Evaluation of Catalase Activity in Organs of Fresh Water Fish, Ctenopharyngodon idella Fed with Vitamin E
Evaluation of Catalase Activity in Organs of Fresh Water Fish, Ctenopharyngodon idella Fed with Vitamin E
Muhammad Shahbaz1,2, Sajid Abdullah1*, Huma Naz3*, Khalid Abbas1, Tanveer Ahmed4, Sana Mehmood1, Muhammad Adeel Hussan5
1Department of Zoology, Wildlife and Fisheries, University of Agriculture, Faisalabad, Pakistan
2Department of Zoology, University of Sialkot, Pakistan
3Department of Zoology, Cholistan University of Veterinary and Animal Sciences Bahawalpur, Pakistan
4Department of Life Sciences, Khwaja Fareed University of Engineering and Information Technology, Rahim Yar Khan, Pakistan
5Department of Parasitology, Cholistan University of Veterinary and Animal Sciences Bahawalpur, Pakistan
Abstract | This work was performed to evaluate the catalase (CAT) activity in Ctenopharyngodon idella fed with vitamin E (VE) supplemented diet. Four different diets viz. T1 (VE-0 mg/Kg Control), T2 (VE-200 mg/Kg), T3 (VE-350 mg/Kg) and T4 (VE-500 mg/Kg) were prepared containing different levels of VE. The C. idella was fed @ 3% of body weight three times in a day for 30 days. At the end of trail fish was dissected and tissues viz. liver, muscle and gills were obtained to analyze the CAT activity. The CAT level was reduced in all treatments when compared control. CAT activity was observed higher in the gills, muscle and liver tissues of T1 diet fed C. idella as 14.68±0.90, 12.76±0.87 and 20.34±0.99 UmL-1, respectively. Comparison among diets for CAT activity showed the trend as T4<T3<T2<T1. The maximum CAT level was noted in liver than that of gills and muscles.
Novelty Statement | This research was conducted to study the impact of Vitamin E on oxidative stress enzyme (catalase) of fish. Vitamin E has proven beneficial in protecting cellular membranes against oxidation and increases the resistance to stress.
Article History
Received: March 12, 2021
Revised: September 15, 2021
Accepted: October 02, 2021
Published: December 20, 2021
Authors’ Contributions
MS executed the research work. SA supervised the research. HN and TA helped in writing article. KA facilitated in conducting the research work in his laboratory.SM and MAH revised the article.
Keywords
CAT, Fish, Tissues, Dietary effect, Vitamin E
Corresponding Authors: Sajid Abdullah and Huma Naz
uaf_sajidabdullah@yahoo.com, dr.humanaz98@gmail.com
To cite this article: Shahbaz, M., Abdullah, S., Naz, H., Abbas, K., Ahmed, T., Mehmood, S., and Hussan, M.A., 2021. Evaluation of catalase activity in organs of fresh water fish, Ctenopharyngodon idella fed with vitamin E. Punjab Univ. J. Zool., 36(2): 171-174. https://dx.doi.org/10.17582/journal.pujz/2021.36.2.171.174
Introduction
Organic substances such as vitamins are very essential micronutrient for proper maintenance, growth, and normal development of animals like fish. These substances cannot be synthesized by the animals rather supplied in diet (Falahatkar et al., 2006). Unlike other single compound vitamins, the vitamin E (VE) is composed of four tocopherols and four tocotrienols (Shiau and Hsu, 2002).
During normal metabolic reactions free radicals are produced known as reactive oxygen species (ROS). Some of these radicals have a key role in transduction of signals (Hancock et al., 2001) but their high level can induce damage in cells of organisms (Wang et al., 2007). Most reactive free radical is hydroxyl which has high ability to cause oxidative damage to any molecule upon interacting with tissues (Castro and Freeman, 2001).
Vitamin E is a fat-soluble compound and act as an antioxidant scavenges of the free radicals (Paul et al., 2004) and save the cell’s membrane lipid-proteins from oxidation (Lee and Shiau, 2004), lipid peroxidation (Paul et al., 2004). These radicals also responsible for damage induce to intracellular components such as enzymes, membrane and DNA/RNA resulted in decreased growth (Paul et al., 2004). Therefore, VE supposed to be a very vital organic compound in body (Blazer, 1992). Many authors have documented the dietary requirement of VE for different fish species including Channel catfish (Wilson, 1984), Carps (Takeuchi, 1993) and Bass (Kocabas, 1999).
Among fish body organs, gills are direct in contact with aquatic environment and are most susceptible to change in water quality. In addition to this, gills are also accountable for respiration and osmoregulation includes acid-base balance and excretion of nitrogenous waste (Au, 2004). Muscles are the most important tissue of fish body used as food and greatly affect the human health (Pirrone and Mahaffey, 2005). Fish liver is a metabolic organ performs the function of excretion, biotransformation and accumulation of toxicants. The physiological condition of animal can be evaluated through suitable biomarkers in fish such as organs (Figueiredo-Fernandes et al., 2006). Therefore, this study was carried out to check the dietary vitamin E impact on catalase activity of Ctenopharyngodon idella.
Materials and Methods
This study was conducted on a 90-day old Ctenopharyngodon idella commonly known as Grass carp. Fingerlings were obtained from local Fish Seed Hatchery and shifted to Fisheries Research Farms, University of Agriculture, Faisalabad, Pakistan. Initially, fish were acclimatized in cemented tanks for few days and then transferred to 70-L glass aquaria for dietary trial. Table 1 show the different diets contained various level of vitamin E and fish fed with these diets at 3% of their body weight. Feed ingredients includes gluten, soybean oil, fish meal, premix and rice polish were ground and mixed with soya oil and then make the paste followed by pellet formation. Fish were fed with experimental diets three times a day at 7:00-8:00AM, 1:00-2:00PM and 6:00-7:00PM for 30 days. At the end, fish were killed and tissues viz. gills, muscles and liver were obtained to check the catalase (CAT) activity. During trail, water quality characteristics such as temperature, total hardness, dissolved oxygen and pH were stabilized as 28oC, 230 mg/L, 5ppm and 7.25, respectively.
CAT extract’s preparation
At the end of the trial, fish was dissected and gills, muscle and liver were isolated. In each organ phosphate buffer of pH 7.0 was mixed, separately, in ratio of 1:4 (w/v) and homogenized for 10 minutes. Homogenate was filtered and centrifuged at 10,000 rpm at 4°C for 12 minutes. Supernatant was collected for catalase study.
CAT assay
Activity of CAT was calculated by adopting the protocol of Chance and Mehaly (1977). The 2 ml of reaction mixture was prepared contained 0.05 mL CAT extract and 1.95 mL of buffered substrate and activity was checked at A240nm.
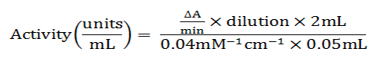
Statistical analysis
Each dietary trial was conducted three times and ANOVA and Tukey’s tests were applied to see the statistical differences and to compare the means among different variables under study (Steel et al., 1997). Statistix version 8.1 software was used for data analyses.
Results and Discussion
The results showed that C. idella fed with VE had lowered CAT activity in organs when compared with T1 (without VE). The lower CAT activity is due to low production of reactive oxygen species (ROS) as VE also act as an antioxidant. It was observed that CAT activity was higher in gills, muscle and liver of fish fed with T1 diet as 14.68±0.90, 12.76±0.87 and 20.34±0.99 UmL-1, respectively (Table 2). Among diets CAT activity showed
Table 1: Composition of experimental diet.
|
Ingredient (g kg−1 diet) |
Compositions of diets |
|||
|
T1 (Control) E-0 mg/Kg |
T2 E-200 mg/Kg |
T3 E-350 mg/Kg |
T4 E-500 mg/Kg |
|
|
Rice Polish g/Kg |
330 |
329.96 |
329.93 |
329.9 |
|
Gluten g/Kg |
320 |
319.96 |
319.93 |
319.9 |
|
Fish meal g/Kg |
300 |
299.96 |
299.93 |
299.9 |
|
Soyabean oil g/Kg |
20 |
19.96 |
19.93 |
19.9 |
|
Premix g/Kg |
30 |
29.96 |
29.93 |
29.9 |
|
α-Tocopherol (mg/kg diet) |
0 |
0.20 |
0.35 |
0.50 |
Table 2: Effect of Vitamin E on CAT activity of C. idella.
|
Diets |
Muscles |
Gills |
Liver |
*Overall mean |
|
T1 VE-0 |
12.76±0.87a |
14.68±0.90a |
20.34±0.99a |
15.93±3.94a |
|
T2 VE-200 |
9.55±0.67b |
10.22±0.73b |
15.65±0.94b |
11.81±3.35b |
|
T3 VE-350 |
7.46±0.53c |
8.10±0.60c |
12.32±0.81c |
9.29±2.64c |
|
T4 VE-500 |
4.28±0.37d |
6.63±0.45d |
8.23±0.68d |
6.38±1.99d |
|
Overall mean |
8.51±3.57c |
9.91±3.51b |
14.14±5.13a |
Means with similar letters in a single row are statistically non-significant at p<0.05.
Table 3: Regression analyses on CAT activity in C. idella
|
Organs |
Regression equation |
SE |
R |
R2 |
|
Muscle |
Activity = 12.9 - 0.0166** (Diets) |
0.0009671 |
0.994 |
0.99 |
|
Gills |
Activity = 14.1 - 0.0161* (Diets) |
0.002126 |
0.975 |
0.95 |
|
Liver |
Activity = 20.4 - 0.0240 **(Diets) |
0.0006957 |
0.998 |
0.997 |
SE= Standard Error; r=Multiple Regression Coefficient; R2; Coefficient of Determination; ** Highly significant; * Significant at P<0.01.
the following trend: T4<T3<T2 (Figure 1). The maximum CAT level was noted in liver of fish than that of gills and muscles. Regression analyses showed the negative relation between activity and VE (Table 3). Similarly, Fatima et al. (2019) also studied the reduced CAT level in Labeo rohita fed with high doses of vitamin E. CAT level significantly decreased in large yellow croaker fed with VE supplemented diet (Wang et al., 2016). Grass carp showed reduced CAT level when fed with VE supplemented diet (Li et al., 2014). Tocher et al. (2002) also studied the lowered CAT level in sea bream fed with diet contained VE1000 as compared to VE0. Vitamin E supplemented diet decreased the CAT activity in gills of C. mrigala (Iqbal et al., 2018). Some authors also observed high level of CAT in liver when fish fed with VE supplemented diet (Zhang et al., 2016). There is little information on fish regarding to VE effect but researcher have done work with other mammals such as Hsu et al. (2001) reported the lowered CAT level in rabbits fed with VE. Vitamin E is also an antioxidant have defensive role against harmful impact of ROS (NRC, 2011), but high level of α-Toc may promote the lipid peroxidation in fish (Kim et al., 2007). It is concluded that vitamin E act as antioxidant and plays role to minimize the oxidative stress in fish.
Conflict of interest
The authors have declared no conflict of interest.
References
Au, D.W.T., 2004. The application of histo-cytopathological biomarkers in marine pollution monitoring: A review. Mar. Pollut. Bull., 48: 817-834. https://doi.org/10.1016/j.marpolbul.2004.02.032
Blazer, V.S., 1992. Nutrition and disease resistance in fish. Annu. Rev. Fish. Dis.,2: 309-323. https://doi.org/10.1016/0959-8030(92)90068-9
Castro, L. and Freeman, B.A., 2001. Reactive oxygen species in human health and disease. Nutrition, 170: 161-165. https://doi.org/10.1016/S0899-9007(00)00570-0
Chance, M. and Mehaly, A.C., 1977. Assay of catalase and peroxidase. Methods Enzymol., 2: 764-817.
Falahatkar, B., Soltani, M., Abtahi, B., Kalbassi, M.R. and Pourkazemi, M., 2006. Effects of dietary vitamin C supplementation on performance, tissue chemical composition and alkaline phosphatase activity in beluga sturgeon (Husohuso). J. Appl. Ichthyol., 22: 283-286. https://doi.org/10.1111/j.1439-0426.2007.00969.x
Fatima, M., Afzal, M. and Shah, S.Z.H., 2019. Effect of high vitamin e dosages on lipid peroxidation and fatty acid profile of Labeo rohita fingerlings. Pakistan J. Zool., 51: 1211-1219. https://doi.org/10.17582/journal.pjz/2019.51.4.1211.1219
Figueiredo-Fernandes, A.A., Fontainhas-Fernandes, E.R. And Reis-Henriques, M.A., 2006. The effect of paraquat on hepatic EROD activity liver and gonadal histology in males and females of Nile Tilapia, Oreochromis niloticus, exposed at different temperatures. Arch. Environ. Contam. Toxicol., 51: 626-632. https://doi.org/10.1007/s00244-005-0208-3
Hancock, J.T., Desikan, R. and Neill, S.J., 2001. Role of reactive oxygen species in cell signaling pathways. Biochem. Soc. Trans., 29: 345-350. https://doi.org/10.1042/bst0290345
Hsu, H., Lee, Y. and Chen, M., 2001. Effects of fish oil and vitamin E on the antioxidant defense system in diet-induced hypercholesterolemic rabbits. Prostagland. other Lipid Mediat., 66: 99-108. https://doi.org/10.1016/S0090-6980(01)00146-0
Iqbal, M., Afzal, M., Anjum, K M., Yaqub, A., Shah, S.Z.H. and Fatima, M., 2018. Growth response, antioxidant status and fatty acid profile of Cirrhinus mrigala fingerlings fed on practical diets after treated with Vitamin E supplementation. Pakistan J. Zool., 13(Suppl.): 120-128.
Kim, H., Lee, H. and Min, D., 2007. Effects and pro-oxidant mechanisms of oxidized a-tocopherol on the oxidative stability of soybean oil. J. Fd. Sci.,72: 223-230. https://doi.org/10.1111/j.1750-3841.2007.00339.x
Kocabas, A.M. and Gatlin, D.M., 1999. Dietary vitamin E requirement of hybrid striped bass (Moronechrysops female × M. saxatilis male). Aquacult. Nutr., 5: 3-7. https://doi.org/10.1046/j.1365-2095.1999.00074.x
Lee, M.H. and Shiau, S.Y., 2004. Vitamin E requirements of juvenile grass shrimp, Penaeusmonodon, and effects on non-specific immune responses. Fish Shellf. Immunol., 16: 475-485. https://doi.org/10.1016/j.fsi.2003.08.005
Li, J., Liang, X.F., Tan, Q., Yuan, X., Liu, L., Zhou, Y. and Li, B., 2014. Effects of vitamin E on growth performance and antioxidant status in juvenile grass carp Ctenopharyngodon idellus. Aquaculture, 430: 21-27. https://doi.org/10.1016/j.aquaculture.2014.03.019
NRC (National Research Council), 2011. Nutrient requirements of fish and shrimp. National Academies Press, Washington, DC.
Paul, B.N., Sarkar, S. and Mohanty, S.N., 2004. Dietary vitamin E requirement of mrigal, Cirrhinus mrigala fry. Aquaculture, 242: 529-536. https://doi.org/10.1016/j.aquaculture.2004.08.037
Pirrone, N. and Mahaffey, K., 2005. Dynamics of mercury pollution on regional and global scales: Atmospheric processes and human exposures around the world. Springer, New York. https://doi.org/10.1007/b105709
Shiau, S.Y. and Hsu, C.Y., 2002. Vitamin E sparing effect bydietary vitamin C in juvenile hybrid tilapia, Oreo chromisniloticus × O. aureus. Aquaculture, 210: 335-342. https://doi.org/10.1016/S0044-8486(01)00853-5
Steel, R.G.D., Torrie, J.H. and Dickey, D., 1997. Principles and procedure of statistics. A biometrical approach 3rd Ed. McGraw Hill Book Co. Inc., New York. pp. 352-358.
Takeuchi, T., Watanabe, K., Satoh, S. and Watanabe, T., 1993. Requirement of grass carp fingerlings for alpha-tocopherol. Nippon Suisan Gakkaishi, 58: 1743-1749. https://doi.org/10.2331/suisan.58.1743
Tocher, D.R., Mourente, G., Van Der Eecken, A., Evjemo, J.O., Diaz, E., Bell, J.G., Geurden, I., Lavens, P. and Olsen, Y., 2002. Effects of dietary vitamin E on antioxidant defence mechanisms of juvenile turbot (Scophthalmus maximus L.), halibut (Hippoglossus hippoglossus L.) and sea bream (Sparusaurata L.). Aquacult. Nutr., 8: 195-207. https://doi.org/10.1046/j.1365-2095.2002.00205.x
Wang, J., Xu, H., Zuo, R., Mai, K., Xu, W., and Ai, Q., 2016. Effects of oxidised dietary fish oil and high-dose vitamin E supplementation on growth performance, feed utilisation and antioxidant defence enzyme activities of juvenile large yellow croaker (Larmichthys crocea). Br. J. Nutr., 115: 1531-1538. https://doi.org/10.1017/S0007114516000398
Wang, J.S., Zhao, M.M., Zhao, Q.Z. and Jiang, Y.M., 2007. Antioxidant properties of papain hydrolysates of wheat gluten in different oxidation systems. Fd. Chem.,4: 1658-1663. https://doi.org/10.1016/j.foodchem.2006.04.024
Wilson, R.P., Bowser, P.R. and Poe, W.E., 1984. Dietary vitamin E requirement of fingerling channel catfish. J. Nutr., 114: 2053-2058. https://doi.org/10.1093/jn/114.11.2053
Zhang, C.X., Huang, F., Li, J., Wang, L., Song, K. and Mai, K.S., 2016. Interactive effect of dietary magnesium and vitamin E on growth performance, body composition, blood parameters and antioxidant status in Japanese seabass (Lateolabrax japonicus) fed oxidized oil. Aquacult. Nut., 22: 708-722. https://doi.org/10.1111/anu.12393
To share on other social networks, click on any share button. What are these?








