of tinda gourd under the research trial were categorized into three groups against NaCl-induced salinity. ‘Round gold’ has more potential for salt stress, ‘Dilpasand’, ‘Indian desi’, and ‘Durga’ were moderately tolerant to NaCl-induced salinity, while the ‘Green ball’ was sensitive to salinity stress. ‘Round gold’ should be cultivated under NaCl salt-affected areas of the world to obtain the maximum yield.
Acknowledgments
The author/co-authors are pleased with the financial assistance of the Higher Education Commission (HEC), Islamabad provided under the vegetable salinity project.
Novelty Statement
The previous studies provided insufficient information for better growth of gourds under saline conditions in the country but the current study would provide sufficient management practices for better growth and development of tinda gourd genotypes.
Author’s Contribution
Muhammad Zeeshan Siddiqui: conducted the research experiment and collected research data.
Mujahid Ali: guided about experimental layout, and statistical, analysis of results.
Shoaib ur Rehman: supervised the research work.
Shahid Iqbal and Malik Abdur Rehman: reviewed the research article.
Saqib Ayyub, Hafiz M. Tayyab Khan, Muhammad Ali, Muhammad Azher Nawaz: they helped in the write-up of the article regarding introduction, objectives, references write-up, and presentation styles etc.
Conflict of interest
The authors have declared no conflict of interest.
References
Abid, M., Ahmad, F., Ahmad, N. and Ahmad, I., 2002. Effect of phosphorous on growth, yield and mineral composition of wheat in different textured saline-sodic soils. Asian Journal of Plant Sciences, 1: 472-475. https://doi.org/10.3923/ajps.2002.472.475
Acosta-Motos, J.R., Ortuño, M.F., Bernal-Vicente, A., Diaz-Vivancos, P., Sanchez-Blanco, M.J. and Hernandez, J.A., 2017. Plant responses to salt stress: Adaptive mechanisms; Adoptive mechanism. Agronomy. 7(1): 18. https://doi.org/10.3390/agronomy7010018
Afzal, L., Basra, S.M.A., Ahmad, N. and Farooq, M., 2005. Optimization of hormonal priming techniques for elevation of salinity in chilies (Capcicum annum L.). Caderno de Pesquisa Ser. Bio. Santa. Cruz. do Sul. 17: 95-109.
Ahmad, P. and Riffat, J., 2005. Effect of salt stress on growth and biochemical parameters of Pisum sativum L. Journal of Agronomy and Soil Science., 51: 665-672. https://doi.org/10.1080/03650340500274151
Arif, Y., Singh, P., Siddiqui, H., Bajguz, A. and Hayat, S., 2020. Salinity induced physiological and biochemical changes in plants: An omic approach towards salt stress tolerance. Plant Physiology and Biochemistry. 156: 64-77. https://doi.org/10.1016/j.plaphy.2020.08.042
Balkaya, A., 2016. Effects of salt stress on vegetative growth parameters and ion accumulations in cucurbit rootstock genotypes. Ekin Journal of Crop Breeding and Genetics, 2(2): 11-24.
Edelstein, M., Plaut, Z. and Ben-Hur, M., 2011. Sodium and chloride exclusion and retention by non-grafted and grafted melon and Curcubita plants. Journal of Experimental Botany. 62: 177-184. https://doi.org/10.1093/jxb/erq255
Elsheery, N.I., Helaly, M.N., Omar, S.A., John, S.V., Zabochnicka-Swiątek, M., Kalaji, H.M. and Rastogi, A., 2020. Physiological and molecular mechanisms of salinity tolerance in grafted cucumber. South African Journal of Botany. 130: 90-102. https://doi.org/10.1016/j.sajb.2019.12.014
Ferreira, F.N., de Lima, G.S., Gheyi, H.R., da Silva Sá, F.V., Dias, A.S. and dos Anjos Soares, L.A., 2022. Production and post-harvest quality of custard apple irrigated with saline water and fertilized with NPK. Comunicata Scientiae, 13: 1-9.
Fita, A., Rodríguez-Burruezo, A., Boscaiu, M., Prohens, J. and Vicente, O., 2015. Breeding and domesticating crops adapted to drought and salinity: A new paradigm for increasing food production. Frontiers in Plant Science. 6: 978. https://doi.org/10.3389/fpls.2015.00978
Fu, C., Khan, M.N., Yan, J., Hong, X., Zhao, F., Chen, L., Ma, H., Li, Y., Li, J., and Wu, H., 2023. Mechanisms of nanomaterials for improving plant salt tolerance. Crop and Environment. https://doi.org/10.1016/j.crope.2023.03.002
Ghani, M.N.O., Awang, Y., and Ismail, M.F., 2018. Effects of NaCl salinity on leaf water status, proline and mineral ion content of four Cucurbitaceae species. Australian Journal of Crop Science. 12(9): 1434. https://doi.org/10.21475/ajcs.18.12.09.PNE1113
GOP (Government of Pakistan). 2019. MNSFR (Ministry of national food security and research economic). Fruit, vegetables and condiments statistics of Pakistan.
Hasegawa, P. M., Bressan, R.A., Zhu, J.K. and Bohnert, H.J., 2000. Plant cellular and molecular responses to high salinity. Annual Review of Plant Biology. 51(1): 463-499. https://doi.org/10.1146/annurev.arplant.51.1.463
Heidari, A., Bandehagh, A. and Toorchi, M., 2004. Effects of NaCl stress on chlorophyll content and chlorophyll fluorescence in Sunflower (Helianthus annuus L.) Lines. Yüzüncü Yıl Üniversitesi Tarım Bilimleri Dergisi. 24(2): 111-120. https://doi.org/10.29133/yyutbd.235924
Heidari, M., 2012. Effects of salinity stress on growth, chlorophyll content and osmotic components of two basil (Ocimum basilicum L.) genotypes. African Journal of Biotechnology. 11(2): 379-384. https://doi.org/10.5897/AJB11.2572
Hoagland, D.R. and Arnon, D.I., 1950. The water-culture method for growing plants without soil. California Agricultural Experiment Station, Circular-347.
İbrahimova, U., Kumari, P., Yadav, S., Rastogi, A., Antala, M., Suleymanova, Z., Zivack, M., Tahjib-ulArif, Md., Hussain, S., Abdelhamid, M., Hajihashemi, S., Yang, S. and Brestic, M., 2021. Progress in understanding salt stress response in plants using biotechnological tools. Journal of Biotechnology. 329: 180-191. https://doi.org/10.1016/j.jbiotec.2021.02.007
Jammes, F., Song, C., Shin, D., Munemasa, S., Takeda, K., Gu, D., Cho, D., Lee, S., Giordo, R., Sritubtim, S., Leonhardt, N., Ellis, B.E., Murata, Y. and Kwak, J.M., 2009. MAP kinases MPK9 and MPK12 are preferentially expressed in guard cells and positively regulate ROS-mediated ABA signaling. Proceedings of the National Academy of Sciences, U.S.A. 106: 20520–20525. https://doi.org/10.1073/pnas.0907205106
Jung, K., Ok, Y.S. and Chang, S.X., 2011. Sulfate adsorption properties of acid-sensitive soils in the Athabasca oil sands region in Alberta, Canada. Chemosphere. 84: 457-463. https://doi.org/10.1016/j.chemosphere.2011.03.034
Kamran, M., Parveen, A., Ahmar, S., Malik, Z., Hussain, S., Chattha, M.S. and Chen, J.T., 2020. An overview of hazardous impacts of soil salinity in crops, tolerance mechanisms, and amelioration through selenium supplementation. International Journal of Molecular Sciences. 21(1): 148. https://doi.org/10.3390/ijms21010148
Kjeldahl, J., 1883. New Method for the Determination of Nitrogen. Chem. News, 48(1240): 101–102.
Kumar, S.B.P., 2020. Salinity stress, its physiological response and mitigating effects of microbial bio inoculants and organic compounds. Journal of Pharmacognosy and Phytochemistry, 9(4): 1397-1303.
Maiti, R.K., Vidyasagar, P., Umashankar, P., Guptal, A., Rajkumar, D. and Gonzalez-Rodríguez, H., 2010. Genotypic variability in salinity tolerance of some vegetable crop species at germination and seedling stage. International Journal of Bio-resource and Stress Management. 23: 204-209.
Maiti, R., Vidyasagar, P. and Banerjee, P.P., 2008. Characterization of salinity tolerance in rice (Oryza sativa L.) genotypes at the germination and seedling stages. Acta Agronomica Hungarica, 56(2): 139-147· https://doi.org/10.1556/AAgr.56.2008.2.3
Moldakimova, N.A., Mukiyanova, G.S., Yarmolinsky, D.G., Brychkova, G.G. Scholthof, H.B., Sagi, M. and Omarov, R.T., 2012. Effect of salinity on viral disease spread in plants. Journal of Stress Physiololgy and Biochemistry. 8: 3-17.
Munns, R. and Tester, M., 2008. Mechanism of salinity tolerance. Annual Review of Plant Biology, 59: 651–681. https://doi.org/10.1146/annurev.arplant.59.032607.092911
Munns, R., Day, D.A., Fricke, W., Watt, M., Arsova, B., Barkla, B.J., Bose, J., Byrt, C.S., Chen, Z.H., Foster, K.J., Gilliham, M., Handerson, S.W., Jenkins, C.L.D., Kronzucker, H.R., Miklavcic, S.J., Plett, D., Roy, S.J., Shahabla, S., Magan C., Sheldon, K.L., Soole, N.L., Tylor, M., Tester, S., Wege, L.H., Wegner, S.D. and Tyerman, S.D., 2020. Energy costs of salt tolerance in crop plants. New Phytologist, 225(3): 1072-1090. https://doi.org/10.1111/nph.15864
Negrão, S., Schmöckel, S.M. and Tester, M., 2017. Evaluating physiological responses of plants to salinity stress. Annals of Botany. 119(1): 1-11. https://doi.org/10.1093/aob/mcw191
Neue Methode zur Bestimmung des Stickstoffs in organischen Korpern. Z. Anal. Chem. 1883. 22, 366–382; En ny Methode til Kvaelstofbestemmelsei organiske Stoeffer. Medd. Carlsberg Lab. 1883, 2 (1), 1–27; Sur une Nouvelle Methode de Dosage de l’Azote dans les Substances Organiques ´ (French summary: Resume du CR Trav. Lab. Carlsberg’; separately paged section) 1883, 2: 1–12. https://doi.org/10.1007/BF01338151
Pakniyat, H. and Armion, M., 2007. Sodium and proline accumulation as osmoregulators in tolerance of sugar beet genotypes to salinity. Pakistan Journal of Biological Sciences. 10(22): 4081-4086. https://doi.org/10.3923/pjbs.2007.4081.4086
Parihar, P., Singh, S., Singh, R., Singh, V.P. and Prasad, S.M., 2015. Effect of salinity stress on plants and its tolerance strategies: a review. Environmental Science and Pollution Research. 22(6): 4056-4075. https://doi.org/10.1007/s11356-014-3739-1
Qu, C., Liu, C., Gong, X., Li, C., Hong, M., Wang, L. and Hong, F., 2012. Impairment of maize seedling photosynthesis caused by a combination of potassium deficiency and salt stress. Environmental and Experimental Botany. 75: 134–141. https://doi.org/10.1016/j.envexpbot.2011.08.019
Roy, S.J., Negrao, S. and Tester, M., 2014. Salt resistant crop plants. Current Opinion in Biotechnology. 26: 115-124. https://doi.org/10.1016/j.copbio.2013.12.004
Schroeder, J.I., Delhaize, E., Frommer, W.B., Guerinot, M.L., Harrison, M.J. and Harrera-Estrella, L., Horie, T., Kochian, L.V., Munns, R., Nishizawa, N.K., Tsay, Y.F. and Sanders, D., 2013. Using membrane transporters to improve crops for sustainable food production. Nature. 497: 60–66. https://doi.org/10.1038/nature11909
Senguttuvel, P., Vijayalakshmi, C., Thiyagarajan, K., Kannanbapu, J.R., Kota, S. and Admavathi, G., 2014. Changes in photosynthesis, chlorophyll fluorescence, gas exchange parameters and osmotic potential to salt stress during early seedling stage in rice (Oryza sativa L.). SABRAO Journal of Breeding and Genetics. 46: 120–135.
Shah, I.H., Manzoor, M.A., Sabir, I.A., Ashraf, M., Haq, F., Arif, S., Abdullah, M., Niu, Q. and Zhang, Y., 2022. Genome-wide identification and comparative analysis of MATE gene family in Cucurbitaceae species and their regulatory role in melon (Cucumis melo) under salt stress. Horticulture, Environment, and Biotechnology, 63(4): 595-612. https://doi.org/10.1007/s13580-021-00413-3
Shahbaz, M., Ashraf, M., Al-Qurainy, F. and Harris. P.J., 2012. Salt tolerance in selected vegetable crops. Critical Reviews in Plant Sciences. 31(4): 303-320. https://doi.org/10.1080/07352689.2012.656496
Shaheen, S., Naseer, S., Ashraf, M. and Akram, N.A., 2013. Salt stress affects water relations, photosynthesis, and oxidative defense mechanisms in Solanum melongena L. Journal of Plant Interactions. 8(1): 85-96. https://doi.org/10.1080/17429145.2012.718376
Shu, S., S. R. Guo, J. Sun and L. Y. Yuan., 2012. Effects of salt stress on the structure and function of the photosynthetic apparatus in Cucumis sativus and its protection by exogenous putrescine. Physiologia Plantarum, 146(3): 285-296. https://doi.org/10.1111/j.1399-3054.2012.01623.x
Singh, H., Kumar, P., Kumar, A., Kyriacou, M.C., Colla, G., and Rouphael, Y., 2020. Grafting tomato as a tool to improve salt tolerance. Agronomy. 10(2): 263. https://doi.org/10.3390/agronomy10020263
Soda, N., Kushwaha, H.R., Soni, P., Singla–Pareek, S.L. and Pareek, A., 2013. A suite of new genes defining salinity stress tolerance in seedlings of contrasting rice genotypes. Funct. Integr. Genomics. 13: 351–365. https://doi.org/10.1007/s10142-013-0328-1
Tavakkoli, E. Fatehi, F., Coventry, S., Rengasamy, P. and McDonald, G.K., 2011. Additive effects of Na+ and Cl– ions on barley growth under salinity stress. Journal of Experimental Botany, 62(6): 2189-2203. https://doi.org/10.1093/jxb/erq422
Toscano, S., Romano, D. and Ferrante, A., 2023. Molecular responses of vegetable, ornamental crops, and model plants to salinity stress. International Journal of Molecular Sciences, 24(4): 3190. https://doi.org/10.3390/ijms24043190
Velmurugan, A., Swarnam, P., Subramani, T., Meena, B. and Kaledhonkar, M., 2020. Water demand and salinity. (Eds) Farhani, M.H.D.A., Vatanpu, V. and Taheri, A.H., Desalination-challenges and opportunities. Book on Demand BOD Publisher, UK, 130. https://doi.org/10.5772/intechopen.88095
Yang, Y., Lu, X., Yan, B., Li, B., Sun, J., Guo, S. and Tezuka, T., 2013. Bottle gourd rootstock-grafting affects nitrogen metabolism in NaCl- stressed watermelon leaves and enhances short-term salt tolerance. Journal of Plant Physiology. 170(7): 653-661. https://doi.org/10.1016/j.jplph.2012.12.013
To share on other social networks, click on any share button. What are these?





