Evaluation the Ovicidal Effect of Zingiber officinale Methanol Extract Against Eggs of Fasciola spp. In Vitro
Research Article
Evaluation the Ovicidal Effect of Zingiber officinale Methanol Extract Against Eggs of Fasciola spp. In Vitro
Hussein Jabar Jasim1*, Amer Murhum Al-Amery2
1College of Veterinary Medicine, Al-Muthanna University, Iraq; 2College of Veterinary Medicine, University of Baghdad, Iraq.
Abstract | The purpose of this study was to compare the effects of Zingiber officinale methanolic extracts on eggs of Fasciola spp. to both positive (Albendazole) and negative (chlorine-free water) control groups in vitro. Microscopic examination of the treated Fasciola spp. eggs with ginger methanolic extract revealed that the various concentrations of Z. officinale methanolic extract used in our study were highly sensitive to it and that their activities differed noticeably from those of the positive (albendazole) and negative (chlorine-free water) controls. The ovacidal influence of ginger extract at a dosage of 5mg/ml with 24, 48, and 72 hr. treatment times was 90.1, 95.8, and 99.2% respectively, compared to the positive and negative control groups, which had ovacidal effects of 83.1 and 16.88%, respectively. But, the ovicidal efficacy of ginger methanolic extract at a dosage of 10 mg/ml is 97.4, 100, and 100% respectively, with treatment times of 24, 48, and 72 hr. While, using ginger extract at doses of 20, 25, and 50 mg/mL with treatment times of 24, 48, and 72 hr. respectively, resulted in 100% ovicidal effectiveness.
Keywords | Ovicidal, Zingiber officinale extract, Fasciola spp. Eggs
Received | January 21, 2023; Accepted | March 15, 2023; Published | March 25, 2023
*Correspondence | Hussein Jabar Jasim, College of Veterinary Medicine, Al-Muthanna University, Iraq; Email: [email protected]
Citation | Jasim HJ, Al-Amery AM (2023). Evaluation the ovicidal effect of Zingiber officinale methanol extract against eggs of Fasciola spp. in vitro. Adv. Anim. Vet. Sci. 11(5):695-700.
DOI | https://dx.doi.org/10.17582/journal.aavs/2023/11.5.695.700
ISSN (Online) | 2307-8316
Copyright: 2023 by the authors. Licensee ResearchersLinks Ltd, England, UK.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Introduction
One of the most significant parasitic zoonotic diseases in the world is fascioliosis, which is caused by the F. hepatica and F. gigantica obligatory endoparasitic trematodes of the Phylum Platyhelminthes-Class Trematoda. These two species are localized in the liver, bile ducts, and gall bladder of a variety of mammals, including cows, buffaloes, sheep, goats, and occasionally equine (Al-Sultan et al., 1999; Shrimali et al., 2016). Also, blood-feeding Fasciola spp. have a two-host life cycle (Fadl et al., 2011). Lymnaea spp., a species of freshwater snail, comprise the majority of the intermediate hosts where its asexual stage develops. Ruminants, who are the specific hosts who contracted the infection after consuming metacercaria, go through the sexual stage (Oleiwi et al., 2017; Mikaeel, 2020). Besides, Fasciolosis has a wide geographic spread as food-borne zoonotic disease. F. gigantica is responsible for the large liver fluke in tropical areas, which is notably prevalent throughout Asia and Africa, because Lymnaea natalensis, its intermediate host, is widely distributed. Whereas, Lymnaea truncatula, the intermediate host of F. hepatica, is only found in a few temperate locations, including Australia, the Americas and Europe (Kanyari et al., 2010; Hassone and Salah, 2019). In addition, Fasciolosis have posing a serious health threat and causing enormous economic losses in livestock industries through death of infected animals, decrease in production, growth retardation, condemnation of affected livers, costs of treatment, and expense for control measures in ruminants (Al-Kubaisee et al., 1999; Abdulwahed and Al-Amery, 2019).
On the other hand, due of growing contraindications in the use of synthetic medications, researchers have recently focused a lot of emphasis on exploring for biologically active plants that can be employed as novel anti-parasitic medicines. Due to their anthelmintic effectiveness and lack of side effects, the use of unprocessed medicinal plants ensures positive health consequences for both humans and animals. The identification of novel compounds with a distinctive structure, high activity, and selectivity can also result from natural product screening; these molecules can subsequently be further improved utilizing synthetic techniques (Al-Bayaty et al., 2006). Moreover, these extracts frequently disrupt the major objectives of parasites, including membrane integrity, microtubules, DNA (intercalation, alkylation) and brain signal transmission (El-Sayed et al., 2012). When examined for anti-parasitic action, a variety of medicinal plant extracts were discovered to be superior to the medicines already in use (Muhammed, 2015). Holistic remedies made from plant extracts are probably going to replace conventional methods of parasite control in veterinary care (Bauri et al., 2015). There are a variety of plants that can be used as antiparasitic, antibacterial and antifungal agents, such as Z. officinale (El-Sayed and El-Saka, 2015).
Materials and Methods
Preparation of Fasciola Spp. eggs
The abattoirs of Samawah in Al-Muthanna province, Iraq, were the source of buffalo gallbladders that were naturally infected with Fasciola spp. The collection of samples was done between March 2022 and December 2022. The abattoirs were visited three times per week, and between 2 and 5 buffaloes were slaughtered daily. The team tested the gallbladders to see how plant extract affected Fasciola mircidium’s capacity to hatch in a controlled environment. After being aseptically placed into glass cylinders, the bile liquid was allowed to set for 30 minutes. The eggs fell to the cylinders’ bottoms. The eggs were collected, numerous times rinsed in PBS solution, and then centrifuged for five minutes at 3000 rpm. The supernatant was discarded, and PBS was used to wash the precipitated eggs multiple times. The eggs were collected, mixed with normal saline in a dark glass container, and stored at 4oC until they were required once again (Moazeni and Khademolhoseini, 2016).
Preparation of ginger (Zingiber officinale) extract
The local herbal market in Muthanna province, south of Iraq, was where we bought the fresh ginger rhizomes. The rhizomes were cut into slices, then dried for a week in the shade, after that mechanically ground into a powder using an industrial electric blender 400 ml of pure methanol and 100 g of dry ginger powder were combined gradually for an hour with a magnetic stirrer to create the methanolic extract. For 24 h the resulting solution was kept at room temperature. The mixture was mixed once more, filtered using Whatman cellulose filter paper, and the solvent was finally evaporated in a rotary evaporator. The leftover semisolid substance was disposed of and stored at 4oC in a clean glass container for future use (Moazeni and Nazer, 2010).
McMaster’s egg counting technique
Using a modified McMaster Counting Chamber, the amount of eggs found in the bile fluid collected from the Fasciolosis-infected buffaloes was determined (Urquhart et al., 1996; Taylor et al., 2016).
Experimental effecting of Z. officinale (Ginger) extract on Fasciola spp. eggs in vitro
Fasciola eggs were incubated with extracts of ginger at various times at doses of 5, 10, 20, 25, and 50 mg/ml. In each experiment, a test tube containing 450 ml of ginger extract with different concentrations was introduced to 50 μl of egg-rich sediment containing roughly 500 unembryonated eggs. At 37oC, the tubes were incubated for 24, 48, and 72 hr. After that, a pipette was used to extract the ginger solution’s supernatant while avoiding the eggs that had hardened up. Each tube’s eggs were placed into specialized little plastic containers containing 3 mL dechlorinated tap water after being thoroughly cleaned. The containers were incubated at 28 oC in the dark for 14 days before being exposed to light for two hours to encourage miracidium hatching. In a control group, 500 eggs from one container were incubated at 28 oC without being exposed to ginger extract. Moreover, albendazole (ABZ; 5 mg/ml) was created by dissolving 25 mg of albendazole in 5 ml of distilled water with 5% DMSO. This was utilized as a positive group. Due of its better effectiveness against Fasciola spp. eggs compared to other anti-Fasciola medications, albendazole was employed as the positive control (Alvarez et al., 2009). The eggs were split into three groups: Those with live miracidia, those with growing cells, and the dead eggs. This allowed researchers to assess the ovicidal action of the ginger extract on each category. Using the proportion of eggs that did not successfully grow and hatch, ovicidal activity was calculated (Vargas-Magaa et al., 2014). It is expressed in the following way:
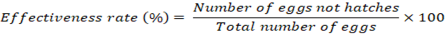
Results and Discussion
The anti-fasciolicide activity of Z. officinale methanolic extract was seen in vitro at various concentrations (5, 10, 20, 25 and 50 mg/mL) for varied times in comparison to positive (Albendazole) and negative (chlorine-free water) control groups (24, 48 and 72 h). The eggs were incubated at 28 oC for 14 days to investigate the effect of the ginger extracts on the miracidial development. Tables 1, 2, and 3 shows the findings of microscopic examinations of the eggs exposed to various doses and durations of ginger extract, respectively. The results revealed that Fasciola spp. eggs are susceptible to Z. officinale methanolic extract at the various concentrations used in our experiment, and that their activities were dramatically different from the negative group (chlorine-free water) and positive (albendazole) controls. According to the Table 1, the ovicidal influence of ginger extract at a dosage of 5 mg/ml with treatments lasting 24, 48, and 72 hr is 90.1, 95.8, and 99.2%, respectively, in comparison to the positive and negative control groups, which are 83.1 and 16.88%, respectively. But, the ovicidal efficacy of ginger extract at concentrations of 10 mg/ml with treatments times 24, 48, and 72 hr is 97.4, 100, and 100%, respectively, as seen in the Table 2. In addition, ginger extract was used at doses of 20, 25, and 50 mg/mL with treatments lasting 24, 48, and 72 hours, respectively, to achieve 100% ovicidal efficacy as displayed in the Table 3. On the other hand, in our experimental study, the eggs were split into three groups: those containing live miracidia, containing growing cells, and dead eggs, as shown in Figure 1. This allowed us to assess the ovicidal activity of the ginger extract.
Table 1: Results the effects of methanolic extracts of Z. officinale against eggs of Fasciola spp. after 24 h in vitro.
|
Concentration of drug and ginger extract (mg/ml) |
No. of examine eggs |
After 14 days |
|||||
|
Eggs containing live miracidia |
% |
Developing eggs |
% |
Dead eggs |
% |
||
|
5 |
418±11.52 |
26±1.00 a |
6.23 |
15±1.00 a |
3.59 |
377±2.88 a |
90.1 |
|
10 |
426±1.00 |
11±1.00 b |
2.58 |
0 |
0 |
415±2.00 a |
97.4 |
|
20 |
438±1.00 |
0 |
0 |
0 |
0 |
438±1.00 |
100 |
|
25 |
411±1.00 |
0 |
0 |
0 |
0 |
411±1.00 |
100 |
|
50 |
426±1.00 |
0 |
0 |
0 |
0 |
426±1.00 |
100 |
|
5 Albendazole positive control |
431±1.00 |
0 |
0 |
70.33±2.51c |
16.8 |
360±2.08a |
83.1 |
|
Distal water negative control |
453±3.60 |
328±1.00 c |
72.1 |
51±1.00 b |
10.7 |
75±2.00c |
16.8 |
Table 2: Results the effects of methanolic extracts of Z. officinale against eggs of Fasciola spp. after 48 hr in vitro.
|
Concentration of drug and ginger extract (mg/ml) |
No. of examine eggs |
After 14 days |
|||||
|
Eggs containing live miracidia |
% |
Developing eggs |
% |
Dead eggs |
% |
||
|
5 |
412±1.00 |
11±1.00 a |
2.9 |
5±1.00 a |
1.21 |
396±1.00 a |
95.8 |
|
10 |
422±1.00 |
0 |
0 |
0 |
0 |
422±1.00 |
100 |
|
20 |
441±1.00 |
0 |
0 |
0 |
0 |
431±8.386 |
100 |
|
25 |
426±1.00 |
0 |
0 |
0 |
0 |
426±1.00 |
100 |
|
50 |
414±1.00 |
0 |
0 |
0 |
0 |
414±1.00 |
100 |
|
5 Albendazole positive control |
431±1.00 |
0 |
0 |
70.33±2.51c |
16.8 |
360.33±2.06b |
83.1 |
|
Distal water negative control |
453±3.60 |
328 b |
71.9 |
50±1.00 b |
10.7 |
75±1.00c |
16.8 |
Table 3: Results the effects of methanolic extracts of Z. officinale against eggs of Fasciola spp. after 72 hr in vitro.
|
Concentration of drug and ginger extract (mg/ml) |
No. of examine eggs |
After 14 day |
|||||
|
Eggs containing live miracidia |
% |
Developing eggs |
% |
Dead eggs |
% |
||
|
5 |
421±1.00 |
3±1.00 a |
0.71 |
0 |
0 |
418±0.00 a |
99.2 |
|
10 |
435±1.00 |
0 |
0 |
0 |
0 |
435±1.00 |
100 |
|
20 |
416±1.00 |
0 |
0 |
0 |
0 |
416±1.0 |
100 |
|
25 |
423±1.0 |
0 |
0 |
0 |
0 |
419±7.234 |
100 |
|
50 |
446±1.00 |
0 |
0 |
0 |
0 |
446±1.00 |
100 |
|
5 Albendazole positive control |
431±1.00 |
0 |
0 |
73±1.00 a |
16.8 |
360.33±2.08b |
83.1 |
|
Distal water negative control |
453±3.60 |
328 b |
71.9 |
51±1.00 b |
10.7 |
75±2.00c |
16.8 |
The use of therapeutic herbs is becoming increasingly common due to the toxicity and side effects of current pharmaceuticals. The discovery of powerful pharmaceutical chemicals is greatly aided by herbal remedies (Ashraf et al., 2020). In vitro, the results of analyze the ovicidal activity of Z. officinale methanolic extracts against eggs of Fasciola spp. at various concentrations and times were agreement with those study by Moazeni and Khademolhoseini (2016), Ghafar et al. (2021), who reached for a similar conclusion that F. hepatica eggs are susceptible to various concentrations of Z. officinale extracts, and that Z. officinale also reduced the production of miracidia in F. hepatica eggs. These investigations also displayed that the anthelmintic activity of Z. officinale extract depends on exposure time and concentration. These findings are explained by the chemical components of Z. officinale extract which act as anthelmintic are tannins, flavonoids, saponins, terpenoids and phenol (Abdullahi et al., 2017; Ghafar et al., 2021). Additionally, the precise mechanism underlying this activity is unknown, but it might be caused by embryonic lysis that takes advantage of the extracts ability to penetrate the Fasciola egg shells. Subsequently, the extracts that have entered the egg may have prevented the development of cells during embryogenesis and the expression of proteins filaments and microtubules in the cytoplasm (Arafa et al., 2015; Hegazi et al., 2018). Moreover, mention Gomes et al. (2016) Saponin, one of the phytochemical components of Z. officinale extract, acts on cell membrane instability and increases permeability, which results in embryonic lysis. These explanations could be supported by results other study by Moxon et al. (2010), those who performed proteome study on F. hepatica eggs and found that from the onset of embryogenesis through the development of the miracidium, a complicated protein expression profile is present. They discovered that a large number of proteins expressed early in embryogenesis play a direct role in cytoskeleton structure and cellular proliferation. Therefore, it is conceivable that phytochemical components in the Z. officinale impede the early phases of embryogenesis and thereby interfere with the protein expression profile. On the other hand, the results of the efficiency of Albendazole against Fasciola spp. eggs in vitro in the current study were consistent with those study by Arafa et al. (2015); Pereira et al. (2016), who showed that Fasciola spp. eggs are sensitive for Albendazole in vitro and the effectiveness of Albendazole against Fasciola spp. eggs depends on the drug concentration, McKellar and Scott (1990), who demonstrated that albendazole activity is restricted to flukes older than 12 weeks, disagree with these findings. The metabolic situation of treated animals, where pathological liver alterations can impair the bioavailability of anthelmintics, poor application, erroneous dose owing to inaccurate weighing, and other factors can all affect an anthelmintic’s effectiveness (Babják et al., 2021). In addition, due to the eggs sensitivity to Albendazole, the drug concentration has an impact on the percentage of eggs that hatch. Albendazole have ability the inhibitory effect to prevent the growth and development of Fasciola spp. eggs suggests that it can penetrate through the egg shell (Arafa et al., 2015). There are two mechanisms that benzimidazoles (BZs) work against nematode eggs, and these include stopping embryonation and preventing hatching (Weston et al., 1984). The ovicidal action of ABZ is similar to that of BZs on nematodes is also correlated. In addition to having an affinity for L-tubulin, ABZ can also pierce the egg shell and accumulate inside. High solubility in lipids is related to the highest action, and this allows ABZ to easily penetrate the eggs’ shell and hinder the growth and development of the embryo. It has been hypothesized that the hydrophobic nature of the drug determines how well it inhibits egg hatching (Robles-Pérez et al., 2014).
Conclusions and Recommendations
Based on the data, results of the current study concluded that Fasciola spp. eggs are highly sensitive to Z. officinale methanolic extract at the different concentrations and times in vitro and that their activities were significantly different from those of the positive (albendazole) and negative (chlorine-free water) controls.
Acknowledgements
The authors are thankful to the head of the veterinary hospital, the medical staff, and the workers at the slaughterhouses in the Al-Muthanna governorate for their assistance during sample collection. The staff of the parasitology departments at the faculty of veterinary medicine at the University of Baghdad, Iraq are also thanked by the authors for their cooperation and the facilities they provided during sample processing.
Novelty Statement
The novelty of the study is focus on physiologically active for Zingiber officinale extract that can be employed as novel anti-parasitic pharmaceuticals due to the lack of an approved vaccination for any parasitic disease and the lack of readily accessible, secure, and efficient medicines for some diseases or parasites that are resistant to synthetic treatments, it is imperative to seek into alternate sources of anti-parasitic medications.
Authors Contribution
These authors each contributed equally.
Conflict of interest
The authors have declared no conflict of interest.
References
Abdullahi H, Karunakaran R, Sankar AU, Aye KM (2017). Anti-inflammatory effect of Zingiber officinale on Sprague Dawley rats. Asian J. Pharm. Clin. Res., 10: 353-355. http://dx.doi.org/10.22159/ajpcr.2017.v10i3.16521
Abdulwahed TK, Al-Amery AM (2019). Morphological and molecular study of Fasciola spp. in sheep in Alkut city. Int. J. Biosci. 14: 121-130. http://dx.doi.org/10.12692/ijb/14.1.121-130
Al-Bayaty MA, Ibrahim FJ, Hayani MW (2006). Evaluation of medicinal constituent (Gingerol) in Iraq cultivated ginger. Iraqi J. Vet. Med., 30(1): 83-90. https://doi.org/10.30539/iraqijvm.v30i1.844
Al-Kubaisee RY, Alwan MJ, Al-Kaisee B (1999). Ecotopic infection of cattle with Fasciola gigantica: Parasitological and pathological studies. Iraqi J. Vet. Med., 23(1): 113–123. https://doi.org/10.30539/ijvm.v23i1.1198
Al-Sultan II, Youkhana SO, Mahran OM (1999). Study on the pathology and parasitic affection of liver in sheep and cattle at Mosul area. Iraqi J. Vet. Med., 23(1): 50-58. https://doi.org/10.30539/ijvm.v23i1.1191
Alvarez L, Moreno G, Moreno L, Ceballos L, Shaw L, Fairweather I, Lanusse C (2009). Comparative assessment of albendazole and triclabendazole ovicidal activity on Fasciola hepatica eggs. Vet. Parasitol., 164(2-4): 211-216. https://doi.org/10.1016/j.vetpar.2009.05.014
Arafa WM, Shokeir KM, Khateib AM (2015). Comparing an in vivo egg reduction test and in vitro egg hatching assay for different anthelmintics against Fasciola species, in cattle. Vet. Parasitol., 214(1-2): 152-158.
Ashraf K, Halim H, Lim SM, Ramasamy K, Sultan S (2020). In vitro antioxidant, antimicrobial and antiproliferative studies of four different extracts of Orthosiphon stamineus, Gynura procumbens and Ficus deltoidea. Saudi J. Biol. Sci., 27(1): 417-432. https://doi.org/10.1016/j.sjbs.2019.11.003
Babják M, Königová A, Burcáková Ľ, Komáromyová M, Dolinská MU, Várady M (2021). Assessing the efficacy of albendazole against fasciola hepatica in naturally infected cattle by in vivo and in vitro methods. Vet. Sci., 8(11): 249. https://doi.org/10.3390/vetsci8110249
Bauri RK, Tigga MN, Kullu SS (2015). A review on use of medicinal plants to control parasites. Indian J. Natl. Prod. Resour., 6: 268-277.
El-Sayed NM, Ismail KA, Ahmed SAG, Hetta MH (2012). In vitro amoebicidal activity of ethanol extracts of Arachis hypogaea L., Curcuma longa L. and Pancratium maritimum L. on Acanthamoeba castellanii cysts. Parasitol. Res., 110(5): 1985-1992.
El-Sayed NM, El-Saka MM (2015). Anti-parasitic activity of Zingiber officinale (Ginger): A brief review. Aperito. J. Bacteriol. Virol. Parasitol., 2(1): 112. https://doi.org/10.14437/2378-7864-2-112
Fadl SR, Kalef DA, Abbas SM (2011). Prevalence of parasitic infection in sheep from different regions in Baghdad. Iraqi J. Vet. Med., 35(1): 204–209. https://doi.org/10.30539/iraqijvm.v35i1.625
Ghafar A, Arbabi M, Mosayebi M, Hooshyar H, Nickfarjam AM (2021). Evaluation of anti-helmintic activity of Zingiber officinale roscoe extract on Fasciola hepatica miracidia in vitro. Int. Arch. Health Sci., 8(1): 45-50.
Gomes DC, de Lima HG, Vaz AV, Santos NS, Santos FO, Dias ÊR, Botura MB, Branco A, Batatinha MJM (2016). In vitro anthelmintic activity of the Zizyphus joazeiro bark against gastrointestinal nematodes of goats and its cytotoxicity on Vero cells. Vet. Parasitol., 226: 10-16.
Hassone WS, Salah MHK (2019). Phylogenetics of Fasciola hepatica in cattle (Iraq). Online J. Vet. Res., 23(8): 776-781.
Hegazi AG, Megeed KNA, Hassan SE, Abdelaziz MM, Toaleb NI, El-Shanawany EE, Aboelsoued D (2018). Comparative ovicidal activity of Moringa oleifera leaf extracts on Fasciola gigantica eggs. Vet. World, 11(2): 215. https://doi.org/10.14202/vetworld.2018.215-220
Kanyari PWN, Kagira JM, Mhoma JRL (2010). Prevalence of endoparasites in cattle within urban and peri-urban areas of Lake Victoria Basin, Kenya with special reference to zoonotic potential. Sci. J. Parasitol., 11: 171–178. http://hdl.handle.net/123456789/2641
McKellar Q, Scott E (1990). The benzimidazole anthelmintic agents. A review. J. Vet. Pharmacol. Ther., 13: 223–247.
Mikaeel FB (2020). Prevalence of Fasciola hepatica in goats and sheep by using ELISA in sera and milk in Duhok, Iraq. Iraq. J. Vet. Med., 44(2): 113–119. https://crerativecommons.org/licenses/by/4.0.
Moazeni M, Khademolhoseini AA (2016). Ovicidal effect of the methanolic extract of ginger (Zingiber officinale) on Fasciola hepatica eggs: An in vitro study. J. Parasit. Dis., 40: 662–666.
Moazeni M, Nazer A (2010). In vitro effectiveness of garlic (Allium sativum) extract on scolices of hydatid cyst. World J. Surg., 34: 2677–2681. https://doi.org/10.1007/s00268-010-0718-7
Moxon JV, LaCourse EJ, Wright HA, Perally S, Prescott MC, Gillard JLJ, Hamilton JV, Brophy PM (2010). Proteomic analysis of embryonic Fasciola hepatica: Characterization and antigenic potential of a developmentally regulated heat shock protein. Vet. Parasitol., 169(1-2): 62-75.
Muhammed DA (2015). Anthelmintic effect of Zingiber officinale (ginger) extract on Toxocara canis infected mice. MSc. thesis Fac. Med. Benha University Egypt. https://doi.org/10.1016/j.vetpar.2009.12.031
Oleiwi KI, Hussein ZS, Salman KO (2017). Detection of Fasciola hepatica in Abu-Ghraib district (Iraq). J. Entomol. Zool. Stud., 5(6): 1067-1072.
Pereira CA, Oliveira LL, Coaglio AL, Santos FS, Cezar RS, Mendes T, Oliveira FLP, Conzensa DG, Lima WS (2016). Anti-helminthic activity of Momordica charantia L. against Fasciola hepatica eggs after twelve days of incubation in vitro. Vet. Parasitol., 228: 160-166.
Robles-Pérez D, Martínez-Pérez JM, Rojo-Vázquez FA, Martínez-Valladares M (2014). Development of an egg hatch assay for the detection of anthelmintic resistance to albendazole in Fasciola hepatica isolated from sheep. Vet. Parasitol., 203(1-2): 217-221. https://doi.org/10.1016/j.vetpar.2013.11.020
Shrimali RG, Patel MD, Patel RM (2016). Comparative efficacy of anthelmintics and their effects on hemato-biochemical changes in Fasciolosis of goats of South Gujarat. Vet. World, 9(5): 524-529.
Taylor MA, Coop RL, Wall RL (2016). Veterinary parasitology, 4th ed. Wiley Blackwell, UK: 480-485.Technicians. Elsevier. USA: 133-135.
Urquhart GM, Armour J, Duncan JL, Dunn AM, Jennings FW (1996). Veterinary parasitology. 2nd Edition, Blackwell Science Ltd., Oxford, pp. 224-234.
Vargas-Magana JJ, Torres-Acosta JF, Aguilar-Caballero AJ, Sandoval-Castro CA, Hoste H, Chan-Perez JI (2014). Anthelmintic activity of acetone–water extracts against Haemonchus contortus eggs: Interactions between tannins and other plant secondary compounds. Vet. Parasitol., 206(3): 322–327. https://doi.org/10.1016/j.vetpar.2014.10.008
Weston KM, O’brien RW, Prichard RK (1984). Respiratory metabolism and thiabendazole susceptibility in developing eggs of Haemonchus contortus. Int. J. Parasitol., 14(2): 159-164. https://doi.org/10.1016/0020-7519(84)90043-2
To share on other social networks, click on any share button. What are these?





