Exogenous Application of Proline to Enhance Rice Tolerance against Heat and Drought Stresses
Research Article
Sajid Hanif1, Abdul Shakoor1,4*, Muhammad Farrukh Saleem1, Ifra Saleem2, Sajid Ali3, Muhammad Awais Ashraf4, Majid Nadeem4, Hira Shair4, Anwar ul Haq5, Rana Abdul Hamid Khan6 and Muhammad Amir Amin7
1Department of Agronomy, University of Agriculture, Faisalabad, 38040, Pakistan; 2Soil Chemistry Section, Institute of Soil Chemistry and Environmental Science, Ayub Agricultural Research Institute Faisalabad, Punjab, Pakistan; 3Vegetable Research Institute Ayub Agricultural Research Institute Faisalabad, Punjab, Pakistan; 4Wheat Research Institute, Ayub Agricultural Research Institute Faisalabad, Punjab, Pakistan; 5Soil and Water Testing Laboratory, Pakpattan, Punjab, Pakistan; 6Maize and Millets Research Institute, Yousaf Wala Sahiwal, Punjab, Pakistan; 7Pulses Research Institute, Ayub Agricultural Research Institute Faisalabad, Punjab, Pakistan.
Abstract | Environmental stresses and changing climate scenarios are the foremost intimidation to sustainable crop production. Among abiotic stresses, heat and drought stresses instigate substantial rice yield reductions. A pot trial was laid out to observe the efficacy of foliar spray of proline on rice in the alleviation of both heat and drought effects in rice. Pot experiment was laid out in Kharif season 2018 inside the glasshouse of University of Agriculture Faisalabad. Treatments viz., control (no stress imposition) and stress treatments viz., drought; heat and combination of drought + heat stress effects were imposed at anthesis. Three different levels of foliar-applied proline included water spray, 10, 20 and 30-mM concentrations. Combined heat and drought stress increased unfertile tillers, unfilled grains and abortive, chalky and opaque kernels, along with declines in chlorophyll and relative leaf water contents (14% over control) the most than individual stress imposition. A 29.76% increment in yield was observed as comparison to to (control) by the use of exogenous proline and its quality attribute due to improved chlorophyll and relative leaf water contents (10% increase) alongside reductions in undesired quality parameters like chalkiness, opaqueness and abortive kernels. Concurrent heat and drought stress were the most hazardous as comparison with individual stress and 30 mM proline utilization gave more amelioration against stress.
Received | July 16, 2021; Accepted | March 20, 2022; Published | June 28, 2022
*Correspondence | Abdul Shakoor, Department of Agronomy, University of Agriculture, Faisalabad, 38040, Pakistan; Email: shakoor2914@gmail.com
Citation | Hanif, S., A. Shakoor, M.F. Saleem, I. Saleem, S. Ali, M.A. Ashraf, M. Nadeem, H. Shair, A. Haq, R.A.H. Khan and M.A. Amin. 2022. Exogenous application of proline to enhance rice tolerance against heat and drought stresses. Pakistan Journal of Agricultural Research, 35(2): 324-333.
DOI | https://dx.doi.org/10.17582/journal.pjar/2022/35.2.324.333
Keywords | Drought, Heat, Rice yield, Grain quality, Proline
Copyright: 2022 by the authors. Licensee ResearchersLinks Ltd, England, UK.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Introduction
Rice is most important cereal crops of more than 50% of the world’s population (Muthayya et al., 2014). Global rice production must rise by 1.0-1.2% yearly to provide food constantly increasing population (Ricepedia, 2020).
Temperature of the ambient atmosphere is increasing day by day and adversely affecting crop growth, development and yield. Elevation in temperature enhances the process of development that ultimately shortening the life cycle of plant. Plants with short life cycle have lower economic yields due to short stature and short reproductive cycle (Jerry and Prueger, 2015). Rice is successfully grown on 27-32°C temperature (Aghamolki et al., 2014). Tenorio et al. (2013) stated, by 1oC increase in temperature from optimum range will cause 10% reduction in yield of rice crop. Rice sensitivity toward preeminent heat stress depends upon the stage of growth, phenotype and rise in daily day and night variation in temperature (Das et al., 2014). Any increase in temperature from optimal 32oC, a week before flowering to during anthesis, decrease the pollen viability and discontinue the process of pollination causing abortion of embryo and development of sterile spikelet that ultimately lowers yield of rice (Reynolds et al., 2016).
Anthesis is considered most sensitive stage toward heat stress factors as it affects the fertilization process and thus causing huge reduction in yield worldwide (Ishimaru et al., 2010). Heat stress not only influence the grain yield and quality but also declines other sensitive traits (Miyahara et al., 2017), that ultimately leads to a dwindling in overall consumer acceptance. Diminution in chlorophyll contents, fragmentation of grana, and escalation in amylolytic activity and interruption in the movement of assimilates happened when plant is imperiled to high temperature stress for extended period (Kozłowska et al., 2007). Higher temperatures along with severe moist deficit in soil abridged the operational efficacy of PSII, enhance protein catabolism, limited nitrogen anabolism and enhanced lipid peroxidation (Iqbal et al., 2020). Relative leave water contents (RLWC) was reduced by the imposition of a-biotic stress factors (Hasanuzzaman et al., 2012). Respiration and water loss from leaves were promoted by heat stress (Duan et al., 2017).
Temperature of the earth is increasing along with water scarcity due to low precipitation. Worldwide rise in temperature and CO2 level is causing a pressure on the availability of water along with increasing population growth and change in land use with other problems (Gray et al., 2016). Drought stress is as lethal as temperature stress for sustainable rice production. It decreases quality and stability of rice when applied at critical growth stages (Jagadish et al., 2012). Anthesis in paddy crop is critical development stage toward heat, drought also affects initiation of flowering mostly by causing panicle sterility which ultimately reduces production of rice (Jagadish et al., 2011). Shortage of water in rice injuriously affect growth, yield and quality (Jagadish et al., 2012)
Whenever there is temperature stress on plant it mostly coincides with drought stress as well, if these two stresses at heading or early flowering stage prolong for almost 14 days, it will result in deceased grain weight (Reynolds et al., 2016). Incidence of drought + heat in mishmash are very brutal (~70%) to the production of crops as their individual occurrence (Prasad et al., 2011). Soil temperature increases when there is heat stress and this period may prolong when plant is facing low water content in soil as well (Sekhon et al., 2010). Combined effect of both stress factors, high temperature and low moisture contents at critical growth stages is detrimental for physiological processes of rice leading towards significant lowering of productivity (Kadam et al., 2014).
Attention is being paid on plant growth regulators in the mitigation of plant stresses. High concentration of proline content helps in osmotic adjustment of different organisms and higher plants (Hayat et al., 2012). Most of the solutes which are produced in plants during various stress conditions proline is common one and unique (Nahar et al., 2016). Foliar spray of proline reduced heat stress in chickpea by minimizing cell injury and increasing the enzymatic activity related to carbon and oxidative metabolism (Kaushal et al., 2011). Hayat et al. (2012) found that by proline application helps to stabilize the subcellular structure of cell, i.e., cellular membranes and important proteins by scavenging the produced free radicles and normalizing the various processes of cell whenever plant faced stressful environment. Growth of Zea mays sown under water limiting conditions was found to be increased when proline was foliar applied at seedling or vegetative stage (Ardabili et al., 2013). Studies has revealed that high proline content reduces the impact of other combined stresses like drought and heat by osmotic adjustment and by increasing the activity of enzyme that ultimately increase the protein metabolism in rice. From the above fact a study was planned to investigate the impact of proline on rice plants under heat and drought stresses, by observing chlorophyll and relative water contents along with quality and yield related attributes.
Materials and Methods
Location
An greenhouse experiment, was laid out in the field area of Faculty of Agriculture, University of Agriculture Faisalabad having sandy loam soil and semi-arid climate during summer 2018.
Plant material and fertilizer
Seed of medium stress tolerant basmati rice cultivar were taken from Rice Research Institute Kala-Shah Kaku. Rice nursery was sown on June 25, 2018 and shifted for transplantation after one month aged into 14 inches high and 12 inches diameter pots having 20 kg sieved soil. Rate of application of fertilizer was 120 kg N, 95 kg P and 75 kg K per hectare. One third 1/3 part of nitrogen along with recommended amount of phosphorous and potassium were give as a basal dose. Left amount of N was applied at 30 and 45 days after sowing in two equal splits. Sources of fertilizer used were ammonium sulphate as source of urea, di ammonium phosphate and sulphate of potassium. Weed free condition was maintained inside the pots by eliminating the weed manually by hands up 45 days of transplanting. A suitable insecticide i.e., lambda cyhalothrin @ 1000ha-1 was sprayed to control the insects.
Experimental design and stress imposition
The current experiment was carried out under CRD with split plot array along with 3 reps. Stress treatments were applied as main factor viz. no stress, heat stress, drought stress and combined effect of heat + drought stress and three levels of exogenous proline viz. P0 = water spray (control), 10 mM, 20 mM and 30 mM were applied as second factor. Saturated condition was maintained in soil till anthesis. Pots were placed in glass house for heat stress imposition for twelve days. For heat stress imposition pots were kept in glass house for twelve days. Morning, noon and evening temperature and humidity were measured with the help of digital thermometer and humidifier probe as shown in Figure 2. Inside and outside glasshouse temperature was measured continuously, average inside temperature was 36±2°C whereas outside was 30±2°C in Figure 3. Drought condition was maintained by cutout irrigation and field capacity was recorded and stabilized 35±5% for twelve days.
Yield contributing traits
Three plants were taken at random from each pot and measuring tape was used for recording final plant height. Fertile and unfertile tillers of each pot were counted, and averaged per plant. Length of panicle was measured with measuring tape of five indiscriminately selected tillers and spikelets of five randomly selected tillers per pot were counted; their mean was computed thereafter. Total, filled and unfilled kernels per panicle of randomly selected five tillers per pot were tallied and average was calculated. Weight of 100-normal grains was noted by using electronic balance and for estimation of yield per pot, total kernels in each pot were separated and weighed.
Quality attributes
On working board twenty grains were placed in front of light (Tungsten filament) and kernels were separated as per specific character and expressed in percentage. Those kernels that did not attain full size due to incomplete fertilization were abortive kernels. When kernels were positioned in front of light bulb and they did not allow light to pass were considered as opaque kernels. Kernels parted on the base of existence of chalkiness on kernels are called chalky kernels. Translucent kernels that attained full size and had compact starch to allow light to pass were regarded as normal kernels. Micro-Kjeldhal apparatus was used for digestion of rice grains followed by ammonia distillation and titration to determine nitrogen concentration. This concentration of nitrogen then multiplied with 5.83 to obtain protein content of grains.
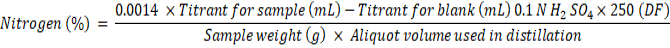
Whereas DF indicte dilution factor if there is any.

Whereas; 5.83 is constant for rice (Bremner and Mulvaney, 1982; Buresh et al., 1982; FAO, 2003).
Chlorophyll contents
After proline application fully extended leaves were collected randomly from each replication for chlorophyll content measurement by using method described by Arnon (1949) and formula is given below:
Chl a (mg per gram FW) = [12.70 (A 663) – 2.690 (A 645)] × V/1000 × W
Chl b (mg per gram FW) = [22.90 (A 645) – 4.680 (A 663)] × V/1000 × W
Where; A is absorbance, W is leaf sample weight, V is sample volume.
Relative leaf relative water contents (RLWC %)
Weatherley (1950) method with some modifications was used to measure leaf water contents. For RWC, 0.5 g leaf sample (FW) was taken from each treatment 48 hours after proline application. The leaves were dipped into distilled water overnight to get turgid leaf weight (TW). Then oven dried the same leaves at 80°C to get the dry weight (DW); using following equation RWC was calculated:
RLWC (%) = [(FW-DW) / (TW-DW)] × 100
Where; DW, Dry weight; FW, Fresh weight; TW, Turgid weight.
Statistical analysis
Fisher’s analysis of variance technique (Steel et al., 1997) was employed to determine significance (F-test) of heat + drought stress and exogenous proline. While means of treatments were compared using Tukey’s HSD test.
Results and Discussion
Yield components and kernels yield
Yield contributing traits of rice is affected due to sole imposition of abiotic stress factors including heat and drought stress and their combination. Plant height (9.06%), fertile tillers (65.88%), panicle length (33.50%), number of spikelet’s per panicle (53.47%), filled grains per kernel (27.23%), 100-kernal weight (30.64%) and yield (61.41%) were significantly decreased by heat and drought stress in concurrent and individual as compared to control shown in Table 1. However, number of unfertile tillers and unfilled grains per panicle was drastically increased where stress was imposed. At individual level, heat stress was more severe than drought stress and deleteriously affected the yield and contributing components. Exogenous application of proline at each level improved plant height (4.73%), fertile tillers (20.95%), panicle length (15.89%), number of spikelets per panicle (28.08%), filled grains per kernel (17.01%), 100-grain weight (4.38%) and yield per plant (29.76%) and lessened the number of unfertile tillers (52.80%) and unfilled grains per panicle (64.18%). Proline at 30 mM rate had significantly better results as compared to other levels.
Among abiotic stresses, the most common ones are high temperature and low moisture contents (drought) under field condition that limits the yield of crops. Drought and heat stress may affect the different various growth stages of crop plants, but the reproductive stages of plants are adversely affected by these stresses (Fahad et al., 2017). The difference in plant height was primarily due to reduction in panicle length due to stress imposition at anthesis stage of rice. This decrease in panicle length may be due to hampered photosynthetic rate during panicle initiation and anthesis stage resulting in less accumulation of photosynthetic assimilates into panicles. Reduced panicle length was observed due to declined photosynthates, proline and sugars buildup in sheath and leaf blades of rice (Mostajeran and Rahimi-Eichi, 2009). Photosynthetic processes are dependent upon stomatal conductance and metabolism of mesophyll cell (Athar and Ashraf, 2005). Exogenous application of proline increased stomatal conductance by maintaining the turgor of cell appropriately (Kamran et al., 2009) which ultimately enhanced the CO2 assimilation at higher rates. This improved photosynthetic efficiency might have led to increased panicle length and increased overall plant height at maturity. Productivity of tillers and indirectly number of filled grains depends upon fertility of panicles that they bear, and fertility of panicles is affected by germination of pollens on stigma and viability and number of pollens (Jagadish et al., 2010). Improvement in number of fertile tillers by exogenous application of proline may be due to increasing amount of proline in pollens, avoiding denaturation of protein and preservation of cellular structures by compatible osmolyte (Chiang and Dandekar, 1995).
Improvement in water balance, enzymatic activities and auxin metabolism due to proline application attributed to improved panicle length and number of spikelets per panicle. Stress application on rice resulted in declined grain yield of rice. The decrease in yield may be due to dwindled weight of individual grain. Result of our experiment was same as that of Virk et al. (2006) who documented that under stress seed weight was declined due to reduction in photosynthates and translocation of materials to economic portion. Regression analysis also indicated strong positive relationships of chlorophyll a and chlorophyll b with grain yield (R2= 0.8754 and 0.9475, respectively of rice in Figure 1A and B). Proline application improved grain yield of rice as reported by Sadak et al. (2015) who documented that under stress proline and other amino acids application in plants resulted in better seed weight.
Table 1: Effect of proline foliar application on yield and yield contributing traits of rice under heat and drought stress.
|
Factors |
Plant height (cm) |
Fertile tillers |
Unfertile tillers |
Panicle length (cm) |
Number of spikelets |
Fertile grains |
Unfertile grains |
100-grain weight (g) |
Yield per plant (g) |
||||||||
|
Stress imposition |
No stress |
81.8 a |
11.33 a |
1.58 c |
23.12 a |
9.93 a |
49.33 a |
8.02 c |
1.62 a |
17.40 a |
|||||||
|
Drought |
78.2 ab |
8.75 b |
3.33 b |
19.71 a |
7.88 b |
41.44 b |
18.16 b |
1.36 b |
13.93 b |
||||||||
|
Heat |
76.8 b |
8.17 b |
3.75 b |
19.4 b |
7.60 b |
40.17 c |
18.3 b |
1.35 b |
12.88 b |
||||||||
|
Heat+Drought |
75.0 b |
6.83 c |
4.92 a |
17.31c |
6.47 c |
38.77 d |
24.45 a |
1.24 c |
10.78 c |
||||||||
|
Tukey’s HSD at p ≤ 0.05 |
3.634 |
0.887 |
0.697 |
1.946 |
0.801 |
0.710 |
1.142 |
0.020 |
1.564 |
||||||||
|
Proline levels |
Control |
76.1 b |
9.58 a |
4.08 a |
18.43 c |
7.05 c |
38.8 d |
21.18 a |
1.37 d |
11.96 d |
|||||||
|
10 Mm |
77.9 ab |
8.92 b |
3.50 b |
19.47 b |
7.68 b |
38.3 c |
18.17 b |
1.38 c |
13.30 c |
||||||||
|
20 mM |
78.0 ab |
8.67 b |
3.33 b |
20.35 b |
8.12 b |
43.5 b |
16.75 c |
1.40 b |
14.21b |
||||||||
|
30 mM |
79.7 a |
7.92 c |
2.67 c |
21.36 a |
9.03 a |
45.4 a |
12.90 d |
1.43 a |
15.52 a |
||||||||
|
Tukey’s HSD at p ≤ 0.05 |
2.976 |
0.601 |
0.450 |
0.984 |
0.514 |
0.849 |
0.766 |
0.011 |
0.491 |
||||||||
|
p = 0.05 |
0.000 |
0.000 |
0.000 |
0.000 |
0.000 |
0.000 |
0.000 |
0.000 |
0.000 |
||||||||
Significant difference occurs where any two means not sharing a common letter at p ≤ 0.05; Mm= Millimolar.
Table 2: Effect of proline foliar application on quality attributes, chlorophyll (a and b) contents and relative leaf water contents of rice under heat and drought stress.
|
Factors |
Normal kernels % |
Abortive kernels % |
Opaque kernels % |
Chalky kernels % |
Grain protein % |
Chlorophyll a (mg g-1 FW) |
Chlorophyll b (mg g-1 FW) |
Relative water content % |
|
|
Stress imposition |
No stress |
77.08 a |
2.38 c |
4.88 c |
4.90 c |
9.37 a |
1.63 a |
0.46 a |
77.13 a |
|
Drought |
66.83 b |
4.34 b |
9.875 b |
13.78 b |
7.73 b |
1.02 b |
0.31 b |
69.48 b |
|
|
Heat |
66.61 b |
4.95 b |
10.06 b |
14.30 b |
7.36 c |
1.01 b |
0.30 b |
68.83 b |
|
|
Heat + Drought |
58.33 c |
6.14 a |
13.69 a |
16.66 a |
6.46 d |
0.80 c |
0.23 b |
66.08 c |
|
|
Tukey’s HSD at p ≤ 0.05 |
0.4574 |
0.9249 |
0.3503 |
0.5560 |
0.2825 |
0.0438 |
0.0291 |
2.0057 |
|
|
Proline levels |
Control |
65.75 d |
5.46 a |
10.81 a |
13.51 a |
6.90 d |
1.02 d |
0.29 d |
66.92 d |
|
10 mM |
66.68 c |
4.83 b |
10.03 b |
12.76 b |
7.36 c |
1.06 c |
0.31 c |
69.17 c |
|
|
20 mM |
67.79 b |
4.18 c |
9.32 c |
12.06 c |
8.10 b |
1.14 b |
0.33 b |
71.36 b |
|
|
30 mM |
68.63 a |
3.34 d |
8.36 d |
11.32 d |
8.55 a |
1.22 a |
0.37 a |
74.08 a |
|
|
Tukey’s HSD at p ≤ 0. |
0.5435 |
0.4064 |
0.4544 |
0.4691 |
0.3496 |
0.0359 |
0.0113 |
2.0658 |
|
|
p = 0.05 |
0.000 |
0.000 |
0.000 |
0.000 |
0.000 |
0.000 |
0.000 |
0.000 |
|
Significant difference occurs where any two means not sharing a common letter at p ≤ 0.05; Mm = Millimolar.
Quality attributes
Rice grains quality was gravely affected by stress imposition as compared to no stress condition. Normal kernel percentage (32.14%) and grain protein contents (45.04%) were significantly diminished whereas percentage of abortive (157.98%), opaque (180.53%) and chalky kernels (240%) was augmented under combined effect of heat and drought stress along with their individually applied stress compared to control are given in Table 2. Increment in normal kernel percentage (4.38%) and decrement in abortive (63.17%), opaque (29.31%) and chalky kernels (19.34%) with application of proline was recorded as compared to no proline application or water spray only. Application of 30 mM proline had differential effects in improving rice kernels quality.
Rice quality was critically affected by stress imposition that might be due to fraught physiological processes such as photosynthesis and transpiration that led to disturbed grain filling.
Reduction in size of rice kernel during unfavorable environment can be result of slower grain filling rate and lower weight of grains can be attributed to limited supply of carbohydrates (Yang and Zhang, 2006). Smaller sized grains may be due to decreased photosynthesis and increased photorespiration under stress and reduction in carbohydrates availability for development of reproductive organ/seed (Laza et al., 2015). Application of proline improved rice kernels quality because it is a source of nitrogen (Szabados and Savoure, 2010) and application of nitrogen upgraded kernel quality (Gravois and Helms, 1996). Exposure of rice crop to stressful environment increased opaque kernels which might be due to possible reduction in grain starch and amylose contents and depressed activity of starch synthase (Terra et al., 2010). Shi et al. (2016) documented that stress enhanced chalkiness, reduced head rice ratio and grain width of rice. Proline application decreased the kernel chalkiness that might be due to its usage as a source of nitrogen and carbon (Szabados and Savoure, 2010). Protein contents of rice grains were reduced during stress that might be due to denaturation of protein contents as a result of amalgam of ROS activities. Application of proline improved protein contents as it acts as solute and protects macromolecules from denaturation and minimizes acidity in cell (Kishor et al., 2005). Also, proline acts as nitrogen and carbon source (Szabados and Savoure, 2010) leading to improved protein contents of rice (Takami et al., 1990).
Chlorophyll contents
Both heat and drought significantly declined chlorophyll contents of rice. The maximum diminution in chlorophyll a (50.9%) and b (50.1%) contents in Table 2 was recorded in both stress conditions followed by individually imposed heat (Chl a 37.4% + Chl b 32.6%) and drought (Chl a 38.0% + Chl b 34.7%) stress as compared with no stress. Differential augmentation in chl (a & b) was recorded with exogenous proline. Exogenous spray of 30 mM proline improved chlorophyll contents (Chl a 16.4% + Chl b 21.6%) to its maximum compared with other levels (Table 2).
Chlorophyll pigments are considered the most susceptible substance to heat stress (Table 2, Berry and Bjorkman, 1980). High-temperature stimulates the degradation of chlorophyll (Zafar et al., 2017), subsequently diminishing the receiving of light quanta and prompting damage to plants (Havaux and Tardy, 1999). Chlorophyll contents in leaves is declined which ultimately hindered the photosynthetic efficacy and growth of plants due to drought (Oneto et al., 2016). Diminution in chlorophyll contents due to stressful environment may be due to impairment in aminolevulinic acid dehydratase and porphobilinogen deaminase activities under excessive ROS. Additionally, augmented conversion of protochlorophyllide to chlorophyllide because of increased activities of protochlorophyllide oxidoreductase under stressful conditions curtailed the chlorophyll contents (Hemantaranjan et al., 2014).
Relative leaf water contents (RLWC)
Stress imposition had substantial effect on RLWC of rice, either combined (14.3%) or individual (10.7%) (Table 2). Concurrent stresses indicated more destructive effect on RLWC followed by sole imposition of both stresses showed statistically similar effects. Proline is a secondary metabolite and involved in mitigation of stresses significantly (9.7%) when applied 30 mM as spray (Table 2).
Relative leaf water content is an indicator of water status of leaf. Reduction in relative leaf water content under stressed environment was most probably due to loss of turgor that resulted in inadequate water accessibility for cell extension processes (Katerji et al., 2011). As per findings of earlier researchers (El-Samad et al., 2011), proline application enhanced relative leaf water contents. Yield of rice was strongly related (R²= 0.914) with improved relative leaf water contents as specified by regression analysis of yield and relative leaf water content in Figure 1c.
Conclusions and Recommendations
Simultaneous imposition of heat and drought stress at anthesis stage triggered more diminution in yield and yield components, quality traits along with chlorophyll and relative leaf water contents of rice as compared to their individual impact. Proline application at 30 mM concentration improved water relations, protein and chlorophyll contents that resulted in better quality kernels and enhanced rice yield.
Acknowledgements
I genially thankful and acknowledged the services provided by Analytical Laboratory, Department of Agronomy, UAF and Medicinal Plant Biochemistry Laboratory, Department of Biochemistry UAF, who ease us in conducting of various biochemical analysis.
Novelty Statement
Information about the Proilne spray on rice under both abiotic stresses heat and drought has been presented in the paper which was not previously studied.
Author’s Contribution
Sajid Hanif: Prepared write-up of the research paper and conducted field trials.
Abdul Shakoor: Helped in data analysis of various traits and paper review etc.
Muhammad Farrukh Saleem: provided guidance for execution of this research experiment.
Ifra Saleem: Helped in collecting data for carious traits
Sajid Ali: Helped in data analysis and write up of this paper.
Muhammad Awais Ashraf: Captiously evaluated the manuscript and made amendments.
Majid Nadeem: Critically reviewed the manuscript for revision.
Hira Shair: Helped the author in write-up of the paper.
Anwar ul Haq: Assisted in data analysis and results and discussion.
Rana Abdul Hamid Khan: Reviewed and edited the final draft.
Muhammad Amir Amin: Assisted in write up of the draft and submitted the manuscript.
Conflict of interest
The authors have declared no conflict of interest.
References
Aghamolki, M.T.K., M.K. Yusop, F.C. Oad, H. Zakikhani, H.Z. Jaafar and S. Kharidah. 2014. Heat stress effects on yield parameters of selected rice cultivars at reproductive growth stages. J. Food Agric. Environ., 12: 741-746.
Alam, R., D.K. Das, M.R. Islam, Y. Murata and M.A. Hoque. 2016. Exogenous proline enhances nutrient uptake and confers tolerance to salt stress in maize (Zea mays L.). Prog. Agric., 27(4): 409-417. https://doi.org/10.3329/pa.v27i4.32120
Ardabili, A.A., O. Sadeghipour and A.R. Asl. 2013. The effect of proline application on drought tolerance of cowpea (Vigna unguiculata L.). Adv. Environ. Biol., 7(14): 4689-4696.
Arnon, D.I., 1949. Copper enzymes in isolated chloroplasts, Polyphenoloxidase in Beta vulgaris. Plant Physiol., 24: 1-15. https://doi.org/10.1104/pp.24.1.1
Athar, H.R. and M. Ashraf. 2005. Photosynthesis under drought stress. In: Handbook of photosynthesis (ed. M. Pessarakli). CRC Press, Taylor and Francis Group, NY, pp. 793-804.
Berry, J. and O. Bjorkman. 1980. Photosynthetic response and adaptation to temperature in higher plants. Ann. Rev. Plant Physiol., 31(1): 491-543. https://doi.org/10.1146/annurev.pp.31.060180.002423
Bremner, R.M. and C.S. Mulvaney. 1982. Nitrogen total. pp. 595-624. In: (ed. A.L. Page) methods of soil analysis, Agron. No. 9. Madison, WI, USA. https://doi.org/10.2134/agronmonogr9.2.2ed.c31
Buresh, R.J., E.R. Austin and E.T. Craswell. 1982. Analytical methods in N-15 research. Fert. Res., 3: 37-62. https://doi.org/10.1007/BF01063408
Chiang, H.H. and A.M. Dandekar. 1995. Regulation of proline accumulation in Arabidopsis during development and in response to desiccation. Plant Cell Environ., 18: 1280-1290. https://doi.org/10.1111/j.1365-3040.1995.tb00187.x
Das, S., P. Krishnan, M. Nayak and B. Ramakrishnan. 2014. High temperature stress effects on pollens of rice (Oryza sativa L.) genotypes. Environ. Exp. Bot., 101: 36-46. https://doi.org/10.1016/j.envexpbot.2014.01.004
Duan, H.W.J., G. Huang, S. Zhou, W. Liu, Y. Liao, X. Yang, Z. Xiao and H. Fan. 2017. Individual and interactive effects of drought and heat on leaf physiology of seedlings in an economically important crop. AoB Plants, 9(1). https://doi.org/10.1093/aobpla/plw090
El-Samad, H.A., M.A.K. Shaddad and N. Barakat. 2011. Improvement of plants salt tolerance by exogenous application of amino acids. J. Med. Plants Res., 5(24): 5692-5699.
Fahad, S., A.A. Bajwa, U. Nazir, S.A. Anjum, A. Farooq, A. Zohaib and M.Z. Ihsan. 2017. Crop production under drought and heat stress: plant responses and management options. Front. Plant Sci., 8: 1147. https://doi.org/10.3389/fpls.2017.01147
FAO (Food and Agriculture Organization of United Nations). 2003. Food energy-methods of analysis and conversion factors. Report of technical workshop Rome 3-6 December 2002. pp. 9.
Gadallah, M.A.A., 1999. Effects of proline and glycinebetaine on Vicia faba responses to salt stress. Biol. Plant, 42(2): 249-257. https://doi.org/10.1023/A:1002164719609
Gravois, K.A. and R.S. Helms. 1996. Seeding rate effects on rough rice yield, head rice, and total milled rice. Agron. J., 88: 82-84. https://doi.org/10.2134/agronj1996.00021962008800010017x
Gray, S.B., O. Dermody, S.P. Klein, A.M. Locke, J.M. McGrath and R.E. Paul. 2016. Intensifying drought eliminates the expected benefits of elevated carbon dioxide for soybean. Nat. Plants, 2: 16132. https://doi.org/10.1038/nplants.2016.132
Hasanuzzaman, M., M.A. Hossain, J.A. Teixeira DaSilva and M. Fujita. 2012. Plant responses and tolerance to abiotic oxidative stress: Antioxidant defense is a key factor. In: Crop stress and its management: Perspectives and strategies. Springer, Dordrecht pp. 261-316. https://doi.org/10.1007/978-94-007-2220-0_8
Havaux, M. and F. Tardy. 1999. Loss of chlorophyll with limited reduction of photosynthesis as an adaptive response of Syrian barley landraces to high-light and heat stress. Funct. Plant Biol., 26(6): 569-578. https://doi.org/10.1071/PP99046
Hayat, S., Q. Hayat, M.N. Alyemeni, A.S. Wani, J. Pichtel and A. Ahmad. 2012. Role of proline under changing environments: A review. Plant Signal. Behav., 7(11): 1456-1466. https://doi.org/10.4161/psb.21949
Hemantaranjan, A., A. Nishant Bhanu, M.N. Singh, D.K. Yadav and P.K. Patel. 2014. Heat stress responses and thermotolerance. Adv. Plants Agric. Res., 1(3): 00012. https://doi.org/10.15406/apar.2014.01.00012
Iqbal, A., Q. Dong, X. Wang, H. Gui, H. Zhang, X. Zhang and M. Song. 2020. High nitrogen enhance drought tolerance in cotton through antioxidant enzymatic activities, nitrogen metabolism and osmotic adjustment. Plants, 9: 178. https://doi.org/10.3390/plants9020178
Ishimaru, T., H. Hirabayashi, M. Ida, T. Takai, Y.A. San-Oh, S. Yoshinaga and M. Kondo. 2010. A genetic resource for early-morning flowering trait of wild rice Oryza officinalis to mitigate high temperature-induced spikelet sterility at anthesis. Ann. Bot., 106(3): 515-520. https://doi.org/10.1093/aob/mcq124
Jagadish, S.V.K., E.M. Septiningsih, A. Kohli, M.J. Thomson, C. Ye, E. Redoña and R.K. Singh. 2012. Genetic advances in adapting rice to a rapidly changing climate. J. Agron. Crop Sci., 198(5): 360-373. https://doi.org/10.1111/j.1439-037X.2012.00525.x
Jagadish, S.V.K., R. Muthurajan, R. Oane, T.R. Wheeler, S. Heuer, J. Bennett and P.Q. Craufurd. 2010. Physiological and proteomic approaches to dissect reproductive stage heat tolerance in rice (Oryza sativa L.). J. Exp. Bot., 61: 143-156. https://doi.org/10.1093/jxb/erp289
Jagadish, S.V.K., R. Muthurajan, Z.W. Rang, R. Malo, S. Heuer, J. Bennett and P.Q. Craufurd. 2011. Spikelet proteomic response to combined water deficit and heat stress in rice Oryza sativa cv N22. Rice, 4(1): 1-11. https://doi.org/10.1007/s12284-011-9059-x
Jerry, L.H. and J.H. Prueger. 2015. Temperature extremes: effects on plant growth and development. Weather Clim. Extremes, 10: 4-10. https://doi.org/10.1016/j.wace.2015.08.001
Kadam, N.N., G. Xiao and R.J. Melgar. 2014. Agronomic and physiological responses to high temperature, drought, and elevated CO2 interactions in cereals. Adv. Agron., 1: 111-156. https://doi.org/10.1016/B978-0-12-800131-8.00003-0
Kamran, M., M. Shahbaz, M. Ashraf and N.A. Akram. 2009. Alleviation of drought-induced adverse effects in spring wheat (Triticum aestivum L.) using proline as a pre-sowing seed treatment. Pak. J. Bot., 41(2): 621-632.
Katerji, N., J.W. Van Hoorn, A. Hamdy, M. Mastrorilli and E.M. Karzel. 2011. Osmotic adjustment of sugar beets in response to soil salinity and its influence on stomatal conductance, growth and yield. Agric. Water Manage., 34(1): 57-69. https://doi.org/10.1016/S0378-3774(96)01294-2
Kaushal, N., K. Gupta, K. Bhandhari, Kumar, P. Thakur and H. Nayyar. 2011. Proline induces heat tolerance in chickpea (Cicer arietinum L.) plants by protecting vital enzymes of carbon and antioxidative metabolism. Physiol. Mol. Biol. Plants, 17(3): 203-213. https://doi.org/10.1007/s12298-011-0078-2
Kishor, P.K., S. Sangam, R.N. Amrutha, P.S. Laxmi, K.R. Naidu, K.S. Rao, S. Rao, K.J. Reddy, P. Theriappan and N. Sreenivasulu. 2005. Regulation of proline biosynthesis, degradation, uptake and transport in higher plants: its implications in plant growth and abiotic stress tolerance. Curr. Sci., pp. 424-438.
Kozłowska, M., M. Rybus-Zajac, J. Stachowiak and B. Janowska. 2007. Changes in carbohydrate contents of Zantedeschia leaves under gibberellin–stimulated flowering. Acta Physiol. Plant, 29: 27-32. https://doi.org/10.1007/s11738-006-0004-3
Laza, M.R.C., H. Sakai, W. Cheng, T. Tokida, S. Peng and Hasegawa. 2015. Differential response of rice plants to high night temperatures imposed at varying developmental phases. Agri. For. Meteorol., 209-210: 69-77. https://doi.org/10.1016/j.agrformet.2015.04.029
Miyahara, K., T. Wada, J.Y. Sonoda, T. Tsukaguchi, M. Miyazaki, M. Tsubone, O. Yamaguchi, M. Ishibashi, N. Iwasawa, T. Umemoto and M. Kondo. 2017. Detection and validation of QTLs for milky-white grains caused by high temperature during the ripening period in japonica rice. Breed. Sci., 67: 333-339. https://doi.org/10.1270/jsbbs.16203
Mostajeran, A. and V. Rahimi-Eichi. 2009. Effects of drought stress on growth and yield of rice (Oryza sativa L.) cultivars and accumulation of proline and soluble sugars in sheath and blades of their different ages leaves. Agric. Environ. Sci., 5(2): 264-272.
Muthayya, S., J.D. Sugimoto, S. Montgomery and G.F. Maberly. 2014. An overview of global rice production, supply, trade, and consumption. Ann. N. Y. Acad. Sci., 1324: 7-14. https://doi.org/10.1111/nyas.12540
Nahar, K., M. Hasanuzzaman and M. Fujita. 2016. Roles of osmolytes in plant adaptation to drought and salinity. In: Osmolytes and plants acclimation to changing environment: Emerging omics technologies. Springer, New Delhi, pp. 37-68. https://doi.org/10.1007/978-81-322-2616-1_4
Oneto, C.D., M.E. Otegui, I. Baroli, A. Beznec, P. Faccio, E. Bossio and D. Lewi. 2016. Water deficit stress tolerance in maize conferred by expression of an isopentenyl transferase (IPT) gene driven by a stress and maturation-induced promoter. J. Biotech., 220: 66-77. https://doi.org/10.1016/j.jbiotec.2016.01.014
Prasad, P.V.V., S.R. Pisipati, I. Momèilovic and Z. Ristic. 2011. Independent and combined effects of high temperature and drought stress during grain filling on plant yield and chloroplast EF-Tu expression in spring wheat. J. Agron. Crop Sci., 197: 430-441. https://doi.org/10.1111/j.1439-037X.2011.00477.x
Reynolds, M.P., E. Quilligan, P.K. Aggarwal, K.C. Bansal, A.J. Cavalieri, S.C. Chapman, S.M. Chapotin, S.K. Datta, E. Duveiller, K.S. Gill, K.S.V. Jagadish, A.K. Joshi, A.K. Koehler, P. Kosina, S. Krishnan, R. Lafitte, R.S. Mahala, R. Muthurajan, A.H. Paterson, B.M. Prasanna, M.W. Rakshit, R.P. Rosegrant, R.P. ISharma, S. Singh, V. Sivasankar, R. Vadez, P.V. Valluru, S. Vara Prasad and O.P. Yadav. 2016. An integrated approach to maintaining cereal productivity under climate change. Glob. Food Sec., 8: 9-18. https://doi.org/10.1016/j.gfs.2016.02.002
Ricepedia, 2020. Food security-Ricepedia.
Sadak, S.H.M., M.T. Abdelhamid and U. Schmidhalter. 2015. Effect of foliar application of aminoacids on plant yield and some physiological parameters in bean plants irrigated with seawater. Acta Biol. Colomb., 20(1): 141-152. https://doi.org/10.15446/abc.v20n1.42865
Sekhon, H.S., G. Singh, P. Sharma and T.S. Bains. 2010. Water use efficiency under stress environments. In: Climate change and management of cool season grain legume crops (eds. S.S. Yadav, D.L. Mc Neil, R. Redden and S.A. Patil). Springer press, Dordrecht, pp. 207-227. https://doi.org/10.1007/978-90-481-3709-1_12
Shi, W., X. Yin, P.C. Struik, F. Xie, R.C. Schmidt and K.S.V. Jagadish. 2016. Grain yield and quality responses of tropical hybrid rice to high night-time temperature. Field Crops Res., 190: 18-25. https://doi.org/10.1016/j.fcr.2015.10.006
Steel, R.G.D., J.H. Torrie and D. Dickey. 1997. Principles and procedures of statistics, a biometrical approach, 3rd ed. McGraw Hill Book Co. Inc. New York. pp. 352-358.
Szabados, L. and A. Savoure. 2010. Proline: A multifunctional amino acid. Trends Plant Sci., 15: 89-97. https://doi.org/10.1016/j.tplants.2009.11.009
Takami, S., T. Kobata and C.H.M. Van Bavel. 1990. Quantitative method for analysis of grain yield in rice. Agron. J., 82: 1149-1153. https://doi.org/10.2134/agronj1990.00021962008200060025x
Tenorio, F., C. Ye, E. Redoña, S. Sierr, M. Laza and M. Argayoso. 2013. Screening rice genetic resources for heat tolerance. Sabrao J. Breed. Genet., 45(3): 371-381.
Terra, T.G.R., T.C.A.D.B. Leal, P.H.N. Rangel, H.B. Barros and A.C.D. Santos. 2010. Tolerance to drought in rice cultivars in Southern Cerrado area from Tocantins state, Brazil. Acta Sci. Agron., 32(4): 715-719. https://doi.org/10.4025/actasciagron.v32i4.5528
Virk, D.S., M. Chakraborty, J. Ghosh and D. Harris. 2006. Participatory evaluation of horse gram (Macrotyloma uniflorum) varieties and their on station responses to on-farm seed priming in Eastern India. Exp. Agric., 42: 411-425. https://doi.org/10.1017/S0014479706003838
Weatherley, P., 1950. Studies in the water relations of the cotton plant I. The field measurement of water deficits in leaves. New Phytol., 49(1): 81-97. https://doi.org/10.1111/j.1469-8137.1950.tb05146.x
Yang, J. and J. Zhang. 2006. Grain filling of cereals under soil drying. New Phytol., 169(2): 223-236. https://doi.org/10.1111/j.1469-8137.2005.01597.x
Zafar, S.A., A. Hameed, A.S. Khan and M. Ashraf. 2017. Heat shock induced morpho-physiological response in indica rice (Oryza sativa L.) at early seedling stage. Pak. J. Bot., 49(2): 453-463.
To share on other social networks, click on any share button. What are these?







