Evaluation of Artificial Insemination Success of Crossbred Friesian Holstein Cow After Foot and Mouth Disease Outbreak
Research Article
Evaluation of Artificial Insemination Success of Crossbred Friesian Holstein Cow After Foot and Mouth Disease Outbreak
Habib Asshidiq Syah1, Aulia Puspita Anugra Yekti1, I Putu Arya Girinata1, Ahmad Fahrudin Husen1, Rizki Prafitri1, Nurul Isnaini1, Nanang Febrianto1, Putri Utami1, Muhaimin Rifa’i2, Trinil Susilawati1*
1Faculty of Animal Science, Universitas Brawijaya, Malang 65145, East Java, Indonesia; 2Faculty of Mathematics and Natural Sciences, Universitas Brawijaya, Malang 65145, East Java, Indonesia.
Abstract | This paper aims to compare and evaluate the success of Artificial Insemination (AI) in Crossbred Friesian Holstein (CFH) cattle before FMD exposure and after FMD exposure in the Pujon sub-district, Malang regency based on non-return rate-1 (NRR-1), non-return rate-2 (NRR-2), and Conception Rate (CR). The material used in this study was 215 CFH cows that became acceptors with a single dose of unsexed semen of 100 before being exposed to FMD (T1) and 115 cows affected by FMD (T2) in Pujon subdistrict, Malang Regency, with a Body Condition Score 2.5-3.5 and aged 1.5-8 years and showing signs of estrus. Using the method of descriptive statistics. The percentage of AI success of T1 and T2 based on NRR-1 values showed no significant difference (P>0.05) 77.00% vs 86.96%, NRR-2 values showed no significant difference (P>0.05) 62.00% vs 73.04%, and CR values showed no significant difference (P>0.05) 49.00% vs 46.09%, respectively. Although AI success based on NRR1, NRR2 and CR showed no significant difference (P>0.05), this study found that AI success in cattle not infected with FMD was higher than in cows infected with FMD.
Keywords | Artificial insemination, Non-return rate, Conception rate, Foot mouth and disease, Dairy cow, Foot and mouth disease
Received | February 20, 2024; Accepted | March 22, 2024; Published | May 18, 2024
*Correspondence | Trinil Susilawati, Department of Animal Science, Universitas Brawijaya, Malang 65145, East Java, Indonesia; Email: tsusilawati@ub.ac.id
Citation | Syah HA, Yekti APA, Girinata IPA, Husen AF, Prafitri R, Isnaini N, Febrianto N, Utami P, Rifa’i M, Susilawati T (2024). Evaluation of artificial insemination success of crossbred Friesian Holstein cow after foot and mouth disease outbreak. Adv. Anim. Vet. Sci., 12(7):1249-1255.
DOI | https://dx.doi.org/10.17582/journal.aavs/2024/12.7.1249.1255
ISSN (Online) | 2307-8316
Copyright: 2024 by the authors. Licensee ResearchersLinks Ltd, England, UK.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
INTRODUCTION
Foot and mouth disease is a virus that is harmful to livestock due to its infection of cloven-hoofed animals, such as cattle, sheep, goats and pigs (Colenutt et al., 2020; Garcia-Pintos et al., 2021). Foot-and-mouth disease is caused by a highly contagious foot-and-mouth virus, which has a devastating economic and social impact (Bravo De Rueda et al., 2015; Grubman and Baxt, 2004). FMD is one of the most expensive animal disease outbreaks ever documented that occurred in 2001 and impacted the UK, Ireland, the Netherlands, France and the UK. The 2007 outbreak cost the public and private sectors € 146 million and € 68 million, respectively, while the UK, which was hit the hardest, suffered an estimated loss of € 5 billion (Anderson, 2008; Feng et al., 2017). Furthermore, calculations using economic parameters in East Java (one of the provinces in Indonesia) in the dairy cattle sector reached IDR 38,892,688 or USD 2,508.27 per farmer (Kusumastuti et al., 2024). Transmission of FMD occurs through contact with infected animals and contaminated objects (Yano et al., 2018). Clinically, FMD-infected cattle are characterized by fever, excessive salivation, difficulty moving or standing, depression, and the appearance of vesicles around the mouth, feet and mammary glands. Furthermore, cows can experience lameness, decreased milk production, weight loss, and possibly even death (Shaban et al., 2022). However, mortality is higher in young cattle (Grubman and Baxt, 2004). FMD virus rapid spread occurs due to the movement and trade of livestock (Knight-Jones and Rushton, 2013). FMD can have a significant impact on dairy cattle reproduction. FMD can lead to increased age at first calving, increased risk of cow replacement due to reproductive failure, and increased time required for first service and conception post-outbreak (Chaters et al., 2018).
After being declared FMD-free for 32 years since 1990, the foot and mouth disease (FMD) outbreak has re-infected hundreds of thousands of livestock in Indonesia. The first case of FMD was discovered in 402 beef cattle on April 28, 2022, in the Gresik Regency, East Java Province, which includes the Surabaya suburbs to the north and west, spanning 5 sub-districts and 22 villages. On May 1, a second case involving 102 beef cattle distributed over 3 sub-districts and 6 villages in Lamongan Regency, East Java Province, west of Surabaya, and 595 beef cattle, dairy cattle, and buffalo distributed over 11 sub-districts and 14 villages in Sidoarjo Regency. On May 11, cases of FMD were also reported in Aceh province’s Tamiang Regency (Sutawi et al., 2023). FMD entered Indonesia in April 2022. As of August 14, 2022, according to the Ministry of Agriculture of the Republic of Indonesia (2022), the number of deaths due to FMD in Indonesia was 6,361 cattle, for a total of 484,772 infected cattle. The Ministry of Agriculture (2022) stated that in Pujon, there were 5,757 cattle infected with FMD as of August 14, 2022.
The impact of FMD on milk production is detrimental, as FMD-affected cows produce less milk, during the period, infected cows reduce feed consumption due to pain caused by sores in the mouth, lips and tongue, leading to lower energy levels and negatively impacting milk production (Lewis et al., 2023). The adverse impact of FMD on cow’s milk production in Indonesia has worsened the current situation, as even under normal conditions, cow’s milk production in Indonesia is insufficient to supply the demand. Furthermore, FMD is an issue for efforts to increase cow’s milk production through population and genetic improvement using artificial insemination (AI). Therefore, the purpose of the study was to determine the success rate of AI before and after an outbreak of FMD based on the conception rate (CR) and non-return rate (NRR).
Materials and Methods
Materials
The FH cows inseminated before and after FMD came from farmers in the same group and region. The materials used in this study were 215 locally adapted Friesian Holstein (CFH) cows owned by SAE Pujon Cooperative (KOP) breeders, Malang District. The 215 cows were divided into 2 treatments, the first treatment (T1) was 100 cows inseminated before FMD. This treatment became the control treatment, the cows in this treatment were not FMD-infected. Meanwhile, in the second treatment (T2), 115 cows were inseminated after FMD, and the cows inseminated after FMD were FMD-infected cows. Artificial insemination after FMD, we selected cows suffering from FMD characterized by fever, excessive salivation, decreased milk production, and lameness. The cows were inseminated after FMD had recovered and been vaccinated. This study was conducted using a purposive sampling method. The cows were selected through a purposive sampling method with the requirements of having given birth, Body Condition Score (BCS) ranging from 2.5 to 3.5 (scale 1-5), aged 2-8 years, and showing signs of estrus. The BCS of 2.5 shows that a cow has had good nutritional consumption, which will affect normal reproductive performance. BCS measures the performance of the nutritional value consumed. Poor BCS conditions and malnutrition can have a detrimental effect on cow reproduction. The nutrients given to dairy cows have an impact on their ability to reproduce and are crucial to the reproductive cycle (Pradhan and Nakagoshi, 2008). Cows that have calved indicate that they have normal reproductive performance, as they have successfully carried the pregnancy to term (Diskin, 2014). The age of cows can also affect reproductive health and their ability to sustain pregnancy. Cows that give birth at an old age may experience reproductive problems such as difficulty reconceiving after giving birth, an increased risk of miscarriage, and a decrease in the number of children produced during their lifetime (Diskin and Kenny, 2014). When a cow shows signs of estrus, the farmer will report to the inseminator. Suppose the cow shows signs of estrus qualified, then the cow will be inseminated 8 hours after the onset of estrus, Semen is inseminated at the corpus uteri position and cows are injected with multivitamins after insemination. After the cows are inseminated, non-return rate 1 will be observed on days 19-22 after insemination, non-return rate 2 will be observed on days 39-42 after insemination and CR will be observed on day 60 after insemination. The method used in this study was to collect primary data by collecting field experiment data by artificial insemination of 115 CFH cows that were inseminated after FMD (T2). Furthermore, T1 insemination of 100 CFH cows was done before the FMD outbreak in Indonesia.
Measured parameters
Statistical analyses
NRR1, NRR2 and CR data at T1 and T2 were entered into MS Excel LTSC as datasets and statistical analyses were performed using the XLSTAT version (<2017.4). The analyses used were descriptive analysis and an unequal two-sample t-test. The level of significance used to identify variations between treatments was set at P<0.05.
Non-return rate
Non-return rate 1 (NRR1) is the percentage of cows that do not return to estrus in the first estrous cycle (days 19–21 after insemination), and non-return rate 2 (NRR-2) is the percentage of cows that do not return to estrus in the first estrous cycle (days 39–42 after insemination) (Susilawati et al., 2023). NRR 1 observations were carried out on days 19–22 after insemination, while NRR2 observations were carried out on days 39–42 after insemination. The calculation of NRR can use the equation:

Cows that did not show signs of estrus during the estrous cycle were assumed to be pregnant, and pregnancy is checked on the 60th day after insemination using the rectal palpation method.
Conception rate
The CR value is obtained from the number of successfully pregnant cows divided by the total inseminated cows times 100% (Jainudeen and Hafez, 2000). The calculation of CR can use the equation:
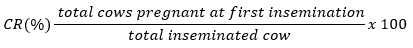
RESULTS AND DISCUSSION
Non-return rate
The evaluation of AI success is determined through NRR and CR observations. The non-return rate (NRR) value is obtained from cows that do not show signs of estrus, and cows that do not show signs of estrus are classified as pregnant (Ansari-Mahyari et al., 2019; Yekti et al., 2022). The success of pregnancy based on NRR can be seen in Table 1. The NRR1 level of T1 in this study was 77.00%, while NRR1 at T2 was 86.96, the NRR1 observation showed no significant difference (P>0.05). According to Taş et al. (2007) NRR in cattle generally has a range of 66.1-79.9%. Therefore, these results are in agreement with the opinion of Hafez and Hafez (2000) which states that the NRR value is generally 70%. However, the NRR1 in this study is lower than 86.55% (Tadesse et al., 2022), and 84% (Damayanti et al., 2023).
Factors affecting the low NRR1 in this study value are inefficient artificial insemination that causes fertilization failure (Sisay et al., 2017). Factors that cause fertilization failure might be due to poor oocyte quality. According to Roth (2017) poor oocyte quality is influenced by disease management, heat stress, physiological status (lactation or non-lactation), and postnatal disorders (Shehab-el-Deen et al., 2010; Walsh et al., 2011). Other factors that cause low NRR are the ability of farmers to recognize signs of estrus and the skills and experience of the inseminator (Ashebir, 2016; Yekti et al., 2022), A normal estrus cycle with clear signs of estrus in cows is essential for insemination to occur at the correct time of ovulation. Walsh et al. (2011) errors in estrus detection are potentially leading to fertilization failure as the timing of ovulation and the age of egg at sperm penetration are critical for conception (Roelofs et al., 2010). The inseminator’s ability in semen handling, semen deposition and timing of insemination also impact fertilization success (Diskin, 2018). Early AI can cause lower fertilization rates, while late AI can reduce embryo quality (Roelofs et al., 2010).
The NRR2 level of T1 in this study was 62.00%, while NRR1 at T2 was 73.04. The NRR2 observation also showed no significant difference (P > 0.05). In this study, both treatments decreased from NRR1 to NRR2, the first treatment decreased from 77.00% to 62.00%, while the second treatment decreased from 86.96% to 73.04%. The NRR1 value has decreased in the NRR2, which may be caused by silent heat and early embryonic death. Silent heat is a situation where the cow is in estrus but does not show normal signs of estrus and has a normal reproductive cycle, which can lead to missed observations (Sammad et al., 2020). Hafez and Hafez (2000) stated that the absence of signs of estrus called silent heat is caused by ovaries that fail to develop due to a deficiency of LH and or GnRH hormones. Meanwhile, embryonic death is caused by low progesterone hormone produced by the corpus luteum, so it cannot maintain the embryo (Parmar et al., 2016).
Conception rate
The conception rate is the percentage of cows successfully pregnant at first insemination as evidenced by rectal palpation (Yekti et al., 2022). Table 1 shows that the conception rate in this study showed no significant difference (P > 0.05) in insemination at T1 (49.00%) and insemination at T2 (46.09%). The significant decrease in CR at T2 was probably due to embryonic death due to low levels of progesterone hormone after contracting FMD. According to Shaban et al. (2022) FMD was proven to reduce progesterone levels in the blood of dairy cows. Progesterone hormone is produced by the corpus luteum which functions to help maintain a thick, nutrient-rich uterine endometrial lining to support embryo growth (Lonergan et al., 2016). Thus, cows that have low progesterone hormone levels might increase the likelihood of early embryonic death.
Table 1: Non-return rate and conception rate in cattle unaffected by FMD and cattle affected by FMD.
|
Variable |
T1 (n=100) |
T2 (n=115) |
P value |
||
|
Not Estrus |
Percentages (%) |
Not Estrus |
Percentages (%) |
||
|
NRR1 |
77 |
77.00 |
100 |
86.96 |
0.061 |
|
NRR2 |
62 |
62.00 |
84 |
73.04 |
0.086 |
|
CR |
49 |
49.00 |
53 |
46.09 |
0.671 |
The pregnancy rate was obtained by dividing the total number of pregnant cows by the total number of inseminated cows (Tadesse et al., 2022). CR in this study was used to determine the number of pregnant cows at the first insemination, but CR cannot be observed quickly after insemination. Previous observations used NRR to evaluate AI success, which can be observed quickly after AI. However, cows assumed to be pregnant based on NRR observations are not accurate (Koch et al., 2022). The NRR observation in this study was used to mark cows that should be checked for pregnancy using the rectal palpation method to determine the conception rate. Another factor that may contribute to lower CR may be due to less careful observation of cattle in estrus due to less than perfect signs of estrus.
After the FMD virus, finding cows showing signs of estrus in this study became more difficult, probably due to the negative impact of the FMD virus and FMD vaccine. FMD-infected cattle consume less feed than normal due to wounds on the mouth, lips, and tongue, which can reduce the energy consumed (Lewis et al., 2023). This condition leads to a decrease in body weight and negatively affects the quality of estrus.
Cow physiology is one of the major factors affecting the success of AI (Rutten et al., 2016). FMD-infected cows showed increased estrogen levels and decreased progesterone levels compared to non-FMD-infected cows. This indicates that FMD infection affects the balance of reproductive hormones in cattle, which has an impact on their reproduction and reproductive health (Shaban et al., 2022). According to Ferreira et al. (2016) the negative impact of the FMD vaccine is delayed ovulation and abortion. Although FMD vaccination leads to decreased pregnancy rates due to delayed ovulation and early embryo loss, prevention of FMD through vaccination is essential.
In this study, we used unsexed semen for T1 and T2 produced by the Singosari artificial insemination center. Yekti et al. (2023) measured the quality of the semen we used based on individual motility, viability, abnormality, concentration, and total motile spermatozoa.
Table 2: The quality of sperm.
|
Parameters |
Value |
|
Individual motility (%) |
54.20 |
|
Viability (%) |
75.35 |
|
Abnormality (%) |
3.38 |
|
Concentration (Million/Straw) |
36.31 |
|
Total motile spermatozoa (10⁶/ml) |
19.55 |
Based on Table 2 spermatozoa used in this study have an individual motility of 54.20%, this value can be categorized as suitable for insemination in cows because according to Standard National Indonesia (SNI4869-1-: 2017), the minimum standard of individual motility is 40%. Sperm motility generally drops by 34% to 46% following the freezing and thawing procedure (Karunakaran et al., 2019). Negative consequences of cryopreservation and the freeze-thaw process include sperm temperature fluctuations and stress brought on by osmotic, chemical, and physical pressure (Borah et al., 2015). The decrease in motility is also due to damaged mitochondria that affect the production of adenosine triphosphate (ATP), which spermatozoa need as an energy source to move (Le et al., 2019). Viability of unsexed semen used in this study was 75.35%. The viability of spermatozoa used in the study was categorized as good. According to Garner and Hafez (2000) the minimum spermatozoa viability value is 60-75%. Decreased sperm viability values may be caused by cryopreservation. The amount of antioxidants in bovine semen was reduced during cryopreservation and was insufficient to shield sperm integrity from oxidative stress. This increases the risk of harm from reactive oxygen species formation during sperm cryopreservation (Ugur et al., 2019). Abnormality in the semen we used was 3.38%, this figure is categorized as good because it has a low abnormality, according to SNI the maximum limit of abnormality in frozen semen is 20%. Abnormalities of the sperm, particularly those of the head, are usually regarded as uncompensated and may affect a bull’s ability to reproduce (Rosyada et al., 2020). Cryopreservation causes sperm morphological changes and mitochondrial damage (Khalil et al., 2018). The concentration of spermatozoa in the semen we used was 36.31 million per straw and the total motile spermatozoa was 19.55 million per straw, according to SNI the minimum limit of spermatozoa concentration per straw is 25 million and the total motile spermatozoa is 10 million per straw. The volume of viable spermatozoa is one of the important factors that can increase the success of artificial insemination (Goodla et al., 2014). So that the semen we used in this study can be categorized as suitable for insemination.
CONCLUSIONS and Recommendations
The results of this study showed that artificial insemination in dairy cows not infected with FMD (T1) and artificial insemination in cows infected with FMD (T2) had no significant difference (P > 0.05). Although there was no significant difference, there was a significant decrease from NRR2 (73.04) to CR (46.09) in artificial insemination of FMD-infected cows (T2). This significant decrease was probably due to a decrease in blood progesterone levels as a negative impact of FMD, which causes high embryo death. This study adds to the evidence that FMD impairs cattle fertility and productivity by increasing embryo mortality and decreasing first-pregnancy success.
ACKNOWLEDGEMENTS
The authors are grateful to the Direktorat Riset, Teknologi, dan Pengabdian Kepada Masyarakat (DRTPM) for supporting this research through Program Magister Menuju Doktor untuk Sarjana Unggul (PMDSU).
NOVELTY STATEMENT
Information on the effect of FMD on the fertilization of CFH cows is still very limited. Furthermore, research on the success of artificial insemination before and after FMD is still rarely conducted in Indonesia and has limited information. Therefore, this study was conducted to provide information on the effect of FMD on the fertilization of Frisian Holstein cows determined from NRRR1, NRRR2 and CR values.
AUTHOR’S CONTRIBUTION
HAS: Conceptualization, drafting the original manuscript, collecting data. APAY: Conceptualization, supervision. IPAG: Collecting data. AFH: Analyze statistics. RP: Conceptualization. NI, MR and TS: Conceptualization, supervision. NF and PU: conceptualization. Each author has reviewed and approved the manuscript’s published form.
Ethical approval
This study has passed the ethical feasibility test from the Institute of Biosciences because the artificial insemination process is carried out by certified and experienced inseminators so that cattle do not experience stress due to the insemination process. Ethical approval was given by the ethics committee of the Institute of Biosciences, Universitas Brawijaya, regarding responsible behavior in the use of female cows for experimental animals. Ethical clearance number 47/EC/KEPK/02/2024.
Conflict of interest
The author declares that there is no conflict of interest with stakeholders related to the material written in this manuscript.
REFERENCES
Anderson I. Foot and Mouth Disease 2007. A Review and Lessons Learned. London: The Stationery Office (2008).
Ansari-Mahyari S, Ojali MR, Forutan M, Riasi A, Brito LF (2019). Investigating the genetic architecture of conception and non-return rates in Holstein cattle under heat stress conditions. Trop. Anim. Health Prod., 51(7): 1847–1853. https://doi.org/10.1007/s11250-019-01875-5
Ashebir G (2016). Status of artificial insemination in tigray regional state, constraints and acceptability under field condition. J. Dairy Vet. Anim. Res., 3(3): 1-6. https://doi.org/10.15406/jdvar.2016.03.00078
Borah BKD, Deka BC, Biswas RK, Chakravarty P, Deori S, Sinha S, Ahmed K (2015). Effect of thawing methods on frozen semen quality of yak (Poephagus grunniens L.) bulls. Vet. World, 8(7): 831–834. https://doi.org/10.14202/vetworld.2015.831-834
Bravo De Rueda C, De Jong MC, Eblé PL, Dekker A (2015). Quantification of transmission of foot-and-mouth disease virus caused by an environment contaminated with secretions and excretions from infected calves. Vet. Res., 46(1). https://doi.org/10.1186/s13567-015-0156-5
Chaters G, Rushton J, Dulu TD, Lyons NA (2018). Impact of foot-and-mouth disease on fertility performance in a large dairy herd in Kenya. Prevent. Vet. Med., 159: 57–64. https://doi.org/10.1016/j.prevetmed.2018.08.006
Colenutt C, Brown E, Nelson N, Paton DJ, Eblé P, Dekker A, Gonzales JL, Gubbins S (2020). Quantifying the transmission of foot-and-mouth disease virus in cattle via a contaminated environment. MBio, 11(4): 1–13. https://doi.org/10.1128/mBio.00381-20
Damayanti DA, Yekti APA, Kuswati, Susilawati T (2023). Successful pregnancy and ovarian condition in Friesian Holstein crossbred dairy cattle with reproductive disorders after single and double dosage artificial insemination. Dev. Modern Livest. Prod. Trop. Countries, pp. 20–24. https://doi.org/10.1201/9781003370048-6
Diskin MG (2014). Achieving high reproductive performance in beef herds. In: Agriculture and food development authority. Grange. Ireland.
Diskin MG (2018). Review: Semen handling, time of insemination and insemination technique in cattle. Animal, 12(1): 75–84. https://doi.org/10.1017/S1751731118000952
Diskin MG, Kenny DA (2014). Optimising reproductive performance of beef cows and replacement heifers. Animal, 8(1): 27–39. https://doi.org/10.1017/S175173111400086X
Feng S, Patton M, Davis J (2017). Market impact of foot-and-mouth disease control strategies: A UK case study. Front. Vet. Sci., 4: 1–10. https://doi.org/10.3389/fvets.2017.00129
Ferreira LCL, Cooke RF, Marques RS, Fernandes HJ, Fernandes CE, Stelato R, Franco GL, Lemos RAA (2016). Effects of vaccination against foot-and-mouth disease virus on reproductive performance of Bos indicus beef cows. J. Anim. Sci., 94(1): 401–405. https://doi.org/10.2527/jas.2015-9537
Garcia-Pintos C, Riet-Correa F, Menchaca A (2021). Effect of foot-and-mouth disease vaccine on pregnancy failure in beef cows. Front. Vet. Sci., 8: 1–8. https://doi.org/10.3389/fvets.2021.761304
Garner D. L and Hafez E.S.E. 2000. Spermatozoa and seminal plasma. In: Reproduction in Farm Animals: 7th edn. Lippincott Williams & Wilkins. USA.
Goodla L, Morrell JM, Yusnizar Y, Stålhammar H, Johannisson A (2014). Quality of bull spermatozoa after preparation by single-layer centrifugation. J. Dairy Sci., 97(4): 2204–2212. https://doi.org/10.3168/jds.2013-7607
Grubman MJ, Baxt B (2004). Foot-and-mouth disease. Clin. Microbiol. Rev., 17(2): 465–493. https://doi.org/10.1128/CMR.17.2.465-493.2004
Hafez ESE, Hafez B (2000). X and Y chromosome bearing spermatozoa in reproduction in farm animals: 7th edn. Lippincott Williams and Wilkins. USA. https://doi.org/10.1002/9781119265306
Jainudeen MR, Hafez ESE (2000). Cattle and buffalo in reproduction in farm animals: 7th edn. Lippincott Williams and Wilkins. USA. https://doi.org/10.1002/9781119265306.ch11
Karunakaran M, Gajare VC, Mandal A, Mondal M, Das SK, Ghosh MK, Rai S, Behera R (2019). Electrophoretic profile of seminal proteins and their correlation with in vitro sperm characters in Black Bengal buck semen. Vet. World, 12(5): 621–628. https://doi.org/10.14202/vetworld.2019.621-628
Khalil WA, El-Harairy MA, Zeidan AEB, Hassan MAE, Mohey-Elsaeed O (2018). Evaluation of bull spermatozoa during and after cryopreservation: Structural and ultrastructural insights. Int. J. Vet. Sci. Med., 6: 49–56. https://doi.org/10.1016/j.ijvsm.2017.11.001
Knight-Jones TJD, Rushton J (2013). The economic impacts of foot and mouth disease - What are they, how big are they and where do they occur? Prevent. Vet. Med., 112(4): 161–173. https://doi.org/10.1016/j.prevetmed.2013.07.013
Koch J, Weber LP, Heppelmann M, Freise F, Klingelmann M, Bachmann L (2022). Effect of different thawing methods for frozen bull semen and additional factors on the conception rate of dairy cows in artificial insemination. Animals, 12(18): 1–17. https://doi.org/10.3390/ani12182330
Kusumastuti TA, Kobayashi I, Juwari A, Ri LDA (2024). Economic losses of foot-and-mouth disease based on business characteristics and regional policies in Indonesia and Japan. Adv. Anim. Vet. Sci., 12(5): 862–872. https://doi.org/10.17582/journal.aavs/2024/12.5.862.872
Le MT, Nguyen TTT, Nguyen TT, Nguyen TV, Nguyen TAT, Nguyen QHV, Cao TN (2019). Does conventional freezing affect sperm DNA fragmentation? Clin. Exp. Reprod. Med., 46(2): 67–75. https://doi.org/10.5653/cerm.2019.46.2.67
Lewis RA, Kashongwe OB, Bebe BO (2023). Quantifying production losses associated with foot and mouth disease outbreaks on large-scale dairy farms in Rift valley, Kenya. Trop. Anim. Health Prod., 55(5): 1–7. https://doi.org/10.1007/s11250-023-03707-z
Lonergan P, Fair T, Forde N, Rizos D (2016). Embryo development in dairy cattle. Theriogenology, 86(1): 270–277. https://doi.org/10.1016/j.theriogenology.2016.04.040
Ministry of Agriculture of the Republic of Indonesia, 2022.https://siagapmk.crisis-center.id/: (Accessed September 2022).
National Standardization Agency (2017). SNI Frozen Semen-Part 1: Bulls. BSN. BSN.
Parmar SC, Parmar C, Vi V (2016). Early embryonic death in bovines: An overview large animal section. Raksha Tech. Rev. Large Anim. Sect., 6(1): 6–12. https://www.researchgate.net/publication/315589534
Pradhan R, Nakagoshi N (2008). Reproductive disorders in cattle due to nutritional status. J. Int. Dev. Cooperat., 14(1): 45–66.
Roelofs J, López-Gatius F, Hunter RHF, van Eerdenburg FJCM, Hanzen C (2010). When is a cow in estrus? Clinical and practical aspects. Theriogenology, 74(3): 327–344. https://doi.org/10.1016/j.theriogenology.2010.02.016
Rosyada ZNA, Ulum MF, Tumbelaka LITA, Purwantara B (2020). Sperm protein markers for holstein bull fertility at national artificial insemination centers in Indonesia. Vet. World, 13(5): 947–955. https://doi.org/10.14202/vetworld.2020.947-955
Roth Z (2017). Effect of heat stress on reproduction in dairy cows: Insights into the cellular and molecular responses of the oocyte. Ann. Rev. Anim. Biosci., 5: 151–170. https://doi.org/10.1146/annurev-animal-022516-022849
Rutten CJ, Steeneveld W, Vernooij JCM, Huijps K, Nielen M, Hogeveen H (2016). A prognostic model to predict the success of artificial insemination in dairy cows based on readily available data. J. Dairy Sci., 99(8): 6764–6779. https://doi.org/10.3168/jds.2016-10935
Sammad A, Umer S, Shi R, Zhu H, Zhao X, Wang Y (2020). Dairy cow reproduction under the influence of heat stress. J. Anim. Physiol. Anim. Nutr., 104(4): 978–986. https://doi.org/10.1111/jpn.13257
Shaban AK, Mohamed RH, Zakaria AM, Baheeg EM (2022). Detection of foot-and-mouth disease virus in raw milk in Menofia Governorate and its effect on reproductive hormones and physiochemical properties of milk. Vet. World, 15(9): 2202–2209. https://doi.org/10.14202/vetworld.2022.2202-2209
Shehab-el-Deen MAMM, Leroy JLMR, Fadel MS, Saleh SYA, Maes D, Van Soom A (2010). Biochemical changes in the follicular fluid of the dominant follicle of high producing dairy cows exposed to heat stress early post-partum. Anim. Reprod. Sci., 117(3): 189–200. https://doi.org/10.1016/j.anireprosci.2009.04.013
Sisay W, Tamene D, Worku G, Kidanu D, Nuraddis I (2017). Evaluation of artificial insemination efficiency in and around Ejere district, Western Shoa Zone, Ethiopia. J. Reprod. Infert., 8(3): 66–71.
Susilawati T, Yekti, AP, Firdaus A, Damayanti Y, Prafitri R, Febrianto N, Kuswatii RA, WahjuningSih S, Isnaini N (2023). The study of artificial insemination with double doses at different times in Friesian Holstein crossbred. Adv. Anim. Vet. Sci., 11(7): 1159–1164. https://doi.org/10.17582/journal.aavs/2023/11.7.1159.1164
Sutawi WA, Malik A, Suyatno HA, Rahayu ID, Hartatie SE (2023). Re-emergence of foot and mouth disease outbreak in Indonesia: A review. Adv. Anim. Vet. Sci., 11(2): 264–271. https://doi.org/10.17582/journal.aavs/2023/11.2.264.271
Tadesse B, Reda AA, Kassaw NT, Tadeg W (2022). Success rate of artificial insemination, reproductive performance and economic impact of failure of first service insemination: A retrospective study. BMC Vet. Res., 18(1): 1–10. https://doi.org/10.1186/s12917-022-03325-1
Taş M, Bacinoglu S, Cirit Ü, Özdaş ÖB, Ak K (2007). Relationship between bovine fertility and the number of spermatozoa penetrating the cervical mucus within straws. Anim. Reprod. Sci., 101(1–2): 18–27. https://doi.org/10.1016/j.anireprosci.2006.08.020
Ugur MR, Saber AA, Evans HC, Gilmore AA, Hitit M, Arifiantini RI, Purwantara B, Kaya A, Memili E (2019). Advances in cryopreservation of bull sperm. Front. Vet. Sci., 6: 1–15. https://doi.org/10.3389/fvets.2019.00268
Walsh SW, Williams EJ, Evans ACO (2011). A review of the causes of poor fertility in high milk producing dairy cows. Anim. Reprod. Sci., 123(3): 127–138. https://doi.org/10.1016/j.anireprosci.2010.12.001
Yano T, Premashthira S, Dejyong T, Tangtrongsup S, Salman MD (2018). The effectiveness of a foot and mouth disease outbreak control programme in Thailand 2008-2015: Case studies and lessons learned. Vet. Sci., 5(4): 1–13. https://doi.org/10.3390/vetsci5040101
Yekti APA, Prafitri R, Kuswati, Huda AN, Kusmartono, Susilawati T (2022). The success of double dose artificial insemination at different times in ongole crossbred cattle. Am. J. Anim. Vet. Sci., 17(1): 26–30. https://doi.org/10.3844/ajavsp.2022.26.30
Yekti APA, Rahayu S, Ciptadi G, Susilawati T (2023). The quality and proportion of spermatozoa X and Y in sexed frozen semen separated with percoll density gradient centrifugation method on Friesian Holstein bull. Adv. Anim. Vet. Sci., 11(3): 371-378. https://doi.org/10.17582/journal.aavs/2023/11.3.371.378
To share on other social networks, click on any share button. What are these?






