Efficacy of Sulfur With Benomyl For Reducing Effect Fungi of Fusarium solani Infecting Rice
Research Article
Efficacy of Sulfur With Benomyl For Reducing Effect Fungi of Fusarium solani Infecting Rice
Qasim H.A. Aljboori* and Saadoon Murad Saadoon
University of Al-Qadisiyah, Al-Qadisiyah, Iraq.
Abstract | In recent years, the importance of sustainable agriculture has been increased and plant diseases management has become one of the most important challenges in agriculture. Moreover, plant diseases remain a major factor limiting agricultural production. F. solani is one of the most important pathogens that causes rot disease in rice and affects plant growth. Many Fusarium species can infect rice plants and affect its growth, in addition to producing various mycotoxins that are dangerous to humans and animals. Therefore, a study was conducted to evaluate the effectiveness of four levels of agricultural sulphur (0,100,200 and 300 g/m2)and the fungicide Benomyl (100 ppm) against F. solani fungus infecting three varieties of rice (Anbar33,Yasmeen and Mishkhab2) grown in Iraq. The study results showed that sulphur reduced the F. solani infecting rice, with the most effective treatment being the (200) g/m2 treatment compared to the (0,100,300) g/m2 treatments. The results showed that varieties had different infection levels with the Anbar33 variety having the lowest infection rate, reaching having the lowest infection severity (18.25%) and Yasmeen variety was more susceptible to the pathogenic fungus F. solani infection severity (32.08%). A study proved that sulphur was effective in reducing the Effect of F. solani to an acceptable level or at least to a level where further control with the fungicide Benomyl is more successful and less expensive.
Received | August 12, 2024; Accepted | October 28, 2024; Published | November 15, 2024
*Correspondence | Qasim H.A. Aljboori, University of Al-Qadisiyah, Al-Qadisiyah, Iraq; Email: abdulkadhim.qh@qu.edu.iq
Citation | Aljboori, Q.H.A. and S.M. Saadoon. 2024. Efficacy of sulfur with benomyl for reducing effect fungi of Fusarium solani infecting rice. Sarhad Journal of Agriculture, 40(4): 1408-1413.
DOI | https://dx.doi.org/10.17582/journal.sja/2024/40.4.1408.1413
Keywords | Anbar33, Benomyl, Rice, Sulphur, Fusarium solani, Yasmee
Copyright: 2024 by the authors. Licensee ResearchersLinks Ltd, England, UK.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Introduction
Globally, rice is the most important grain crop source for humans. Rice is food for half of the world’s population Rice is grown in 114 countries and is grown excellently in most of the provinces of Iraq. Rice is food for half of the world’s population (Sarwar et al., 2022) and is grown in a wide range of ecosystems (Gopala et al., 2022). Loss crop of grain due to fungi diseases is a long-problem for grains worldwide. One of the most significant groups of phytopatho genic fungi are F. solani .They infects a wide range of crops globally, causing massive economic losses owing to yield decreases and mycotoxin pollution. F. solani has substantial effects on the growth of rice in every country, F. solani significantly reduces rice production. Pramunadipta et al. (2022) addition to mycotoxins, it is a serious problem for human health and the International economy (Altomare et al., 2021; Lee and Ryu, 2017). F. solani is one pathogen that affects cereal crops worldwide such as wheat, rice, and maize in temperate and subtropical regions of Asia, and North America. South Africa and Europe (Moreira et al., 2020; Shi et al., 2016; Wiśniewska et al., 2014; Hafez et al., 2022). F. solani disease is one of the significant obstacles to plant growth and is difficult to control (Wei et al., 2022; Wang et al., 2022) F. solani is a pathogenic plant rot disease belonging to a group of fungi related to a group of diseases such as root rot and stem root (Patel et al., 2022). Diseases caused by F. solani have surpassed F. oxysporum, providing an example of Japan’s recent F. solani disease epidemic (Arie, 2019). Fusarium disease mostly causes inhibits seed rice germination (Eğerci et al., 2022). Sulfur is a micro and macronutrient that is required by all living things. Their multi-fold effectiveness against fungus necessitates their further exploitation in the agricultural sector. Their influence on seed quality further supports their investigation and suitable application as a seed treatment agent, which has the potential to considerably stimulate and expedite seed germination and seedling growth (Sidhu et al., 2017) Fungicides play a role in the control of plant diseases and increase yields as sulfur is used in various forms for crop protection (Rao and Paria, 2013). Benomyl is a highly efficient fungicide that inhibits fungal development and is used in various South American nations to reduce fungal growth in cereals (Llorent-Martínez et al., 2012). Benomyl effectiveness may be reduced under specific circumstances, but at comparable or lower levels to other evaluated fungicides. The findings showed that climate change may have an even greater detrimental impact on climatic situations by lowering the efficiency of antifungal used to control pathogens infections and toxigenic fungus species (Cruz et al., 2014).
Materials and Methods
This laboratory study was conducted during 2023-2024 at the University of Al-Qadisiyah. Iraq to evaluate the effectiveness of the fungicide (Benomyl @ 100 ppm.Shanghai Lavaur Chemical Co. Ltd, China) and agricultural sulphur with the fungicide benomyl in reducing the infestation of the fungus F. solani that infects the rice plants. The study was conducted with a completely randomized design (CRD) with three replicates and Agricultural sulfur was used in the experiment rate of (0, 100, 200 and 300 g/m2). Plant material: Study included three varieties of rice (Anbar33, Yasmeen and Mishkhab2) grown in south-central Iraq. F. solani fungi were isolated from rice grain using the agar plate technique (International Seed Testing Society, 1976). Mixed soil samples were taken at a depth of 25 cm from rice fields. Soil samples were air-dried for 7 days at a room temperature of 25 and were sterilized by steam for 20 min under a pressure of 1.0 bar and 120°. Rice seeds were sterilized with 2% sodium hypochlorite for 2 min. Sulphur was added to the sterilized soil 30 days before planting except for the control sample at the level of (100,200 and 300 g/m2) with three replicates in all experimental treatments.100 P.D.A/g containing F. solani fungi, CFU 200/g was mixed with the soil. Rice seeds were inoculated with a suspension of F. solani fungi using a rotary vibrator at a speed of 70 rpm for 60 min. then air dried, and seeds were planted in plastic pots with dimensions of 100 × 100 × 50 cm containing soil infected with F. solani fungi, and then were watered. After germination of the seeds, they were irrigated as needed. Fertilizer (P2O5 46%) was added to soil at a rate of 100 kg/ ha-1, while nitrogen fertilizers were added at a rate of 100 kg/ ha-1 in the form of urea (N 46%) in two batches, the first in the germination stage and the second in the branching stage. After the plants reached the vegetative growth stage, the recommended dose of benomyl fungicide was used at a concentration of 100 ppm once every 10 days. After the plants reached the age of 40 days, the data regarding plant height, fungal infection severity, germination rate were recorded. A six-point scale was used to determine the severity of infection according to ( Liu et al., 1995). As follows:
0 = Uninfected plant.
1 = 25% of plant leaves are yellowed and wilted.
2 = 26-50% of plant leaves are yellowed and wilted.
3 = 51-75% of plant leaves are yellowed and wilted.
4 = 76-100% of plant leaves are yellowed and wilted.
5 = Dead plants, according to the severity of infection according to the equation:
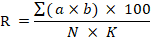
R= Infection severity % ∑ (a×b) = Sum of the number of infected plants (a) multiplied by the corresponding degree on the scale (b). N= Total number of plants. K= Highest degree on the scale and equals 5. The germination rate index was calculated according to (Awasthi et al., 2016). length plant was measured using a ruler on the last day of germination, and the results were analysed and compared using the least significant difference (LSD) test.at P < 0.05 level of significance. The statistical analysis program SPSS was used for statistical interpolation of the data.
Results and Discussion
Table 1 showed the effectiveness of sulfur in inhibiting the growth of the plant pathogenic fungus F. solani. There was a significant effect of sulfur on the germination rate of rice seeds, and it was found that the sulfur treatment at the level of 200 g/m2 + benomyl 100 ppm significantly affected the germination rate, effectiveness of sulfur in inhibiting activity of F. solani fungi, where it reached the highest germination with a value of 85.33 compared to the treatment 0 g/m2. The lowest germination rate was a value of 60.59%, as the high activity of F. solani fungi affected the vitality of rice seeds, which led to a reduction in the germination rate, and compared with treatment 100 g/m2, it was a value of 78.89, where sulfur did not effectively affect the activity of F. solani due to the low content of sulfur in the soil compared to the treatment 200 g/m2, and compared with treatment 300 g/m2 + benomyl 100 ppm, the germination rate decreased to a value of 70.99 due to the high sulfur content in the soil and its effect on the rice plant. The demonstrated variety Anbar33 elevated germination rate value of 77.42% compared to variety Yasmeen, which demonstrated a value of 69.33%. Variety Mishkhab2, which registered a value of 75.33%, and overlapping between treatments, variety Anbar33 200 g/m2 demonstrated the highest germination rate of 88.67% sd compared to treatment 0 g/m2 variety Yasmeen exhibited the lowest germination rate of 56.33%. This is due to the difference in genetic genes of the plant varieties in disease resistance.
It was also found that sulfur had a significant effect on the plant height of rice (Table 2) whereas feeding with sulfur promoted the growth of roots and shoots of the rice plant. These results are in line with some of previous studies. For instance, Zandi et al. (2020) and Thomas et al. (2003) have demonstrated that the sulfur treatment at the level of 200 g/m2 increased the plant height rate by 39.00 cm due to the presence of an optimal amount of sulfur in the soil that effects of F. solani fungi growth and increases rice plant growth compared to the treatment (0.100, and 300 g/m2)+ benomyl 100 ppm and that the sulfur treatment of 300 g/m2 reduced the plant height rate This may be due to root hair burns because of direct contact with sulfur with the root, or rice is sensitive to sulfur at level 300g/m2, as sulfur toxicity affects rice growth and development (Johnson, 2023). While exhibiting sulfur treatment 0g/m2 the lowest plant height rate, equivalent to 29.11 cm due to the absence of sulfur in the soil and the increased activity of F. solani fungi and its effect on the growth of rice plants compared to treatment (100, 200, 300 g/m2)+ benomyl 100 ppm. demonstrated variety Anbar33 most elevated plant height rate value of 39.17cm compared to variety Yasmeen, which showed a value of 32.25 cm, and Variety Mishkhab2 which registered a value of 33.08cm results showed overlapping between treatments, Variety Anbar33 200g/ m2+ benomyl 100 ppm demonstrated highest plant height rate of 42.32 cm compared to treatment 0g/m2 variety Yasmeen which demonstrated the lowest plant height rate 24.67cm.
Table 1: Effect of benomyl and sulfur on the germination percentage of rice seeds under laboratory conditions.
|
Rice varieties |
Benomyl 100 ppm + Sulfur g /m2 |
Means |
|||
|
0 |
100 |
200 |
300 |
||
|
Anbar33 |
63.67 |
84.00 |
88.67 |
73.33 |
77.42 |
|
Yasmeen |
56.33 |
78.00 |
80.67 |
62.33 |
69.33 |
|
Mishkhab2 |
62.67 |
74.67 |
86.67 |
77.33 |
75.33 |
|
٦٠.٥٩ |
78.89 |
85.33 |
70.99 |
||
|
V×T=4.89 |
T= 2.83 |
V= 2.45 |
L.S.D |
||
Note: The germination percentage of rice seeds is shown in percentage.
Table 2: Effect of benomyl and sulfur on the plant height of rice under laboratory conditions.
|
Rice varieties |
Sulfur g/m2 + Benomyl 100 ppm |
Means |
|||
|
0 |
100 |
200 |
300 |
||
|
Anbar33 |
35.00 |
41.00 |
42.33 |
38 .33 |
39.17 |
|
Yasmeen |
24.67 |
32.33 |
39.00 |
33 .00 |
32.25 |
|
Mishkhab2 |
27.67 |
30.33 |
35.67 |
38.67 |
33.08 |
|
٢٩.١١ |
34.55 |
39.00 |
36.00 |
||
|
V×T= 3.34 |
T=1.93 |
V= 1.67 |
L.S.D |
||
Note: The height of rice plants is shown in centimeters.
Table 3 demonstrated a significant effect on the rate of infection of F. solani in rice Sulfur is considered an organic pesticide to combat fungal diseases that affect plants (Sokovic et al., 2013). due to the soil containing a level of sulfur that reduces the activity of F. solani fungi and its effect on the plant compared to
Table 3: Effect of benomyl and sulfur on the infection of rice plants by Fusarium solani under laboratory conditions.
|
Rice varieties |
Sulfur g/m2 + Benomyl 100 ppm |
Means |
|||
|
0 |
100 |
200 |
300 |
||
|
Anbar33 |
25.33 |
18.67 |
14.33 |
14.67 |
18.25 |
|
Yasmeen |
36.67 |
32.33 |
29.00 |
30.33 |
32.08 |
|
Mishkhab2 |
29.33 |
25.67 |
19.33 |
19.67 |
23.50 |
|
٣٠.٤٤ |
25.55 |
20.88 |
21.55 |
||
|
V=0.97 |
T= 1.12 |
V ×T=1.9 |
L.S.D |
||
Note: infection of rice plants by F. solani percentage is shown in percentage.
(0.100 g/m2), where the infection rate of F. solani fungi value of 30.44% when treating sulfur at (rate 0 g/m2) and 25.55% at the level of 200 g/m2 due to the low sulfur content in the soil. Compared to the treatment of (300) g/m2 + Benomyl 100 ppm, the infection rate increased to a value of 21.55 due to the high percentage of sulfur in the soil and its effect on growth of the rice plant compared to the treatment of 200 g/m2. Results showed variety Anbar33 lowest infection rate of fungi F. solani value of 18.25 compared to variety Yasmeen, which demonstrated the highest infection F. solani value of 32.25% Variety Mishkhab2 demonstrated an infection rate value of 23.50% and It was found that the interaction between the treatments in the experiment showed that the Anber33 variety recorded the lowest infection rate of 14.33% at the sulfur level (200 g/m2+ benomyl 100 ppm) compared to the Yasmeen variety, which recorded the highest infection rate of 36.67% at the sulfur level 0g/m2. These results are due to the effect of sulfur in soil, which affected the balance of fungi present in the soil (Jiao and Lu, 2020).
Conclusions and Recommendations
Sulphur can reduce the growth and infection of F. solani to an acceptable level or at least to a level where further control with the fungicide Benomyl is more successful and less expensive. Sulfur is an important element that stimulates plant growth and forms part of the plant’s defense against pathogen infection and invasion. Sulfur has shown antifungal activity against F. solani. According to the research results, the Variety differed in their level of infection. Among the varieties, Anbar33 was the least infected by F. solani, while variety Yasmeen was the most infected by F. solani. Rice was negatively affected by Benomyl (@ 300g/m2) for all rice varieties (Anbar33,Yasmeen and Mishkhab2).
Acknowledgements
The authors a grateful for the support from University of Al-Qadisiyah for conducting the study.
Novelty Statement
This study determines the best level of sulfur g/m2 effective with the fungicide Benomyl in reducing the effect of F. solani infecting three varieties of rice (Anbar33, Yasmeen and Mishkhab2).
Author’s Contribution
Qasim H.A. Aljboori: Planned the experiment and wrote the manuscript
Saadoon, S.M: Analyzed the data.
Conflict of interest
The authors have declared no conflict of interest.
References
Altomare, C., A.F. Logrieco and A. Gallo. 2021. Mycotoxins and mycotoxigenic fungi: Risk and management. A challenge for future global food safety and security. https://doi.org/10.1016/B978-0-12-819990-9.00032-9
Arie, T., 2019. Fusarium diseases of cultivated plants, control, diagnosis, and molecular and genetic studies. J. Pestic. Sci., 44(4): 275-281. https://doi.org/10.1584/jpestics.J19-03
Awasthi, P., H. Karki, K. Bargali and S.S. Bargali. 2016. Germination and seedling growth of pulse crop (Vigna spp.) as affected by soil salt stress. Curr. Agric. Res. J., 4(2): 159-170. https://doi.org/10.12944/CARJ.4.2.05
Cruz, A., P. Marin, N. Magan and M.T. Gonzalez-Jaen. 2014. Combined effects of benomyl and environmental factors on growth and expression of the fumonisin biosynthetic genes FUM1 and FUM19 by Fusarium verticillioides. Int. J. Food Microbiol., 191: 17-23. https://doi.org/10.1016/j.ijfoodmicro.2014.08.026
Eğerci, Y., P.K. Teksür and A.U. Morca. 2022. Identification of Fusarium andiyazi associated with the Bakanae disease of rice in Turkey. Curr. Microbiol., 79(10): 291. https://doi.org/10.1007/s00284-022-02962-x
Gopala, K.S., K.K. Vinod, P.K. Bhowmick, H. Bollinedi, R.K. Ellur, R. Seth and A.K. Singh. 2022. Rice breeding. In: Fundamentals of field crop breeding. Singapore: Springer Nature Singapore. pp. 113-220. https://doi.org/10.1007/978-981-16-9257-4_3
Hafez, M., R. Gourlie, M. Telfer, N. Schatz, T.K. Turkington, B. Beres and R. Aboukhaddour. 2022. Diversity of Fusarium solani associated with wheat node and grain in representative sites across the Western Canadian Prairies. Phytopathology, 112(5): 1003-1015. https://doi.org/10.1094/PHYTO-06-21-0241-R
International Seed Testing Association, 1976. International rules for seed testing. Seed Sci. Technol., 4: 51-77.
Jiao, S. and Y. Lu. 2020. Abundant fungi adapt to broader environmental gradients than rare fungi in agricultural fields. Glob. Change Biol., 26(8): 4506-4520. https://doi.org/10.1111/gcb.15130
Johnson, K., 2023. The effect of sulfate contamination of water on wild rice nutrient composition (Doctoral dissertation, University of Minnesota).
Lee, H.J. and D. Ryu. 2017. Worldwide occurrence of mycotoxins in cereals and cereal-derived food products: Public health perspectives of their co-occurrence. J. Agric. Food Chem., 65(33): 7034-7051. https://doi.org/10.1021/acs.jafc.6b04847
Liu, L., J.W. Kloepper and S. Tuzun. 1995. Induction of systemic resistance in cucumber against Fusarium wilt by plant growth-promoting rhizobacteria. Phytopathology, 85(6): 695-698. https://doi.org/10.1094/Phyto-85-695
Llorent-Martínez, E.J., M.L. Fernández-de Córdova, A. Ruiz-Medina and P. Ortega-Barrales. 2012. Fluorimetric determination of thiabendazole residues in mushrooms using sequential injection analysis. Talanta, 96: 190-194. https://doi.org/10.1016/j.talanta.2011.12.072
Moreira, G.M., F.J. Machado, C.B. Pereira, D.L. Neves, D.J. Tessmann, T.J. Ward and E.M. Del Ponte. 2020. First report of the Fusarium tricinctum species complex causing Fusarium head blight of wheat in Brazil. Plant Dis., 104(2): 586-586. https://doi.org/10.1094/PDIS-03-19-0552-PDN
Patel, R., K. Mehta, J. Prajapati, A. Shukla, P. Parmar, D. Goswami and M. Saraf. 2022. An anecdote of mechanics for Fusarium biocontrol by plant growth promoting microbes. Biol. Contr., pp. 105012. https://doi.org/10.1016/j.biocontrol.2022.105012
Pramunadipta, S., A. Widiastuti, A. Wibowo, H. Suga and A. Priyatmojo. 2022. Identification and pathogenicity of Fusarium solani. associated with the sheath rot disease of rice (Oryza sativa) in Indonesia. J. Plant Pathol., 104: 251–267. https://doi.org/10.1007/s42161-021-00988-x
Rao, K.J. and S. Paria. 2013. Use of sulfur nanoparticles as a green pesticide on Fusarium solani and Venturia inaequalis phytopathogens. RSC Adv., 3(26): 10471-10478. https://doi.org/10.1039/c3ra40500a
Sarwar, N., H. Ali, A. Wasaya, O. Farooq, K. Mubeen, M. Dawood and S. Ahmad. 2022. Smart nutrient management in rice crop. In: Modern techniques of rice crop production. Singapore: Springer Singapore. pp. 85-103. https://doi.org/10.1007/978-981-16-4955-4_7
Shi, W., Y. Tan, S. Wang, D.M. Gardiner, S. De Saeger, Y. Liao and A. Wu. 2016. Mycotoxigenic potentials of Fusarium species in various culture matrices revealed by mycotoxin profiling. Toxins, 9(1): 6. https://doi.org/10.3390/toxins9010006
Sidhu, A., H. Barmota and A. Bala. 2017. Antifungal evaluation studies of copper sulfide nano-aquaformulations and its impact on seed quality of rice (Oryzae sativa). Appl. Nanosci., 7: 681-689. https://doi.org/10.1007/s13204-017-0606-7
Soković, M.D., J.M. Glamočlija and A.D. Ćirić. 2013. Natural products from plants and fungi as fungicides. Fungicides-showcases of integrated plant disease management from around the world, pp. 185-232.
Thomas, S.G., T.J. Hocking and P.E. Bilsborrow. 2003. Effect of sulphur fertilisation on the growth and metabolism of sugar beet grown on soils of differing sulphur status. Field Crops Res., 83(3): 223-235. https://doi.org/10.1016/S0378-4290(03)00075-3
Wang, Y., R. Wang and Y. Sha. 2022. Distribution, pathogenicity and disease control of Fusarium tricinctum. Front. Microbiol., 13. https://doi.org/10.3389/fmicb.2022.939927
Wei, F., N. Ma, H.A. Haseeb, M. Gao, X. Liu and W. Guo. 2022. Insights into structural and physicochemical properties of maize starch after Fusarium verticillioides infection. J. Food Compos. Anal., 114: 104819. https://doi.org/10.1016/j.jfca.2022.104819
Wiśniewska, H., L. Stępień, A. Waśkiewicz, M. Beszterda, T. Góral and J. Belter. 2014. Toxigenic Fusarium species infecting wheat heads in Poland. Central Eur. J. Biol., 9: 163-172. https://doi.org/10.2478/s11535-013-0262-1
Zandi, P., J. Yang, X. Xia, Y. Tian, Q. Li, K. Możdżeń and Y. Wang. 2020. Do sulfur addition and rhizoplane iron plaque affect chromium uptake by rice (Oryza sativa L.) seedlings in solution culture? J. Hazard. Mater., 388: 121803. https://doi.org/10.1016/j.jhazmat.2019.121803
To share on other social networks, click on any share button. What are these?








