Efficacy of Locally Available Anthelmintics against Helminths of Dairy Cows in Gazipur, Bangladesh
Research Article
Efficacy of Locally Available Anthelmintics against Helminths of Dairy Cows in Gazipur, Bangladesh
Md. Aminul Islam1*, SM Mostafizur Rahaman Sumon1, ANM Aminoor Rahman2, Fahima Khatun3
1Department of Medicine, Faculty of Veterinary Medicine and Animal Science, Bangabandhu Sheikh Mujibur Rahman Agricultural University, Gazipur 1706, Bangladesh; 2Department of Gynecology, Obstetrics and Reproductive Health, Faculty of Veterinary Medicine and Animal Science, Bangabandhu Sheikh Mujibur Rahman Agricultural University, Gazipur 1706, Bangladesh; 3Department of Pathobiology, Faculty of Veterinary Medicine and Animal Science, Bangabandhu Sheikh Mujibur Rahman Agricultural University, Gazipur 1706, Bangladesh.
Abstract | Parasitic diseases are crucial factors in reducing dairy production. To find out the efficacy of locally available anthelmintics against helminths, anthelmintic treatments were given to dairy cows in five different farms of Gazipur, Bangladesh and the helminth parasitic loads of dairy cows were determined before and after treatment by Modified Stoll’s Dilution Technique using a McMaster counting chamber. A total of 85 faecal samples from five different dairy farms were examined. The overall infection rate of helminths in dairy cows is 37.65%. A total of five different types of nematodes were observed in all the investigated dairy farms. The highest infection rate (11.76%) and the lowest infection rate (4.71%) was found for Neoascaris spp. (Toxocara spp.) and Fasciola spp., respectively. Hemonchus spp. were fully (100%) susceptible to Ivermectin, Fenbendazole, and Levamisole. Neoascaris spp. were shown 33.33% and 16.67% resistant against Ivermectin and Fenbendazole, respectively. The Fasciola spp. was 66.67% resistant against Triclabendazole and the Trichuris spp. was the highest resistant against Ivermectin injection (100%) in all the investigated dairy farms. These findings are crucial for the selection of anthelmintics for the treatment of various types of nematodes in dairy cows and might be crucial in preventing anthelmintic resistance in dairy cows.
Keywords | Anthelmintic resistance, Dairy cows, Effectiveness, Helminth infections, Local anthelmintics
Received | October 21, 2022; Accepted | November 15, 2022; Published | November 30, 2022
*Correspondence | Md. Aminul Islam, Department of Medicine, Faculty of Veterinary Medicine and Animal Science, Bangabandhu Sheikh Mujibur Rahman Agricultural University, Gazipur 1706, Bangladesh; Email: [email protected]
Citation | Islam MA, Sumon SMMR, Rahman ANMA, Khatun F (2022). Efficacy of locally available anthelmintics against helminths of dairy cows in Gazipur, Bangladesh. Adv. Anim. Vet. Sci. 10(12): 2630-2640.
DOI | http://dx.doi.org/10.17582/journal.aavs/2022/10.12.2630.2640
ISSN (Online) | 2307-8316

Copyright: 2022 by the authors. Licensee ResearchersLinks Ltd, England, UK.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Introduction
Dairy is an essential part of the global food circulation system and it plays a crucial role in the sustainability of rural areas in particular. Globally, about one billion people are living on dairy farms (Shamsuddin et al., 2007). The dairy industry actively contributes to the economies of many communities, regions, and countries (IDF, 2013; Islam et al., 2018). The dairy sector can play an important role in providing jobs for rural communities (Islam et al., 2019; Haque et al., 2021). Dairy production and processing provide employment, not only to people who work on dairy farms or in dairy plants but also to the whole sector, from upstream (inputs and services providers…) to downstream (marketing of finished products…) (Shamsuddin et al., 2007; Islam et al., 2020a). The dairy sector is a dynamic global industry, with growing production (since 2000 on an average +2.2% growth annually) which is forecast to continue in the long-term (IDF, 2013). Consumption of dairy products is consequently expected to increase by 20% in future, according to FAO and OECD (DLS, 2008). In this context, dairy production and dairy processing appear as industries of utmost importance in contributing to the global challenge of today’s food security and for decades to come (IDF, 2013; Islam et al., 2019a).
Although the dairy sector has lots of potentials, these dairy animals are infected with lots of infectious, metabolic, nutritional, and parasitic diseases. During the last decade, evidence has been generated that gastrointestinal helminth negatively affects the performance of adult dairy animals (Vercruysse and Claerebout, 2001). The effects of parasitism can be separated into two types – subclinical and clinical (Islam et al., 2020). Losses in animal productivity (milk production, weight gain, altered carcass composition, conception rate, etc.) are subclinical effects; whereas, visible, disease-like symptoms (roughness of coat, anaemia, oedema, diarrhoea) are clinical effects (Charlier et al., 2009). Many trials have estimated the effects of gastrointestinal helminths by measuring the association between infection levels and production parameters or by assessing the effect of anthelmintic treatment on production traits (Vercruysse and Claerebout, 2001; Smith, 1997). The distribution of parasites in adult dairy cows is overdispersed (Agneessens et al., 2000; Borgsteede et al., 2000; Islam et al., 2015) and the prevalence of gastrointestinal helminths in dairy cows were determined previously in different areas of Bangladesh (Alim et al., 2012; Islam et al., 2015; Rahman et al., 2018). It is evident from either artificial infection or anthelmintic control studies that helminths have several adverse impacts on dairy cows, including effects on milk production, suckler calf weaning weight and fertility (Hawkins, 1993). The subclinical effects are of major economic importance to the producer and can induce a decrease in milk production of up to and even over 11% and could be responsible for chronic and insidious economic losses in adult dairy cows (Charlier et al., 2009; Gross et al., 1999; Sanchez et al., 2004).
Due to the negative effects of parasites on dairy cows, it is essential to control parasitic diseases in dairy animals. In the absence of other alternatives, parasite control continues to rely on anthelmintics because of their high performance and their use is likely to continue in the foreseeable future as the first and foremost line of defence against parasites (Martin, 1985; Rahman et al., 2018). Because of the haphazard and extensive use of anthelmintics, the helminth parasites become resistant to commonly used anthelmintics and ultimately treatment failure occurs (Besier, 2007). Thus, the determination of anthelmintic resistance against helminths is crucial for the development and application of new helminth control methods. Furthermore, the determination of the underlying mechanisms for the development of anthelmintic resistance in dairy cows is also crucial (Cotter et al., 2015).
There is a scarcity of studies to determine the resistance of locally available anthelmintics against helminths in Bangladesh (Rahman et al., 2018; Hoque et al., 2003). By considering the above facts, the present work has been undertaken to obtain detailed information on the parasitic infestation in dairy cows and evaluate the efficacy of some locally available anthelmintics and the degree of resistance (if any) against helminth parasites in some selected dairy farms of Gazipur district, Bangladesh. The first objective is to determine the common gastrointestinal helminths infecting small-scale dairy cows in the Gazipur district of Bangladesh. The second objective is to treat infected dairy cows in vivo using locally available specific anthelmintics against helminths to determine the efficacy of commonly used anthelmintics against helminths to interpret the degree of anthelmintic resistance (if any) at Gazipur, Bangladesh. The findings are essential in the selection of effective anthelmintics for the treatment of helminths infections in dairy cows. The findings might be crucial for the prevention of anthelmintic resistance in dairy cows globally.
Materials and Methods
The present study is designed to determine the efficacy of locally available anthelmintics against helminths, anthelmintic treatments, which given to dairy cows in five different dairy farms.
Study area and period
The research was undertaken on 5 (five) different dairy farms in the Gazipur district of Bangladesh. Gazipur is the industrial city of Bangladesh and is considered a highland city of Bangladesh. The dairy farms in Gazipur are mostly small-scale and consist of 5–10 dairy cows. The Bangabandhu Sheikh Mujibur Rahman Agricultural University (BSMRAU) dairy farm is established for teaching and research purposes. The small-scale dairy farms are owned by the farmers. All the selected farms were fed dairy cows mostly rice straw and concentrate consisting of rice polish, wheat bran, maize crush, and oil cake (Table 1). The cows were supplied with limited green grass with zero grazing in the pasture land. The age of all the cows was 2–4 years. Among all the tested farms, only the BSMRAU dairy farm was routinely dewormed by different types of locally available anthelmintics to all the dairy cows without identifying parasite infected cows. Before conducting the field experiment, the BSMRAU dairy farm treated dairy cows 3 (three) times subsequently at 3–4 months intervals. Except for the BSMRAU dairy farm, the other 4 (four) farms did not routinely deworm dairy cows (Table 1). All the cows of all five farms were apparently healthy and did not show any visible signs of helminth infections. The study was conducted from July 2015 to June 2016.
Questionnaire
Each dairy cattle farm owner was asked basic questions regarding the use of anthelmintics for deworming dairy cows, their properties, specifically, which anthelmintics had been used and the policy determining when the dairy cows were treated. Information about feeding and grazing was also gathered from the record book (Table 1).
Sample collection and storage
About 10–20g of fresh faecal samples were collected from every animal by inserting the hand directly into the animal’s rectum. The faecal samples were then put into a sample collection vial with 10% formalin solution and shifted to the laboratory & kept refrigerated until examination (Islam et al., 2020). All the cows of all the dairy farms were sampled for gastrointestinal helminths.
Sample examination
The quantitative estimation of helminth eggs was done by employing ‘Modified Stoll’s Dilution Technique’ by using a McMaster counting chamber as described previously (Soulsby, 1982). In short, three grams of well-mixed faecal sample was put into a 100 (hundred) mL beaker containing 42 (forty-two) mL of zinc sulfate solution with a specific gravity of 1.25–1.30. Then some glass beads were added to the beaker. Then the mixture was thoroughly mixed with a stirrer. The mixture then was strained with a coffee strainer, shaken well and 0.15 mL was taken using 1 (one) mL special pipette and put on two chambers of the McMaster slide and allowed the counting chambers to stand for 5 (five) minutes. Care was taken to avoid bubble formation during putting the sample on the slide. The slide was then examined under a compound microscope at 10 × 10 magnification. The eggs of specific helminths were identified based on morphological features and counted (Soulsby, 1982; Hendrix, 2006; Valero et al., 2009). The counts were made from each sample and the total number of eggs of parasites found in the slide was multiplied by 100 to get the eggs per gram of faeces (EPG). The EPG was calculated using Eq. (1).
EPG = 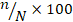 (1).
(1).
Where n is the number of eggs on a smear and N is the times/frequency of 0.15 mL samples examined (1–7 smears in this study). A subsequent 7 (seven) smears were examined before considering a sample as a negative sample.
Anthelmintic treatment
Anthelmintic resistance tests were conducted in vivo only with slight modifications from previous studies (Coles et al., 1992; Coles et al., 2006). The weight of the individual dairy animals were measured using the scale. After identifying infected dairy cattle, the infected cattle were divided into four groups and treated by using commonly used local anthelmintics (Rahman et al., 2017) and are as follows:
(i) Oral Fenbendazole at 7.5 mg per kg body weight (Trade name: Peraclear bolus: Techno drugs) for nine infected dairy animals. Each bolus contains Fenbendazole 250 mg.
(ii) Injectable Ivermectin subcutaneously at 0.2 mg per kg body weight (Trade name: Vermic injection: Techno drugs Ltd., Ivermectin 10 mg/mL) for eight infected dairy animals.
(iii) Oral Levamisole and Triclabendazole combination at 6.75 mg per kg body weight (Trade name: Levex bolus: ACME Laboratories Ltd) for 10 infected dairy animals. Each bolus contains Levamisole 600 mg and Triclabendazole 900 mg.
In the local market, Levamisole orally at 8 mg per kg body weight (Trade name: Levavet bolus: ACME Laboratories Ltd., Each bolus contains Levamisole 600 mg) were used for the treatment of nematodes and Triclabendazole 12 mg/kg body weight (Trade name: Fasinex bolus: Novartis, Bangladesh Ltd., Each bolus contains Triclabendazole 900 mg) were used for the treatment of Fasciola spp. During the experiment period, due to the unavailability of the Levavet bolus (Levamisole only) and Fasinex bolus (Triclabendazole only), Levex bolus (combination of Levamisole and Triclabendazole) is used for the treatment of both nematodes and Fasciola spp. Anthelmintic treatments were done as per the recommendation of the company.
(iv) The control group (untreated). Five infected dairy animals from five different dairy farms.
Except for the control group, all the positive 27 (twenty seven) cows were treated with various types of anthelmintics. After 14 (fourteen) days of treatment, again faecal samples were collected, stored (Flanagan et al., 2011) and examined by the same technique described previously (Soulsby, 1982) and the efficacy of different anthelmintics were compared by using faecal worm egg count reduction test (FWCRT) using the Eq. 2.
Efficacy =  (2).
(2).
Where FWCO is= the faecal worm egg count before anthelmintic treatment (0 days) and FWC14 is the faecal worm egg count after anthelmintic treatment (at 14 days).
Interpretation of resistance
The reduction percentage of eggs of specific helminths after treatment were determined by using Eq. 2. If the egg reduction after treatment is < 95%, then the parasite is considered to be resistant to that specific anthelmintic
Table 1: Descriptive characteristics and management factors of the dairy farms
| Name of dairy farms |
Total number of dairy animals |
Grazing in the pasture land |
Roughage supplied |
Concentrate supplied |
Anthelmintic treatment |
Supplied vitamin- mineral premixes |
| Hedayet dairy farm | 13 | No | Rice straw, limited green grass | Rice polish, wheat bran | No anthelmintic treatment | Table salt with concentrate daily orally |
| BSMRAU dairy farm | 41 | No | Rice straw, sufficient green grass | Rice polish, wheat bran, maize crush, oil cake | Regular anthelmintic treatment at 3–4 months intervals using locally available anthelmintics | Parenteral multivitamin-mineral premix at 3–4 months intervals and oral table salt & vitamin-mineral premix with concentrate daily |
| Porabari moddho para dairy farm | 11 | No | Rice straw, limited green grass | Rice polish, wheat bran | No anthelmintic treatment | Oral table salt with concentrate daily |
| Abu Taleb dairy farm | 09 | No | Rice straw, limited green grass | Rice polish, wheat bran | No anthelmintic treatment | Table salt with concentrate daily orally |
|
Mojibor dairy farm |
11 | No | Rice straw, limited green grass | Rice polish, wheat bran | No anthelmintic treatment | Table salt with concentrate daily orally |
as described previously (Cotter et al., 2015). Data analysis were performed in MS Excel in Windows version 10.
Results
A total of 85 (eighty five) samples were collected from 5 (five) different dairy farms in the Gazipur district, Bangladesh. On ‘Modified Stoll’s Dilution Technique’ ova of 5 (five) different types of helminths were found. The EPG (egg per gram) of different helminths was 20–100. The mean ± SD of EPG of Hedayet dairy farm, BSMRAU dairy farm, Porabari moddho para dairy farm, Abu Taleb dairy farm, and Mojibor dairy farm were 25.75 ± 5.38, 34.05 ± 20.47, 29.67 ± 10.80, 22.50 ± 2.89, and 22.50 ± 2.89, respectively. Among 85 samples, 32 samples were positive for different helminths. The overall infection of different types of helminths was 37.65% (Table 2). The infection rate of Neoascaris spp., Hemonchus spp., Trichuris spp., Capillaria spp., and Fasciola spp. was 11.76, 8.24, 7.06, 5.88 and 4.71%, respectively in different farms (Table 2). A total of 27 treatments were given in different 5 (five) farms (leaving five positive animals as a positive control on five farms) using different types of local anthelmintics against five different types of helminths (Table 2). The mean efficacy of Ivermectin injection against Hemonchus spp., Neoascaris spp., Trichuris spp., and Capillaria spp. were 100, 66.67, 0, and 100%, respectively and the mean resistance of Ivermectin injection against Hemonchus spp., Neoascaris spp., Trichuris spp., and Capillaria spp. were 0, 33.33, 100, and 0%, respectively. The mean efficacy of Fenbendazole tablets against Hemonchus spp., Neoascaris spp., and Trichuris spp were 100, 83.83, and 100%, respectively, and the mean resistance of Fenbendazole tablets against Hemonchus spp., Neoascaris spp., and Trichuris spp. were 0, 16.17, and 0%, respectively. The mean efficacy of Levamisole bolus against Neoascaris spp., Hemonchus spp., Capillaria spp., and Trichuris spp., were 100, 100, 100, and 100%, respectively and mean resistance of Levamisole bolus against Neoascaris spp., Hemonchus spp., Capillaria spp., and Trichuris spp., were 0, 0, 0, and 0%, respectively (Table 2). The mean efficacy of Triclabendazole against Fasciola spp. was 33.33% and mean resistance of Triclabendazole against Fasciola spp. was 66.67%. Except for the BSMRAU dairy farm, all other 4 (four) farms were fully susceptible to all the tested anthelmintics. The highest resistance (66.67%) was observed for Triclabendazole against Fasciola spp. in all the investigated dairy farms (Figure 1). The BSMRAU dairy farm alone was shown 75, 100, and 25% resistant Ivermectin, Triclabendazole, and Fenbendazole, respectively against different types of helminths e.g., Neoascaris spp., Trichuris spp., and Fasciola spp. (Figure 2).
Discussion
At present, anthelmintic and/or antimicrobial drug resistance-free livestock production is one of the critical challenges of sustainable animal agriculture production (Islam et al., 2021; Haque et al., 2020; Naide et al., 2018). Effective surveillance, specific diagnosis, and accurate treatment are prerequisites for the control of gastrointestinal helminths in dairy cattle to boost production from dairy animals. The overall infection rate of gastrointestinal helminths in the
Table 2: Farm wise distribution of different helminths and the efficacy of different anthelmintics against helminths in Gazipur, Bangladesh
| Name of dairy farm |
Total samples |
Infec tion (%) |
Name of parasites |
Anthelmintic treatment |
EPG |
Anthe lmintic efficacy/ reduction (%) |
|||||
| Exam ined |
Positive for para sites |
Vermic injection |
Pera clear tablet |
Lev ex bolus |
Before treat ment |
After treat ment |
Control sample |
||||
| Hedayet dairy farm | 13 | 4 | 30.77 |
Hemonchus spp. |
✔ |
× | × | 25 | 0 |
Capillaria spp. (EPG 20) |
100 |
|
Hemonchus spp. |
× | ✔ | × | 25 | 0 | 100 | |||||
|
Neoascaris spp. |
× | × | ✔ | 33 | 0 | 100 | |||||
| BSMRAU dairy farm | 41 | 14 | 34.15 |
Neoascaris spp. |
× | ✔ | × | 33 | 0 |
Trichuris spp. (EPG 25) |
100 |
|
Neoascaris spp. |
× | ✔ | × | 100 | 50 | 50 | |||||
|
Hemonchus spp. |
× | ✔ | × | 33 |
0 |
100 | |||||
|
Neoascaris spp. |
✔ | × | × | 25 | 0 | 100 | |||||
|
Hemonchus spp. |
× | × | ✔ | 33 | 0 | 100 | |||||
|
Trichuris spp. |
✔ | × | × | 20 | 17 | 15 | |||||
|
Neoascaris spp. |
× | ✔ | × | 50 | 0 | 100 | |||||
|
Fasciola spp. |
× | × | ✔ | 33 | 20 | 39 | |||||
|
Capillaria spp. |
× | × | ✔ | 20 |
0 |
100 | |||||
|
Trichuris spp. |
× | × | ✔ | 20 | 0 | 100 | |||||
|
Fasciola spp. |
× | × | ✔ | 25 | 17 | 32 | |||||
|
Trichuris spp. |
✔ | × | × | 33 |
17 |
50 | |||||
|
Neoascaris spp. |
✔ | × | × | 33 | 17 | 50 | |||||
| Porabari moddho para dairy farm | 11 | 6 | 54.55 |
Hemonchus spp. |
× | × | ✔ | 50 | 0 |
Hemonchus spp. (EPG 25) |
100 |
|
Fasciola spp. |
× | × | ✔ | 33 | 0 | 100 | |||||
|
Neoascaris spp. |
× | ✔ | × | 25 | 0 | 100 | |||||
|
Trichuris spp. |
× | ✔ | × | 25 | 0 | 100 | |||||
|
Hemonchus spp. |
✔ | × | × | 20 | 0 | 100 | |||||
| Abu Taleb dairy farm | 9 | 4 | 44.44 |
Neoascaris spp. |
✔ | × | × | 25 | 0 |
Fasciola spp. (EPG 20) |
100 |
|
Neoascaris spp. |
× | ✔ | × | 25 | 0 | 100 | |||||
|
Capillaria spp. |
× | × | ✔ | 20 | 0 | 100 | |||||
| Mojibor dairy farm | 11 | 4 | 36.36 |
Neoascaris spp. |
× | ✔ | × | 25 | 0 |
Capillaria spp. (EPG 20) |
100 |
|
Trichuris spp. |
× | × | ✔ | 25 | 0 | 100 | |||||
|
Capillaria spp. |
✔ | × | × | 20 | 0 | 100 | |||||
|
Total/ range
|
85 | 32 | 37.64 |
6 = Hemonchus spp. 10 = Neoascaris spp. 5 = Trichuris spp. 3 = Fasciola spp. 3 = Capillaria spp. |
8 | 9 | 10 | 20–100 | 0–50 | 20–25 |
15–100 |
✔ Indicates treatment and × indicates no treatment
present study is 37.65% (Table 2) which is considered a moderate infection. Previously helminth infections in dairy cattle were also reported (Keyyu et al., 2006; Epherem, 2007; Alim et al., 2012) which supports our present study. This moderate infection could be considered an insidious enemy of dairy cows because the moderate infection sometimes goes unnoticed without any clinical signs. This type of moderate infection deprives nutrition from dairy cows and reduces the production of meat, milk, etc. from dairy cows (Khatun et al., 2021). Thus, moderate helminth infection might cause huge economic losses for the dairy industry due to reduced earnings from dairy farms.
The EPG of different parasites in the present study (20–100) is comparatively lower than in previous studies (Islam et al., 2015; Geurden et al., 2015). This is because of the differences in geography (high land), management (zero-grazing in pasture land with limited green grass), and age of the cows (Hoste et al., 2005; Pfukenyi and Mukaratirwa, 2013; Tulu and Lelisa, 2016). The geography, management, nutrition, and age of cows, etc. have profound effects on the load of the parasites (Talukder et al., 2015; Islam et al., 2020b). Before establishing infection, adult dairy cows expel parasites that are ingested and usually acquire immunity (Dunn, 1978) that’s why the EPG in the present study might be lower.
At present, dairy cows were infected predominantly with Neoascaris spp. (11.76%) (Table 2). Previously the higher infection rate of dairy cows with Neoascaris spp. was also reported (Rajakaruna and Warnakulasooriya, 2011) which supports our present study. Neoascaris spp. are very critical helminth parasites for dairy animals. They might be responsible for producing diarrhoea, biliary and intestinal obstruction, poor performance, and death of dairy cows. Besides these the migrating larvae of Neoascaris spp. damages many internal organs of dairy cows including the lungs and might predispose them to secondary bacterial infections. They can transmit to the dairy calves through milk (Khatun et al., 2021). Along with diarrhoea, colic, inappetence, and weight loss, these helminths could cause intestinal obstruction and death of dairy calves. So effective preventive measures are essential for the prevention of Neoascaris spp. infection in dairy cows.
The infection caused by Hemonchus spp., Trichuris spp., and Capillaria spp. in the present study were considered moderate. These helminths are crucial for the maintenance of the health of adult dairy cows. They could cause diarrhoea, inappetence, rough coat due to loss of nutrition, and ultimately reduced production from dairy cows. A relatively lower rate of infection (4.71%) was observed in dairy cows with Fasciola spp. which is almost similar to the findings
of a previous study (Höglund et al., 2010). This lower rate of infection does not mean that Fasciola spp. infection in small-scale dairy cattle could be overlooked. Fasciola spp. is an important trematode in dairy cows that affects the liver of the animals and is responsible for global production losses from dairy animals and negatively affects the world economy (Beesley et al., 2018; Schweizer et al., 2005). Furthermore, it was reported that Fasciola spp. infected cattle are more prone to Salmonella dublin infection (Vaessen et al., 1998). So measures should be taken to prevent infection of Fasciola spp in dairy cows.
Among all the 5 (five) investigated farms, except the BSMRAU dairy farm, all the cows of the other 4 (four) farms were fully susceptible to all the tested anthelmintics (Table 2). Thus Fenbendazole, Ivermectin, Triclabendazole, and Levamisole are cent percent effective against Hemonchus spp., Neoascaris spp., Capillaria spp., Fasciola spp., and Trichuris spp. in naïve dairy cows. This indicates that there was no development of anthelmintic resistance against used anthelmintics in the other four farms (Table 2) except the BSMRAU dairy farm. The most likely reason is that the farmers of those 4 (four) farms had not used any anthelmintics for the routine treatment of the dairy cows (Table 1) maintaining an adequate level of helminth refugia (proportion of helminth population that are not exposed to used anthelmintics of this study). Thus Hemonchus spp., Neoascaris spp., Capillaria spp., Fasciola spp., and Trichuris spp. of four farms were not exposed to Fenbendazole, Ivermectin, Triclabendazole, and Levamisole previously. As a result, the anthelmintic resistance genes were not developed in helminths of other 4 (four) farms as evidenced by a previous study (Shalaby, 2013). As a result, these helminths of 4 (four) farms (except BSMRAU dairy farm) are fully susceptible to anthelmintics used in the present study.
Among all the 5 (five) farms, the BSMRAU dairy farm showed < 95% egg reduction of Neoascaris spp., Trichuris spp., and Fasciola spp. against Fenbendazole & Ivermectin, Ivermectin, and Triclabendazole (Table 2 and Figure 2) respectively. These findings indicate that there is the development of anthelmintic resistance helminths in the BSMRAU dairy farm. This form of anthelmintic resistance against different helminths could be considered as a limitation for the effective control of these nematodes in dairy cows. The probable reason is that this farm was dewormed three times to all the dairy cows routinely (3–4 months interval) before our experiment without identifying parasite-infected cattle (Table 1). From the present findings we can conclude that a subsequent third-time indiscriminate treatment in dairy cows using different types of anthelmintics might cause the development of resistant Neoascaris spp., Trichuris spp., and Fasciola spp. against Fenbendazole, Ivermectin, and Triclabendazole, respectively in dairy cows significantly. Previously development of anthelmintic resistant helminths (Geurden et al., 2015; Kelley et al., 2020; Cristel et al., 2017) and antibiotic-resistant bacteria (Sumon et al., 2018; Islam et al., 2008; Islam et al., 2011) due to indiscriminate use of anthelmintics and antibiotics, respectively have also been reported which supports our present findings.
This type of indiscriminate use of anthelmintics will create serious conditions for the maintenance of the health of dairy cows along with public health implications. For overcoming this problem, detection of infected cattle and specific anthelmintic treatment of the infected cattle are suggested (Vercruysse et al., 2009). Anthelmintic treatments using a combination of two or three anthelmintics might delay the development of anthelmintic resistance in dairy cows as evidenced by previous studies (Leathwick and Besier, 2014; Leathwick et al., 2012; Bartram et al., 2012; Dobson et al., 2011). Besides these, the development and application of non-anthelmintic methods of helminth control could be considered as one of the best time demanding approaches to avoid indiscriminate treatment using various anthelmintics and subsequent avoidance of anthelmintic and/or drug resistance in farm animals.
The resistant helminth against Levamisole in BSMRAU dairy farm was not developed after a third time indiscriminate treatment using different types of anthelmintics which indicates that Levamisole resistance develops at a slower rate than Fenbendazole, Ivermectin, and Triclabendazole in dairy cows. The probable reason for the different findings on Levamisole might be due to the immune stimulant properties of Levamisole (Mansour, 2018). These findings indicate that Levamisole is still effective for the treatment of dairy cows in Gazipur, Bangladesh. Previously it was also reported that Levamisole was fully effective against Cooperia spp. in dairy cows in Western Australia (Cotter et al., 2015), Europe (Demeler et al., 2009), and Argentina (Suarez and Cristel, 2007) which is similar to our present findings.
Anthelmintic resistance is a global problem in the livestock industry with severe consequences in emerging countries because of indiscriminate use and huge consumption of anthelmintics in livestock farming, lack of education, lack of awareness, compromised immunity, inability to purchase more effective but costly anthelmintics, underdosing, undernutrition, and a habit of self-administration of anthelmintics to the livestock by farmers (Rahman et al., 2018). Previously resistance of Fasciola spp. against triclabendazole was also reported (Kelley et al., 2020; Brockwell et al., 2014) which supports our present findings. Resistance to macrocyclic lactone compounds such as Ivermectin and benzimidazoles has also been reported in dairy cattle to nematodes previously (Sutherland and Leathwick, 2011; Geurden et al., 2015; Cristel et al., 2017). So, the choice of these resistant anthelmintics for the treatment of helminths of dairy cows is not judicious. This might cause treatment failure and ultimately create more severe consequences. Choice of alternate anthelmintics, limited use of anthelmintics following strict regulations, educating the farmers and health care professionals about the negative effects of anthelmintics might reduce anthelmintic resistant helminths in the livestock production systems.
One of the limitations of the present study is the fewer samples. Future studies should be directed to finding the effectiveness of the local anthelmintics in dairy cows using huge samples. Determination of anthelmintic resistance using faecal worm egg reduction count alone is prone to uncertainty in cattle (Geurden et al., 2015). Factors such as the quality of anthelmintic drugs, determination of the weight of the animal for the selection of accurate dose to avoid underdosing, and loss of anthelmintics during drenching are also crucial. But the authors believe the findings and views expressed in this paper are crucial in reducing anthelmintic resistance in dairy farming systems. But every nation should undertake and effectively implement an anthelmintic efficacy testing program following standard guidelines to detect new anthelmintic resistant helminths to prevent the spread of resistant helminths globally through animals, animal products such as milk, meat, and the environment.
Conclusions
Zero grazing in pasture land and limited green grass supply from highland in small-scale dairy farming causes moderate helminth infections in dairy cows. Naive dairy cows are fully susceptible to Ivermectin, Fenbendazole, Levamisole, and Triclabendazole therapy. A subsequent third-time indiscriminate treatment using anthelmintics did not develop Levamisole-resistant helminths in dairy cows. But a subsequent third-time indiscriminate use of different types of anthelmintics for the treatment of helminths might cause the development of Ivermectin, Fenbendazole, and Triclabendazole resistant Neoascaris spp., Trichuris spp., and Fasciola spp. significantly in dairy cows. Thus, non-anthelmintic robust and sensitive approaches to helminth control are crucial to developing anthelmintic free dairy production systems and to avoiding the emergence of anthelmintic resistant helminths in dairy cattle globally.
Funding
This research was funded by Research Management Committee (RMC), Bangabandhu Sheikh Mujibur Rahman Agricultural University, grant number RMC Project No. UGC/RMC/49 (section 7) 2015-16” and “special allocation for R&D projects supported by the Ministry of Science and Technology, Government of the People’s Republic of Bangladesh, grant number 39.00.0000.009.06.009.20-1331/150BS (08 December 2020) for the fiscal year 2020-2021.
Data Availability Statement
All data of this manuscript are included herewith the manuscript.
Acknowledgments
The support from dairy farmers and their attendants during the field experiment was greatly acknowledged.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Author Contributions
Conceptualization, M.A.I.; methodology, M.A.I.; software, M.A.I.; validation, M.A.I., S.M.M.R.S., and F.K.; formal analysis, M.A.I.; investigation, M.A.I.; resources, M.A.I; data curation, M.A.I.; writing—original draft preparation, M.A.I.; writing—review and editing, M.A.I., S.M.M.R.S., and A.N.M.A.R.; visualization, M.A.I.; supervision, M.A.I.; project administration, M.A.I.; funding acquisition, M.A.I. All authors have read and agreed to the published version of the manuscript.
References
Agneessens J., Claerebout E., Dorny P., Borgsteede F.H.M., Vercruysse, J. (2000). Nematode parasitism in adult dairy cows in Belgium. Vet. Parasitol. 90: 83–92. https://doi.org/10.1016/S0304-4017(00)00232-6
Alim M.A., Das S., Roy K., Sikder S., Mohiuddin., Masuduzzaman M., Hossain M.A. (2012). Prevalence of gastrointestinal parasites in cattle of Chittagong division, Bangladesh. Wayamba J. Anim. Sci. 4: 1–8.
Bartram D.J., Leathwick D.M., Taylor M.A., Geurden T., Maeder S.J. (2012). The role of combination anthelmintic formulations in the sustainable control of sheep nematodes. Vet. Parasitol. 186: 151–158. https://doi.org/10.1016/j.vetpar.2011.11.030
Beesley N.J., Caminade C., Charlier J., Flynn R.J., Hodgkinson J.E., Martinez‐Moreno A., Martinez‐Valladares M., Perez J., Rinaldi L., Williams D.J.L. (2018). Fasciola and fasciolosis in ruminants in Europe: Identifying research needs. Transbound. Emerg. Dis. 65: 199–216. https://doi.org/10.1111/tbed.12682
Besier R.B. (2007). New anthelmintics for livestock: the time is right. Trend. Parasitol. 23: 21–24. https://doi.org/10.1016/j.pt.2006.11.004
Borgsteede F.H.M., Tibben J., Cornelissen J.B., Agneessens J., Gaasenbeek C.P. (2000). Nematode parasites of adult dairy cattle in The Netherlands. Vet. Parasitol. 89: 287–296. https://doi.org/10.1016/S0304-4017(00)00219-3
Brockwell Y.M., Elliott T.P., Anderson G.R., Stanton R., Spithill T.W., Sangster N.C. (2014). Confirmation of Fasciola hepatica resistant to triclabendazole in naturally infected Australian beef and dairy cattle. Int. J. Parasitol. Drugs Drug Resist. 4: 48–54. https://doi.org/10.1016/j.ijpddr.2013.11.005
Charlier J., Höglund J., Samson-Himmelstjerna G., Dorny P., Vercruysse J. (2009). Gastrointestinal nematode infections in adult dairy cattle: impact on production, diagnosis and control. Vet. Parasitol. 164: 70–79. https://doi.org/10.1016/j.vetpar.2009.04.012
Coles G.C., Jackson F., Pomroy W.E., Prichard R.K., von Samson-Himmelstjerna G., Silvestre A., Taylor M.A., Vercruysse J. (2006). The detection of anthelmintic resistance in nematodes of veterinary importance. Vet. Parasitol. 136 (3-4): 167–185. https://doi.org/10.1016/j.vetpar.2005.11.019
Coles G.C., Bauer C., Borgsteede F.H.M., Geerts S., Klei T.R., Taylor M.A., Waller P.J. (1992). World Association for the Advancement of Veterinary Parasitology (W.A.A.V.P.) methods for the detection of anthelmintic resistance in nematodes of veterinary importance. Vet. Parasitol. 44: 35–44. https://doi.org/10.1016/0304-4017(92)90141-U
Cotter J.L., Burgel A.V., Besier R.B. (2015). Anthelmintic resistance in nematodes of beef cattle in south-west Western Australia. Vet. Parasitol. 207: 276–84. https://doi.org/10.1016/j.vetpar.2014.11.019
Cristel S., Fiel C., Anziani O., Descarga C., Cetrá B., Romero J., Fernández S., Entrocasso C., Lloberas M., Medus D., Steffan P. (2017). Anthelmintic resistance in grazing beef cattle in central and northeastern areas of Argentina —An update. Vet. Parasitol. Region. Stud. Rep. 9: 25–28. https://doi.org/10.1016/j.vprsr.2017.04.003
Demeler J., Van Zeveren A.M.J., Kleinschmidt N., Vercruysse J., Hoglund J., Koopman R., Caberet J., Claerebout E., Areskog M., von Samson-Himmelstjerna G. (2009). Monitoring the effects of ivermectin and albendazole against gastrointestinal nematodes of cattle in Northern Europe. Vet. Parasitol. 160: 109–115. https://doi.org/10.1016/j.vetpar.2008.10.030
DLS. (2008). Annual report on livestock, Division of Livestock Statistics, Ministry of Fisheries and Livestock, Farmgate, Dhaka, Bangladesh.
Dobson R.J., Barnes E.H., Tyrrell K.L., Hosking B.C., Larsen J.W.A., Besier R.B., Love S., Rolfe P.F., Bailey J.N. (2011). A multi-species model to assess the impact of refugia on worm control and anthelmintic resistance in sheep grazing systems. Australian Vet. J. 89: 200–208. https://doi.org/10.1111/j.1751-0813.2011.00719.x
Dunn A.M. (1978). Veterinary Helminthology. 2nd edition. London: William Heinemann Medical Books.
Epherem W. (2007). Prevalence of Bovine GI helminths in selected Dairy farms of Addis Ababa. DVM Thesis, Jimma University, Jimma, Ethiopia.
Flanagan A., Edgar H.W.J., Gordon A., Hanna R.E.B., Brennan G.P., Fairweather I. (2011). Comparison of two assays, a faecal egg count reduction test (FECRT) and a coproantigen reduction test (CRT), for the diagnosis of resistance to triclabendazole in Fasciola hepatica in sheep. Vet. Parasitol. 176: 170–176. https://doi.org/10.1016/j.vetpar.2010.10.057
Geurden T., Chartier C., Fanke J., di Regalbono A.F., Traversa D., von Samson-Himmelstjerna G., Demeler J., Vanimisetti H.B., Bartram D.J., Denwood M.J. (2015). Anthelmintic resistance to ivermectin and moxidectin in gastrointestinal nematodes of cattle in Europe. Int. J. Parasitol. Drugs Drugs Resist. 5: 163–171. https://doi.org/10.1016/j.ijpddr.2015.08.001
Gross S.J., Ryan W.G., Ploeger H.W. (1999). Anthelminthic treatment of dairy cows and its effect on milk production. Vet. Rec. 144: 581–587. https://doi.org/10.1136/vr.144.21.581
Haque M.H., Islam M.A., Karim M.R., Kayesh M.E.H., Sarker S., Nazir K.H.M.N.H., Anwer M.S. (2021). Coronavirus disease 2019 and future pandemics: Impacts on livestock health and production and possible mitigation measures. Vet. World. 14 (9): 2434–2443. https://doi.org/10.14202/vetworld.2021.2434-2443
Haque M.H., Sarker S., Islam M.S., Islam M.A., Karim M.R., Kayesh M.E.H., Shiddiky M.J.A., Anwer, M.S. (2020). Sustainable Antibiotic-Free Broiler Meat Production: Current Trends, Challenges, and Possibilities in a Developing Country Perspective. Biology. 9(11): 411. https://doi.org/10.3390/biology9110411
Hawkins J.A. (1993). Economic benefits of parasite control in cattle. Vet. Parasitol. 46: 159–173. https://doi.org/10.1016/0304-4017(93)90056-S
Hendrix S. (2006). Laboratory Procedures for Veterinary Technicians, 5th Ed., 812-814.
Hoque M.N., Begum N., Nooruddin M. (2003). Albendazole resistance in gastrointestinal nematode parasites of cattle in Bangladesh. Trop. Anim. Health Prod. 35: 219–222.
Höglund J., Dahlström F., Engström A., Hessle A., Jakubek E.B., Schnieder T., Strube C., Sollenberg S. (2010). Antibodies to major pasture borne helminth infections in bulk-tank milk samples from organic and nearby conventional dairy herds in south-central Sweden. Vet. Parasitol. 171: 293–299. https://doi.org/10.1016/j.vetpar.2010.04.002
Hoste H., Torres-Acosta J.F., Paolini V., Aguilar-Caballero A., Etter E., Lefrileux Y., Chartier C., Broqua, C. (2005). Interactions between nutrition and gastrointestinal infections with parasitic nematodes in goats. Small Rumin. Res. 60(12): 141–151. https://doi.org/10.1016/j.smallrumres.2005.06.008
IDF. (2013). The Economic Importance of Dairying. IDF Factsheet – February 2013.
Islam M.A., Alam M.M., Uddin M.S., Kobayashi N., Ahmed M.U. (2011). Detection of Methicillin-Resistant Staphylococcus aureus (MRSA) from animal and human origin in Bangladesh by Polymerase Chain Reaction (PCR). Bangladesh J. Vet. Med. 9 (2): 161–166. https://doi.org/10.3329/bjvm.v9i2.13472
Islam M.A., Alam M.M., Choudhury M.E., Kobayashi N., Ahmed M.U. (2008). Determination of minimum inhibitory concentration (MIC) of cloxacillin for selected isolates of methicillin-resistant Staphylococcus aureus (MRSA) with their antibiogram. Bangladesh J. Vet. Med. 6 (1): 121–126. https://doi.org/10.3329/bjvm.v6i1.1350
Islam M.T., Saurov M.S.J., Rahman M.A., Talukder A.K., Islam M.A., Haider M.G., Rahman A.N.M.A. (2020b). A retrospective study of common poultry diseases at Gazipur district of Bangladesh. Bangladesh J. Ecol. 2 (2): 113–120.
Islam M.A., Uddin M.S., Islam M.J., Ahmed M.U., Alam M.M.
(2021). Investigation of antibiotic resistance pattern of Staphylococcus aureus in clinical samples of animals and humans from selective areas of Bangladesh. Bangladesh J. Vet. Med. 19 (1): 1–11.
Islam M.A., Ikeguchi A., Naide T. (2018). Aerosols and bacteria concentration in different types of Japanese dairy milking houses. In 10th international livestock environment symposium (ILES X). Sponsored by ASABE, Omaha. USA: Nebraska. https://doi.org/10.13031/iles.18-117
Islam M.A., Ikeguchi A., Naide T. (2019). Concentrations of aerosol numbers and airborne bacteria, and temperature and relative humidity, and their interrelationships in a tie-stall dairy barn. Animals. 9(12): 1023. https://doi.org/10.3390/ani9121023
Islam M.A., Ikeguchi A., Naide T. (2020a). Influence of temperature and humidity on the dynamics of aerosol numbers and airborne bacteria in a dairy calf house. Biosyst. Engineer. 194: 213–226. https://doi.org/10.1016/j.biosystemseng.2020.04.003
Islam M.A., Ikeguchi A., Naide T. (2019a). Relationships between aerosol particles and airborne bacteria and their dependence on environmental factors in a dairy calf house. Transact. ASABE. 62(6): 1819–1828. https://doi.org/10.13031/trans.13570
Islam M.A., Rahman A.N.M.A., Alam M.S., Islam M.T. (2020). Gastrointestinal nematodiasis in quail in Bangladesh. Vet. Sci. Res. Rev. 6 (2): 138–142. https://doi.org/10.17582/journal.vsrr/2020.6.2.138.142
Islam M.M., Islam M.S., Howlader M.M.R., Lucky N.S. (2015). Prevalence of Gastrointestinal Nematodiasis and Comparative Efficacy of Anthelmintics on Body Weight of Cattle in Bangladesh. Int. J. Scient. Res. Agric. Sci. 2: 61–75 https://doi.org/10.12983/ijsras-2015-p0061-0075.
Kelley J.M., Rathinasamy V., Elliott T.P., Rawlin G., Beddoe T., Stevenson M.A., Spithill T.W. (2020). Determination of the prevalence and intensity of Fasciola hepatica infection in dairy cattle from six irrigation regions of Victoria, South-eastern Australia, further identifying significant triclabendazole resistance on three properties. Vet. Parasitol. 277: 109019. https://doi.org/10.1016/j.vetpar.2019.109019
Keyyu J.D., Kassuku A.A., Msaliilwa P.L., Monrad J., Kyvsgaard N.C. (2006). Cross sectional Prevalence of Helminth Infections in Cattle on Traditional, small scale and Large-scale Dairy Farms in Iringa District, Tanzania. Vet. Res. Commun. 30: 45–55. https://doi.org/10.1007/s11259-005-3176-1
Khatun F., Maruf A.A., Rahman M.M., Yasmin A., Zinnah M.A., Islam M.A., Alam M.S. (2021). Incidence of gastrointestinal parasitism in cattle in Gazipur, Bangladesh. Vet. Sci. Res. Rev. 7 (2): 109–114. https://doi.org/10.17582/journal.vsrr/2021.7.2.109.114
Leathwick D.M., Besier R.B. (2014). The management of anthelmintic resistance in grazing ruminants in Australasia – strategies and experiences. Vet. Parasitol. 204: 44-54. https://doi.org/10.1016/j.vetpar.2013.12.022
Leathwick D.M., Waghorn T.S., Miller C.M., Candy P.M., Oliver A.-M.B. (2012). Managing anthelmintic resistance – Use of a combination anthelmintic and leaving some lambs untreated to slow the development of resistance to ivermectin. Vet. Parasitol. 187: 285–294. https://doi.org/10.1016/j.vetpar.2011.12.021
Martin R.J. (1985). Gamma-Aminobutyric acid- and piperazine-activated single-channel currents from Ascaris suum body muscle. Brit. J. Pharmacol. 84: 445–461. https://doi.org/10.1111/j.1476-5381.1985.tb12929.x
Mansour A.M.A. (2018). Effect of levamisole administration on immunogenic and protective capacity of Brucella abortus RB51. Nat. J. Physiol. Pharm. Pharmacol. 8 (5): 635–639. https://doi.org/10.5455/njppp.2018.8.1143004122017
Naide T., Ikeguchi A., Islam M.A., Miyazaki A., Katusda K., Kawashima K., Nakakubo R. (2018). Relationship between aerosol concentration and airborne microbe including porcine sapelovirus concentration in Japanese weaning swine houses. In 10th international livestock environment symposium (ILES X). Sponsored by ASABE, Omaha, Nebraska, USA. https://doi.org/ 10.13031/iles.18-118.
Pfukenyi D.M., Mukaratirwa. (2013). A review of the epidemiology and control of gastrointestinal nematode infections in cattle in Zimbabwe. Onderstepoort J. Vet. Res. 80: 12. https://doi.org/10.4102/ojvr.v80i1.612
Rahman T.M.M., Dey A.R., Islam S., Hossain M.S., Talukder M.H., Alam M.Z. (2018). Anthelmintic resistance to cattle gastrointestinal nematodes in selected dairy farms of Mymensingh and Sirajganj districts of Bangladesh. Res. Agric. Livest. Fisher. 5 (1): 87–92. https://doi.org/10.3329/ralf.v5i1.36556
Rahman M.M., Kabir A., Islam M.K., Rahman M.S., Yasmin M.S., Alom F., Islam M.A., Ahmed S., Islam M.R., Islam M.H. (2017). Prospects and genesis of the versatile antiparasitic drug Ivermectin and its unsurpassed beneficial impact in Human and Veterinary Medicine. Int. J. Nat. Sc. 6 (3): 132–140.
Rajakaruna R.S., Warnakulasooriya K.N. (2011). Gastrointestinal parasites in dairy cattle in Kandy district in Srilanka. Annu. Res. J. SLSAJ. 11: 92–99.
Sanchez J., Dohoo I., Carrier J., DesCôteaux L. (2004). A meta-analysis of the milk-production response after anthelminthic treatment in naturally infected adult dairy cows. Prevent. Vet. Med. 63: 237–256. https://doi.org/10.1016/j.prevetmed.2004.01.006
Schweizer G., Braun U., Deplazes P., Torgerson P.R. (2005). Estimating the financial losses due to bovine fasciolosis in Switzerland. Vet. Rec. 157: 188–193. https://doi.org/10.1136/vr.157.7.188
Shamsuddin M., Alam M.M., Hossein M.S., Goodger W.J., Bari F.Y., Ahmed T.U., Hossain M.M., Khan A.H.M.S.I. (2007). Participatory rural appraisal to identify needs and prospects of market-oriented dairy industries in Bangladesh. Trop. Anim. Health Prod. 39: 567–581. https://doi.org/10.1007/s11250-007-9062-9
Smith G. (1997). The economics of parasite control: obstacles to creating reliable models. Vet. Parasitol. 72: 437–444. https://doi.org/10.1016/S0304-4017(97)00109-X
Shalaby H.A. (2013). Anthelmintics Resistance; How to Overcome it? Iranian J. Parasitol. 8 (1): 18–32.
Suarez V.H., Cristel S.L. (2007). Anthelmintic resistance in cattle nematodes in western Pampeana Region of Argentina. Vet. Parasitol. 144: 111–117. https://doi.org/10.1016/j.vetpar.2006.09.016
Sutherland I.A., Leathwick D.M. (2011). Anthelmintic resistance in nematode parasites of cattle: a global issue? Trend. Parasitol. 27: 176–181. https://doi.org/10.1016/j.pt.2010.11.008
Sumon S.M.M.R., Haider M.G., Islam M.A., Siddiki S.H.M.F., Karim M.R. (2018). Prevalence and antibiogram profile of Staphylococcus aureus isolated from milk samples of lactating cows with subclinical mastitis in Gazipur, Bangladesh. Ann. Bangladesh Agric. 22 (1): 51–60.
Soulsby E.J.L. (1982). Helminths, Arthropods and protozoa of domesticated animals, 7th edition, Bailliere Tindall, London, United Kingdom, pp. 764–766.
Tulu D., Lelisa K.A. (2016). Study on Major Gastro-Intestinal Helminths Parasites of cattle in Tulo District, West Hararghe Zone, South-Eastern Ethiopia. Austin J. Vet. Sci. Anim. Husban. 3(2): 1027.
Talukder A.K., Rahman M.A., Islam M.A., Islam M.T., Selim A.S.M., Paul A.K., Rahman M.A. (2015). Evaluation of health care and husbandry system of calves at buffalo farms in southern Bangladesh. SAARC J. Agric. 13(2): 108–120 https://doi.org/10.3329/sja.v13i2.26572.
Valero M.A., Perez-Crespo I., Periago M.V., Khoubbane M., Mas Coma S. (2009). Fluke egg characteristics for the diagnosis of human and animal fascioliasis by Fasciola hepatica and F. gigantica. Acta Trop. 111: 150–159. https://doi.org/10.1016/j.actatropica.2009.04.005
Vaessen M.A., Veling J., Frankena K., Graat E.A., Klunder T. (1998). Risk factors for Salmonella dublin infection on dairy farms. Vet. Quart. 20: 97–99. https://doi.org/10.1080/01652176.1998.9694848
Vercruysse J., Claerebout E. (2001). Treatment vs non-treatment of helminth infections in cattle: defining the threshold. Vet. Parasitol. 98: 195–214. https://doi.org/10.1016/S0304-4017(01)00431-9
Vercruysse J., Jackson F., Besier B., Pomroy B. (2009). Novel solutions for the sustainable control of nematodes in ruminants (PARASOL). Vet. Parasitol. 164(1): 1–2. https://doi.org/10.1016/j.vetpar.2009.04.025
To share on other social networks, click on any share button. What are these?





