Efficacy of Plant Seed Oils Against Callosobruchus maculatus L. on Chickpea Grains
Research Article
Efficacy of Plant Seed Oils Against Callosobruchus maculatus L. on Chickpea Grains
Agha Mushtaque Ahmed1*, Fahad Nazir Khoso1, Ali Zachi Abdulqader Alhilfi2, Sohail Ahmed Otho1, Qurban Ali3, Din Muhammad Soomro1 and Zubair Ahmed Soomro1
1Department of Entomology, FCPT, Sindh Agriculture University Tando Jam, Pakistan; 2Department of Plant Protection, College of Agriculture, University of Basrah, Iraq; 3Hubei Key Laboratory of Plant Pathology, Huazhong Agricultural University, Wuhan, Hubei 430070, P.R. China.
Abstract | Pulse beetles (Callosobruchus maculatus L.) often damage the stored grains to such an extent that a huge quantity of total production is significantly affected. For last few years, a substantial consideration has been given globally to different botanical oils due to their pesticidal properties and environmental safety. In this context, we evaluated the insecticidal effect of four botanical oils against C. maculatus including neem seeds (Azadirachta indica A. Juss), rocket seeds (Erucas sativa Mill.), cotton seeds (Gossypium hirsutum L.) and mustard seeds (Brassica nigra L.). The effect of these oils was recorded on insect mortality, number of adult insects, seed damage (punctured seeds and weight loss of chickpea seeds), seed germination percentage, root length (cm) and seed vigour index. The insect mortality was recorded at different intervals such as 24, 48, 72 hrs and 1 week) and the results indicated that E. sativa gave a consecutively higher mortality at each interval, meanwhile the highest at one week (50.00%) as compared to other treatments followed by A. indica (45.35%). Besides, E. sativa oil did not allow the pest to breed freely thus few numbers of adults C. maculatus (71.67±14.92) on chickpea seeds with minimum seed damage (punctured seeds 75.44 ±9.67; seed weight loss 3.18±1.37) was conclusively observed for three months. The results regarding the effect of botanical oils against C. maculatus and on seed characteristics showed that E. sativa and A. indica oils were comparatively better in order to control the pest population and are recommended for stored grains pest management.
Received | April 26, 2021; Accepted | August 31, 2022; Published | December 02, 2022
*Correspondence | Agha Mushtaque Ahmed, Department of Entomology, FCPT, Sindh Agriculture University Tando Jam, Pakistan; Email: aghamushtaq@gmail.com
Citation | Ahmed, A.M., F.N. Khoso, A.Z.A. Alhilfi, S.A. Otho, Q. Ali, D.M. Soomro and Z.A. Soomro. 2022. Efficacy of plant seed oils against Callosobruchus maculatus L. on chickpea grains. Sarhad Journal of Agriculture, 38(5): 222-233.
DOI | https://dx.doi.org/10.17582/journal.sja/2022/38.5.222.233
Keywords | Botanical oils, Pulse beetle, Seed characters, Pest population, Chickpea
Copyright: 2022 by the authors. Licensee ResearchersLinks Ltd, England, UK.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Introduction
Chickpea Cicer arietinum (L.) is ranked among legume crops in the world in terms of cultivated area of 14.56 million hectares and production of 11.56 million metric tons (Merga and Haji, 2019; FAOSTAT, 2018-19). It is an essential dietary component which provides sufficient food around the world particularly in south Asia (Varshney et al., 2013). It does only enrich the soil health because of their nitrogen fixing ability but also having ability to grow in drought-tolerant area; hence play a vital role in sustainable agriculture (Khan et al., 2020). In Pakistan, chickpea is cultivated on about 960,000 hectares with an average production of 441.5 metric tons per year (GoP, 2017). During storage, a significant loss to chickpea seeds occurs due to different insect pests, mites, diseases and abiotic factors like temperature and relative humidity (Ahmad et al., 2001; Upadhyay and Ahmad, 2011; Befikadu, 2014). Cicer arietinum is mainly infested by two species i.e., Callosobruchus maculatus and C. chinensis of bruchid beetles (Kashiwaba et al., 2003). These beetles do not only cause physical damage to the seeds by consuming seeds (with loss of 55% seed weight) but also affect their nutritional quality (with loss of 45% protein content) which results in considerable economic loss worldwide (Sharma and Thakur, 2014). During early onset of infestation, young ones of insects are not generally seen in seeds; later the visible symptoms like round holes in the seeds occur due to the emergence of the insect through windows which resulted in complete damage and loss of seeds (Moreira et al., 2012). Furthermore, insect’s excreta cause bad odors, fermentation and organic changes of the food seeds due to poisonous chemical substances like Aflatoxin secrets from the moulds grown in the damaged stored products cause liver cancer in human being (Magan, 2006; Magan and Aldred, 2007; Stejskal et al., 2018). Mostly, these stored seed pests control worldwide through an application of synthetic insecticides including fumigation of permethrin, methyl bromide, phosphine, and pirimiphos-methyl (Singal and Singh, 1990; Boyer et al., 2012; Stejskal et al., 2021). Undoubtedly, these chemicals as fumigants against stored seed pests have been reported relatively efficient in overseeing its population, but due to risk of chemical exposure to non-targeted organisms and human health, efforts have been taken to avoid usage of these chemicals (Kosini and Nukenine, 2017). Besides, several stored seed insect pests exhibited a higher extent (200-fold) of resistance to various insecticides (Ahmad, 2007; Alyokhin et al., 2008) and created an alarming situation for pest controlling programs.
Presently, awareness of these risks has enlarged the urgent requirement to explore, develop and apply cost-effective, environmental-friendly, and secure pesticides for protecting stored seeds (Saxena and Sayyed, 2018). Nevertheless, natural plant products with insecticidal activity have recently gained a prominence due to their largely low toxicity particularly against non-targeted pests and mammalian, environmentally safe and broad public adoption (Isman, 1994; Dey and Gupta, 2016). In this connection, several plant derivatives have been reported effective against various stored seed pests (Raja, 2014). The neem (Azadirachta indica A. Juss) and its extracted oil, cotton seed oil (Gossypium hirsutum L.), mustard oil (Brassica nigra L.) and rocket seed oil (Eruca sativa Mill.) are an excellent botanical product having anti-ovipositional, growth inhibitors, antifeedants, insecticidal and insect repellent effects against various stored seeds pests including bruchid beetles (Sithisut et al., 2011; Regnault-Roger et al., 2012; Radha and Susheela, 2014). The mustard oil extracts from its seeds contain allyl isothiocyanate (AITC) which is greatly volatile in nature with vapor density of 3.4 times higher than the air and assure flavor and pungency. Due to high vapor density of AITC, these are quite toxic to various stored seed pests including Rhyzopertha dominica F., Tribolium confusum Jacuelin du Val. and Lasioderma serricorne F (Tsao et al., 2002) and similarly for field crop pests including lepidopteran (Konecka et al., 2018) but these investigations remained limited only to adult insects and still requires more detail study against various stored seed pests at their different life stages (Paes et al., 2011). Cotton seed oil has good physiochemical properties and generally considered as the most insecticidal of the vegetable oils because it contains a good amount of oleic acid that has been observed toxic against many insect pests including stored grain pests but did not provide any additional data (Cranshaw and Baxendale, 2013). The rocket seed oil contains high fatty acids (82.1%) with enough alpha lenolenic acid (9.34%) and oleic acid (15.53%) (Abozid et al., 2014).
Although, these plant derivatives have been studied for having good insecticidal efficacy but there is still lack of additional data using rocket seed oil and its efficacy against stored seed pests. According to Hebeish et al. (2008) and Hieu et al. (2010) limonene as an essential part of citrus lubricate and contain highly insecticidal effects. Similarly, Tripathi et al. (2003) explored and detailed reviewed by Singh et al. (2021) that d-Limonene was best as a contact and fumigant toxicity, ovicidal effects, oviposition-deterrent, development inhibition, and feeding-deterrent activities against R. dominica, Sitophilus oryzae (L.) and T. castaneum. Previously, it has been well addressed about neem and its byproducts (purified/ raw), including bioactive compounds (i.e., azadirachtin) affecting survival, exploiting behavior and inhibiting growth and development of various stored seed insects pests and other field insects (Singh, 1993; Morgan, 2009; Chaudhary et al., 2017; Pasquoto-Stigliani et al., 2017; Kilani-Morakchi, 2021). In addition, volatile extracts of neem as well as other aromatic plants contain numerous persistent and fumigant effect which can directly be applicable to various stored seed seeds against insects (Tamgno and Ngamo, 2014). Although, these plants and their derivatives are quite effective and used as insecticides but there is still lack of information regarding their proper application as bio-insecticides against bruchid beetles. Therefore, this study has been proposed to observe the effect of different plant seed oils against C. maculatus on chick seeds.
Materials and Methods
Insect culture
The stock culture of C. maculatus was taken from Seed Storage Research Laboratory, University of Karachi, Sindh, Pakistan and was further reared on chickpea seeds at 30±2°C with relative humidity of 65±5%. All insect cultures were properly maintained in a growth chamber in the laboratory. The extracted oils of four different plant species A. indica (Azadirachtin) A. Juss., E. sativa L. (fatty acids), G. hirsutum L. (polyphenolic component i.e., Gossypol) and B. nigra L. (allyl isothiocyanate) were used as seed protectant against C. maculatus. The prepared oil of these plant species was provided by Agriculture Research Institute (A.R.I) Tando Jam.
Mortality assessment
The chickpea seeds were sterilized by storage for 1 week at −20±2°C, air dried to avoid mould and adjusted to stabilise moisture content at 11–12% prior to the experiment. Initially, 50 adults (25 pairs those were identified on morphological characters) of 1-2 days old adults were introduced in a jar (500 mL) having chickpea seeds (300 g). The jars were tightened with ventilated lid and were incubated for a week to let them mate. Later, the parent stocks were separated and seeds having eggs were transferred to fresh chickpea seeds in insect rearing jars. Such procedure was carried out to multiply our stock and to get F1 generation for subsequent experimentation. The insecticidal activity of botanical oils against C. maculatus adults was evaluated through residual/contact method (Fatope and Mann, 1995; Bacci et al., 2009). The chickpea seeds were treated separately (except control treatment which were only treated with petroleum ether at similar dose) with each oil at concentration of 10 mL kg−1 diluted with petroleum ether. The grains and oils were stirred continuously for few minutes until a uniform coating of applied oil on each seed appears clearly. Next, these jars were kept for 2-3 h at 30± 2°C for complete evaporation of the solvent. Twenty newly emerged beetles (10 pairs male and female) of C. maculatus were transferred on treated seeds (100 g) with three replicates and kept in plastic jars. Each jar was covered with a muslin cloth and tighten with an elastic rubber band to avoid any escaping and kept at 30±2 °C. The corrected mortality of insects was recorded after exposure for 24, 48, 72 hrs and 1 week of treatment. Insects were examined daily and insects those did not response/move were considered as dead. The mortality data were corrected using the formula proposed by Schneider -Orelli (1947). For corrected mortality, mortality in treatments were comparatively calculated from control, thus no need to present mortality for control treatment.
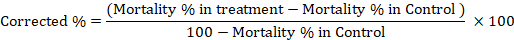
Damage assessment through seed weight loss
After recording the mortality at different intervals, all the treatments including control along with their replications were incubated at 30±2°C and further observed for seed damage (i.e., punctured chickpea seeds and seed weight loss) and number of adult insects at monthly interval until three months of the experiment. The seed weight (100 g) for weight loss was measured with electrical balance (Model EEB1105, China) after subtracting the consumed part of seeds by insect. The extent of damage to the seeds in each treatment was assessed using the exit-hole method with little modification (Ajayi and Adedire, 1996); the punctured seeds with holes were counted and weight loss expressed in percentage using following formula (Chowdhury et al., 2008).
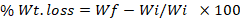
Where, Wt= total weight; Wi = Initial weight and Wf= final weight.
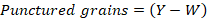
Where; W=number of punctured seeds per treatment and Y= total number of seeds per treatment.
Ten pairs of C. maculatus per 100 g chickpea treated seeds by four different botanicals (and fifth control treatment) with three replicates were set out. Further, means of all three months (1st, 2nd and 3rd months) of experiment were pooled for all parameters to know the exact information. Four botanicals and control were considered five treatments and months were considered as replicates (three replications). Analysis of variance test (with single factor) was performed for each parameter (i.e., number of adults emerged, punctured seeds and weight loss). The primary data for punctured seeds and weight loss were prior calculated with the formula as given above.
Seed vigour index (V.I)
The effect of extracted oils on chickpea seed was recorded through vigour index. The treated seeds (100 g) with each extract oils were kept separately in four different jars (250 mL) along with control treatment “untreated seeds” (5th jar) those replicated thrice. These jars were kept until three months at 30 ± 2 °C in insect growth chamber (Classic 300 HIT, UK) and every month three samples of 10 seeds randomly were taken from replicates of each treatment for observing seed germination percentage and root length (cm). The seeds were placed in a Petri dish (10×15cm2) having jute moisturized bed and kept at room temperature in laboratory. The roots were measured with simple ruler and vigour index of seeds was calculated using the following formula given by (Abdul-Baki and Anderson, 1973).

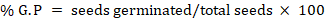
Where; V.I= Vigour Index and G.P= Germination percentage
Statistical analysis
All the collected data (i.e. percent corrected mortality, weight loss percentage, number of punctured seeds and Vigor index) were calculated according to the mentioned formulae. The data were statistically analyzed using one way analysis of variance (ANOVA) and mean differences also compared by Least Significant Difference (LSD) test at p<0.05 using Statistix software (ver 8.1) for all other parameters.
Results and Discussion
Effect of botanical oils on corrected mortality of C. maculatus
The effect of botanical oils on corrected mortality of C. maculatus found significant (P<0.05) at different intervals under laboratory condition and presented in Table 1. The highest mortality (45.45%) of C. maculatus was recorded at E. sativa oil followed by A. indica (21.21%). However, the lowest mortality percentage (16.16%) was similarly recorded at G. hirsutum and B. nigra after 24 h. The increased time intervals further enhanced the efficacy of E. sativa oil and mortality of C. maculatus was remained highest (50.50%) after 48 h as compared to other botanical oils. Although, the efficacy of other three oils also showed higher increment in mortality percentage of C. maculatus after 48 h (34.11% at A. indica, 29.08% at G. hirsutum and 25.71% B. nigra) than mortality recorded on E. sativa oil, but overall mortality was remined highest at E. sativa. It could be due to late effects of these oils and on the other hand majority C. maculatus was already died by E. sativa oil at 24 h thus only few insects left survived. We observed similar trend in mortality percentage of C. maculatus after 72 h and one week as observed 48 h. All these botanical oils gradually and steady killed the C. maculatus but E. sativa and A. indica were the most prominent botanical oils those killed the highest percentage of C. maculatus.
Table 1: Effect of botanical oils on corrected mortality (%) of C. maculatus at different intervals (n=3).
|
Treatments |
24h |
48h |
72h |
One week |
|
Azadirachta indica |
21.21 |
37.11 |
41.94 |
42.35 |
|
Eruca sativa |
45.45 |
52.58 |
51.16 |
50.00 |
|
Gossypium hirsutum |
16.16 |
36.08 |
38.71 |
40.91 |
|
Brassica nigra |
16.16 |
25.71 |
32.26 |
38.73 |
Corrected percentage for mortality was calculated using Schneider Orelli (1947) formula. Ten pairs were taken, and mortality were recorded after exposure for 24, 48, 72 hrs and 1 week of treatment. For corrected mortality, mortality in treatments were comparatively calculated from control thus no need to mortality for control treatment.
Effect of botanical oils on adult emergence of C. maculatus in response to punctured seed and seed weight loss of chickpea seeds
The adult emergence of C. maculatus, punctured seeds and weight loss of chickpea seeds were significantly different (P<0.05) as mentioned in Table 2. The lowest mean adult emergence of pulse beetle (13.13±1.45)
Table 2: Effect of botanical oils on pest population of C. maculatus and seed damage containing punctured seeds and weight loss of chickpea seeds (Means ± S.E and n=3).
|
Treatment |
No. of pulse beetles |
Punctured seed |
(Weight loss from 100g) |
|
1st month |
|||
|
Azadirachta indica |
30.33±0.88c |
6.67±0.33bc |
0.86±0.03bc |
|
Eruca sativa |
13.13±1.45d |
4.67±0.67c |
0.63±0.03dc |
|
Gossypium hirsutum |
37.67±0.88b |
9.00±0.57b |
1.06±0.05b |
|
Brassica nigra |
31.67±0.88c |
8.67±1.45b |
1.06±0.03b |
|
Control |
69.67±0.67a |
34.33±1.76a |
4.36±0.12a |
|
2nd month |
|||
|
Azadirachta indica |
121.33±1.45d |
147.67±0.88b |
6.91±0.05d |
|
Eruca sativa |
88.67±0.88e |
105.67±2.40c |
3.56±0.08e |
|
Gossypium hirsutum |
158.33±1.20b |
166.33±0.88a |
12.16±0.54b |
|
Brassica nigra |
144.00±0.57c |
161.33±0.88a |
9.26±0.20c |
|
Control |
175.33±1.20a |
165.00±0.57a |
19.33±0.12a |
|
3rd month |
|||
|
Azadirachta indica |
191.33±1.20d |
169.33±0.88b |
8.86±0.32c |
|
Eruca sativa |
112.67±1.45e |
116.0±0.57c |
5.36±0.12d |
|
Gossypium hirsutum |
279.00±0.57b |
266.0±0.57a |
15.76±0.08b |
|
Brassica nigra |
267±1.76c |
266.0±0.57a |
15.11±0.05b |
|
Control |
323.67±0.88a |
270.33±0.33a |
20.16±0.17a |
Ten pairs of C. maculatus per 100 g chickpea treated seeds by four different botanicals (and fifth control treatment) with three replicates were set out. Anova test (with single factor) was performed for each parameter (i.e. number of adults emerged, punctured seeds and weight loss). The primary data for punctured seeds and weight loss were prior calculated with the formula as given in methodology. All the means were separated by applying least significant difference (LSD) post-hoc test at p<0.05 and represented by different letters within the same column.
Table 3: The pooled population of C. maculatus and seed damage containing number of punctured seeds and weight loss of chickpea seeds for 1-3 months (Means ± S.E, n=3).
|
Treatments |
No. of pulse beetles |
Punctured seed |
Seed weight loss |
|
Azadirachta indica |
114.33±23.31c |
107.89±15.50b |
5.54±2.13c |
|
Eruca sativa |
71.67±14.92d |
75.44±9.67c |
3.18±1.37d |
|
Gossypium hirsutum |
153.56±34.91ab |
147.11±26.33a |
9.66±4.42b |
|
Brassica nigra |
152.89±34.85ab |
145.33±25.84a |
8.47±4.07b |
|
Control |
189 .89±36.83a |
156.44±22.24a |
14.61±5.13a |
Means of all three months (1st, 2nd and 3rd months) of experiment were further pooled for all parameters to know the exact information. Four botanicals and control were considered five treatments and months were considered as replicates (three replications). Anova test (with single factor) was performed for each parameter (i.e. number of adults emerged, punctured seeds and weight loss). All the means were separated by applying least significant difference (LSD) post-hoc test at p<0.05 and represented by different letters within the same column.
with less extent of damage to seeds in results of minimum punctured seeds (4.67±0.67) and the lowest seed weight loss (0.63±0.03 g) were recorded on E. sativa oil in the first moth of seed treatment. However, the effect of other seeds treated with different oils showed significant results in inhibiting egg laying on seeds and seed damage as compared to control treatment, but the extent was bit low in comparison to the seeds treated with E. sativa oil. In the second month of seed treatment, the trend remained same with the lowest mean number of adults 88.67±0.88 at E. sativa oil followed by 121.33±1.45 by A. indica were recorded. Similarly, the extent of damage in puncturing seeds (105.67±2.40) and seed weight loss (3.56±0.08g) were lowest at E. sativa oil followed by 147.67±0.88 and 6.91±0.05g at A. indica. However, in the third month of seed treatment, an extensive difference was recorded in mean number of adult pulse beetle with seed damage particularly in control except E. sativa. The results showed a nominal extension in number of adult pulse beetles (112.67±1.45) and seed damage (punctured seeds 116.0±0.57 with minimum weight loss of 5.36±0.12g), respectively recorded on E. sativa as compared to the first and the second months of experimentation but on the other hand the remaining treatments almost lost their effectiveness and became susceptible against C. maculatus which resulted with higher number of adult beetles and seed damage.
The overall pooled results for standardized parameters for three months as in Table 3 showed a significant different (P<0.05) in all parameters. The lowest adult mean number (71.67 ± 14.92) of C. maculatus with minimum seed damage (punctured seeds 75.44±9.67; weight loss of 3.18±1.37) of chickpea seed were recorded on seeds treated with E. sativa. Meanwhile, the results regarding protection of chickpea seeds against pulse beetle with application of A. indica oil were also satisfactory after E. sativa oil during whole period of experimentation.
The effect of botanical oils on seed vigour index of chickpea seeds
The result regarding vigour index consisting of germination and root length are described in Table 4. The germination of chickpea seeds with application of different botanical oils showed significantly good after one month of seed treatment. The treatment of chickpea seeds with A. indica and control treatments gave 100% germination next by 93.33% at E. satvia.
Table 4: Effect on botanical oils on vigour index of chickpea seeds containing germination % and root length (Means± S.E, n=3).
|
Treatment |
Germination (%) |
Root length (cm) |
Vigour index |
|
1st month |
|||
|
Azadirachta indica |
100.00 |
2.771± 0.31ab |
277.00 |
|
Eruca sativa |
93.33 |
2.413±0.35bc |
225.17 |
|
Gossypium hirsutum |
90.00 |
2.160±0.13c |
194.31 |
|
Brassica nigra |
86.67 |
2.270±0.10bc |
196.74 |
|
Control |
100.00 |
3.263±0.06a |
326.33 |
|
2nd month |
|||
|
Azadirachta indica |
83.33 |
1.843±0.03bc |
153.49 |
|
Eruca sativa |
86.67 |
2.040±0.03b |
176.84 |
|
Gossypium hirsutum |
80.00 |
1.767±0.08c |
141.52 |
|
Brassica nigra |
80.00 |
1.943±0.03bc |
155.47 |
|
Control |
100.00 |
2.803±0.16a |
280.33 |
|
3rd month |
|||
|
Azadirachta indica |
81.33 |
1.54±0.03bc |
131.14 |
|
Eruca sativa |
84.67 |
1.87±0.10b |
150.87 |
|
Gossypium hirsutum |
78.33 |
1.34±0.16c |
111.78 |
|
Brassica nigra |
73.67 |
1.58±0.02bc |
139.64 |
|
Control |
100.00 |
2.44±0.050a |
244.00 |
Four botanicals and control were considered five treatments and replicated thrice. Three samples of 10 seeds randomly were taken from replicates of each treatment for observing seed germination percentage and root length (cm). A basic formula was used for calculating germination percentage as mentioned in methodology and similarly for recording vigour index. However, Anova test (with single factor) was performed for root length based on similar samples. All the means were separated by applying least significant difference (LSD) post-hoc test at p<0.05 and represented by different letters within the same column.
After two months of seed treatments, the germination of chickpea seeds was slightly lower but still satisfactory for seed production however the maximum germination (100%) was recorded in control treatment followed by E. sativa (86.67%). After three months, we observed a pronounced effect in seed germination except in control treatment (100%). A significant different was noted in root length (P<0.05) of chickpea seeds after application of botanical oil seed in all three months. In the first month, maximum root length was 3.263±0.06 cm in control treatment next 2.771±0.31 cm at A. indica and almost similar 2.413±0.35 cm at E. sativa. In the second month, the root length of chickpea seeds was slightly lower but still good at E. sativa (2.040±0.03 cm) after control treatment (2.803±0.16 cm). The lowest root length (1.767±0.08 cm) was recorded at G. hirsutum. However, after three months, we also observed a pronounced effect in root length too except in control treatment (2.44±0.050 cm); but still E. sativa as compared to other treatments gave better results with root length of 1.87±0.10 cm. The vigour index of chickpea seeds in the first month was higher 277.00 at A. indica after control treatment (326.33) but in the second month, better results 176.84 were observed at E. sativa after control treatment (280.33) and similar trend remained continued until third month. However, the vigiour index of chickpea seed decreased dramatically in all treatments except in control (244.00).
Due to the toxicity of most pesticides and their residual effects, alternative control measures are prerequisite to eradicate the stored seeds pests. In this context, the use of botanical oils as pesticide is an apex site of attraction now a days. The local botanical plants are well recognized and documented for their performance as a repellent, seed protectant and toxic against many stored seed pests. In the present study, we evaluated the four botanical seed oils from neem seeds (A. indica), rocket seeds (E. sativa), cotton seeds (G. hirsutum) and mustard seeds (B. nigra) and used against C. maculatus which is to be considered as one of the major insect pests of pulses. The oils were used because most botanical oils have great potency being a toxic to many stored seed pests (Ahmd et al., 2003). Though, our findings indicated that all botanical oils played an imperative role against pulse beetle, but E. sativa provided the highest mortality followed by A. indica. Similarly, these results are in line with Abdul et al. (2017) who stated that most plant extracts show insecticidal activity which elicit complete mortality of insects, inhibition of adult’s emergence and any other physiological disorder. Similarly, Obeng-Ofori et al. (1998) stated that the high doses of eugenol provide 100% mortality of Triboleum castaneum H. An effective insecticidal impact of different plant extracts against various stored seed pests through topical application have also been previously well recorded (Tripathi et al., 2004; Suthisut et al., 2011) and similarly noted in present study. Moreover, Ahmed et al. (2016) determined the potential insecticidal effects of ten plant extracts (olive, tea, bhang, elephanta, neem, dharek, fruit of garlic, cloves, black pepper, and red chilies) against C. maculatus. Whereas the leaf extracts of black pepper showed highest efficiency in controlling chickpea beetle as compared to cloves, neem, and garlic. It showed that other botanicals in comparison with our most conventional botanical i.e., neem could perform better impact on store seeds and similarly we observed in present study when E. sativa performed more effectively than A. indica. The mortality percentage was noted increased at different interval but overall, one week mortality was remained highest at E. sativa because maximum mortality of C. maculatus was already died at 24 h and only few insects could survive; whereas A. indica was steady in killing the insects with the second best correctly mortality percentage; thus, both these botanical oils were found best as controlled almost 50% of adults pulse beetles. The extracts from different aromatic plants namely, pyrethrin (from Chrysanthemum cinerariaefolium), horseradish (Armoracia rusticana) oil, azadirachtin (from A. indica), allyl isothiocynate (from B. nigra) and carvone (from Carum carvi) have been acknowledged globally as these essential oils could be utilized as a protective additive as these are cheap to afford and easy to apply and slower in action when used within 24 hrs of their application. But it is important to evaluate first as all the botanical oils are not effective against every insect pest (Singh et al., 2012).
In addition to the direct mortality of C. maculatus, the effect of these botanical oils was tested for number of emerged adults and seed damage including punctured seeds and weight loss of chickpea seed. To testify the shelf life of these oils, we kept insects on treated seeds for three months consecutively. Those insects which survived during the first experiment of corrected mortality were further observed for extension of their next progeny on treated seeds. During the first month, the adults C. macularus were limited and their damage on seeds was also negligible as compared to the control treatment. However, in the second and third months, a significant raise in adult beetles of C. maculatus on G. hirsutum and B. nigara after control treatment was observed. Conversely, E. sativa and A. indica controlled the pest population with less seed damage as these bruchid beetles were less successful in puncturing the chickpea seeds treated particularly with E. sativa and similarly consumed the less part of seed kernels. Overall, we recorded in pooled data of three months that as pest population increased; the seed damage with number of punctured seeds and weight loss of chickpea seeds also roused. These results are in accordance with previous findings of (Varma and Anandhi, 2010) who studied the biology of C. chinensis and its management with different botanical extracts. They found that botanical pesticides played a crucial role to repel and kill the number of adult beetles. Thus, a smaller number of punctured seeds and seeds weight losses was noted. Sarswati et al. (2011) also studied the competency of plant oils (neem oil, soybean oil, mustard oil, coconut oil and sesame oil) against C. maculatus on mung bean (Vigna radiate L.) with a smaller number of adults pulse beetles as compared to control treatment. The phenomenon by which essential oil safeguards the seeds is not utterly clear, but we presume that it helps in inhibiting egg laying and disturb the larval (embryo) development on seed surface which results in death of premature larvae inside the egg before hatching of eggs and could bore into the seed (Singh et al., 2012). In some case, oil directly destroy/kill the insect eggs and if somehow, they manage to lay on seeds, the oil coating prevents the exchange of gaseous and the immature larvae inside the egg or kernel may die due to shortage of oxygen supply. But, if seeds are not sufficiently coated with botanical oils, then larvae do manage to enter the seed and will grow normally (Singh et al., 2012). Most of these researchers have recommended best dose of these botanical oils ranging from 5-10ml/kg of grins as seed protectant against pulse beetles for inhibiting their next progeny, loss in seed weigh and with shelf life up to 66 days (Bhatnagar et al., 2001).
The selection of botanical oils is also equally important based on their possible effects against insects without damaging seed quality and viability. Thus, in the present study, seed quality containing seed germination, root length and seed vigour index were observed. We noticed that the germination of chickpea seeds was less affected with application of these botanical oils for three months and particularly was excellent for two months with E. sativa and A. indica. Similarly, root length and vigour index were also satisfactory in parallel to control treatment on E. sativa and A. indica. In contrary, the chickpea seeds treated with G. hirsutum and B. nigara showed their bit adverse effect on seed characters. A similar finding has also been previously reported by Tariq et al. (2017) who observed leaf powders of five plants i.e., Syzygium cumini, Citrus limon, Momordica charantia, Eucalyptus globulus and Piper nigrum against C. chinensis and found no adverse effect of these botanical powders on germination of chickpea grains. Khinchi et al. (2017) reported different plant part powders viz., neem leaf, garlic bulb, garlic leaf and onion bulb. According to them all these were effective particularly neem leaf against pulse beetle but did not damage the quality and viability of chickpea seeds. Hossain et al. (2014) stated that plant extracts including tobacco leaf powder (TLP) had no consequence on seed enlargement of chickpea. A high mortality rate was reported in C. chinensis and other insect pests from vital oils of Citrus spp. such as C. reticulata, C. sinensis, C. paradise and C. grandis (Zia et al., 2013). Rahman and Talukder (2006)he oil treatment (oil from nishinda, eucalyptus, bankalmi, neem, sesame, safflower and ash of bablah Acacia arabica L. wood) used against pulse beetle did not show adverse effects on germination capability of black gram, Vigna mungo, seeds. Law-Ogbomo (2007) kept three varieties of maize seeds treated with different plant products in Petri dishes against Sitophilus zeamais (Motsch) in the laboratory under ambient room temperature (30±2oC) for observing the effect of plant oils on seed characters. They observed that after the germination, the seed further growth properly. Thus, the results of current study suggested that the botanical oils especially E. sativa and A. indica can be recommended to treat the seeds to avoid insect pests when stored for longer times. in
Conclusions and Recommendations
We conclude the results with these findings, though all the botanical oils against C. maculatus found effective but E. sativa and A. indica were the more prominent. These both botanical oils did not only kill the adults pulse beetle successfully but also protected the chickpea seeds with a smaller number of adult beetles and minimum seed damage including less punctured seeds and minimum seed weight loss. Furthermore, the preservation of chickpea seeds for consecutive two months were better with all these botanical oils. However, few effects of these oils on seed characteristics including seed germination, root length and vigour index were noted after three months of storage but still found the lowest deleterious effect on seed characters by E. sativa oil as compared to other treatments.
Acknowledgments
We highly acknowledge Prof. Dr. Imran Khatri, Chairman, Department of Entomology, FCPT, SAU Tando Jam, Pakistan for his kind perusal and support during this research work.
Novelty Statement
The present work will be helpful to reduce the usage of synthetic chemicals. These botanicals are safe for human health, environmentally friendly and effective against targeted insect pests. Botanical oils as a control strategy for stored grain pests are not yet in practice as chemicals by local farmers and still many botanicals are still needed to explore. Thus, this is another attempt to do research using different botanical oils in comparison to neem oil and observe their possible side effects on seed characteristics.
Author’s Contribution
AMA and QR: Designed and executed this research work.
AZAA and FNK: Prepared this manuscript with scientific writing.
SAO: Helped in data taking.
JDH: Assisted in manuscript with scientific writing.
DMS and ZAS: Performed lab work during research work.
Conflict of interest
The authors have declared no conflict of interest.
References
Abdul, A., F. Haque, M. Akter, N. Islam and S.A. Rahman. 2017. Screening and biological activity of indigenous plant extracts against pulse beetle Callosobruchus maculatus Bruchidae: Coleoptera). J. Agric. Biol., 5(2): 99-106.
Abdul-Baki, A.A., and J.D. Anderson. 1973. Vigour determination in soybean by multiple criteria. J. Chem. Sci., 13(1): 630-633. https://doi.org/10.2135/cropsci1973.0011183X001300060013x
Abozid, M.M., Y.A. Ashoush, A.A. Sakr, K.M. Taha, and E. Ayimba. 2014. Evaluation of Egyptian rocket seed oil as a source of essential fatty acids and its hypolipidemic effect in rats fed on high fat diet. Int. J. Adv. Res., 2(7): 434-441.
Ahmad, M., 2007. Insecticide resistance mechanisms and their management in Helicoverpa armigera (Hübner). A review. J. Agric. Res., 45(4): 319-335.
Ahmed, K.S., T. Itino and T. Ichikawa. 2003. Duration of developmental stages of Callosobruchus chinensis (Coleoptera: Bruchidae) on Adzuki bean and the effects of neem and sesame oils at different stages of their development. Pak. J. Biol. Sci., 6(10): 932-935. https://doi.org/10.3923/pjbs.2003.932.935
Ahmed, K.S., Y. Yasui and T. Ichikawa. 2001. Effects of neem oil on mating and oviposition behavior of adzuki bean weevil, Callosobruchus chinensis L. (Coleoptera: Bruchidae). Pak. J. Biol. Sci., 4: 1371-1373. https://doi.org/10.3923/pjbs.2001.1371.1373
Ahmed, Z., A. Muhammad, F. Naz and I. Muhammad. 2016. Bio-efficacy of some plant extracts against chickpea beetle, Callosobruchus maculatus L. (Coleoptera: Bruchidae) attacking chickpea. J. Entomol., 88(2): 192-202.
Ajayi, O.E. and C.O. Adedire. 1996. Insecticidal activity of an underutilized tropical plant seed oil, Hura crepitans L. on cowpea seed beetle, Callosobruchus maculatus. (F) (Coleoptera: Bruchidae). Niger. J. Entomol., 20: 74–81.
Alyokhin, A., M. Baker, D. Mota-Sanchez, G. Dively, and E. Grafius. 2008. Colorado potato beetle resistance to insecticides. Am. J. Pot. Res., 85: 395-413. https://doi.org/10.1007/s12230-008-9052-0
Bacci, L., M.C. Picanço, E.C. Barros, J.F. Rosado, G.A. Silva, V.F. Silva and N.R. Silva. 2009. Physiological selectivity of insecticides to wasps (Hymenoptera: Vespidae) preying on the diamondback moth. Sociobiology, 53: 151–167.
Befikadu, D., 2014. Factors affecting quality of grain stored in Ethiopian traditional storage structures and opportunities for improvement. Int. J. Sci. Basic Appl. Res., 18(1): 235-257.
Bhatnagar, A., N.S. Bhadauria and S.S. Jakhmola. 2001. Efficacy of vegetable oils against pulse beetles Callosobruchus maculatus in cowpea. Indian J. Entomol., 63: 23.
Boyer, S., H. Zhang, and G. Lempérière. 2012. A review of control methods and resistance mechanisms in stored-product insects. Bull. Entomol. Res., 102(2): 213-229. https://doi.org/10.1017/S0007485311000654
Chaudhary, S., R.K. Kanwar, A. Sehgal, D.M. Cahill, C.J. Barrow, R. Sehgal and J. R. Kanwar. 2017. Progress on Azadirachta indica based biopesticides in replacing synthetic toxic pesticides. Front. Plant Sci., 8: 610. https://doi.org/10.3389/fpls.2017.00610
Chowdhury, J.U., B.M.N. Islam and M. Yusuf. 2008. Chemical composition of the leaf essential oils of Murraya koenigii (L.) Spreng and Murraya paniculata (L.) Jack. Bangladesh J. Pharmacol., 3: 59-63. https://doi.org/10.3329/bjp.v3i2.841
Cranshaw, W.S., and B. Baxendale. 2013. Insect control: Horticultural oils. Fact sheet 5569. Ft Collins, CO: Colorado State University.
Dey, D., and, M.K. Gupta. 2016. Use of essential oils for insect pest management. A review. Innov. Farming, 1(2): 21-29.
FAOSTAT, F., 2018-19. FAOSTAT statistical database. Publisher: FAO (Food and Agriculture Organization of the United Nations), Rome, Italy.
Fatope, M.O., and A. Mann. 1995. Cowpea weevil bioassay: a simple prescreen for plants with seed protectant effects. Int. J. Pest Manage., 41: 84-86. https://doi.org/10.1080/09670879509371928
GoP, 2017. Production of gram. Pakistan Statistical Survey, 2015-16. Bureau of Statistics, Govt. of Pak, Islamabad.
Hebeish, A., M.M. Fouda, I.A. Hamdy, S.M. El-Sawy, and F.A. Abdel-Mohdy. 2008. Preparation of durable insect repellent cotton fabric: Limonene as insecticide. Carbohydr. Polym., 74(2): 268-273. https://doi.org/10.1016/j.carbpol.2008.02.013
Hieu, T.T., S.I. Kim, H.W. Kwon, and Y.J. Ahn. 2010. Enhanced repellency of binary mixtures of Zanthoxylum piperitum pericarp steam distillate or Zanthoxylum armatum seed oil constituents and Calophyllum inophyllum nut oil and their aerosols to Stomoxys calcitrans. Pest Manage. Sci., 66(11): 1191-1198. https://doi.org/10.1002/ps.1993
Hossain, M.A., M.A.A. Bachchu, K.S. Ahmed and M.A. Haque. 2014. Effectiveness of indigenous plant powders as seed protectant against Callosobruchus maculatus (L.) in stored chickpea (Cicer arietinum). Bangladesh J. Agric. Res., 39(1): 93-103. https://doi.org/10.3329/bjar.v39i1.20146
Isman, M.B., 1994. Neem botanical insecticides commercialization. Phytoparasitica, (25): 339–344. https://doi.org/10.1007/BF02981099
Kashiwaba, K., N. Tomooka, A. Kaga, O.K. Han and D.A. Vaughan. 2003. Characterization of resistance to three bruchid species (Callosobruchus spp., Coleoptera, Bruchidae) in cultivated rice bean (Vigna umbellata). J. Econ. Ent., 96: 207-213. https://doi.org/10.1093/jee/96.1.207
Khan, S., Z. Shah, I.A. Mian, K. Dawar, M. Tariq, B. Khan, M. Mussarat, H. Amin, M. Ismail, S. Ali, and T. Shah. 2020. Soil fertility, N2 fixation and yield of chickpea as influenced by long-term biochar application under mung–chickpea cropping system. Sustainability, 12(21): 9008. https://doi.org/10.3390/su12219008
Khinchi, S.K., M.M. Sharma, M.K. Khinchi, R.P. Naga, D. Acharya and R.C. Asiwal. 2017. Studies on efficacy of certain plant part powders against pulse beetle, Callosobruchus chinensis Linn. on chickpea, Cicer arietinum (L). Jr. Ent. Zoo. Stud., 15(3): 575-578.
Kilani-Morakchi, S., H. Morakchi-Goudjil, and K. Sifi. 2021. Azadirachtin-based insecticide: Overview, risk assessments and future directions. Front. Agron., 3: 32. https://doi.org/10.3389/fagro.2021.676208
Konecka, E., A. Kaznowski, W. Marcinkiewicz, D. Tomkowiak, M. Maciag, and M. Stachowiak. 2018. Insecticidal activity of Brassica alba mustard oil against lepidopteran pests Cydia pomonella (Lepidoptera: Tortricidae), Dendrolimus pini (Lepidoptera: Lasiocampidae) and Spodoptera exigua (Lepidoptera: Noctuidae). J. Plant Prot. Res., 58(2).
Kosini, D. and E.N. Nukenine. 2017. Bioactivity of novel botanical insecticide from Gnidia kaussiana against Callosobruchus maculatus in stored Vigna subterranean seeds. J. Insect Sci., 17(1): 1–7. https://doi.org/10.1093/jisesa/iex004
Law-Ogbomo, K.E., 2007. Reduction of Post-Harvest Loss Caused by Callosobruchus maculatus (F.) in three varieties of cowpea treated with plant oils. J. Entomol., 4: 194-201. https://doi.org/10.3923/je.2007.194.201
Magan, N., 2006. Mycotoxins in Europe: Prevention and early detection strategies. Mycopathologia, 162(3): 245-253. https://doi.org/10.1007/s11046-006-0057-2
Magan, N. and D. Aldred. 2007. Postharvest control strategies: minimizing mycotoxins in the food chain. Int. J. Food Microbiol., 119(1-2): 131-139. https://doi.org/10.1016/j.ijfoodmicro.2007.07.034
Merga, B. and J. Haji. 2019. Economic importance of chickpea: Production, value, and world trade. Cogent Food Agric., 5(1): 1615718. https://doi.org/10.1080/23311932.2019.1615718
Moreira, M.D., M.C. Picanço, L.C. de Almeida Barbosa, R.N.C. Guedes, M. Ribeiro de Campos, G.A. Silva and J.C. Martins. 2012. Plant compounds insecticide activity against Coleoptera pests of stored products. Pesq. Agropec. Bras. Brasılia, 42: 909-915. https://doi.org/10.1590/S0100-204X2007000700001
Morgan, E.D., 2009. Azadirachtin, a scientific goldmine. Bio-organ. Med. Chem., 17(12): 4096-4105. https://doi.org/10.1016/j.bmc.2008.11.081
Obeng-Ofori, D., C.H. Reichmuth, A.J. Bekele and A. Hassan. 1998. Toxicity and protectant potential of camphor, a major component of essential oil of Ocimum kilimandscharicum against four stored product beetle. Int. J. Pest Manage., 44: 203–209. https://doi.org/10.1080/096708798228112
Paes, J.L., L.R.D. Faroni, M.A. Martins, O.D. Dhingra and T.A. Silva. 2011. Diffusion and sorption of allyl isothiocyanate in the process of fumigation of maize. Rev. Bras. Eng. Agríc. Ambient., 15: 296e301. https://doi.org/10.1590/S1415-43662011000300011
Pasquoto-Stigliani, T., E.V.R. Campos, J.L. Oliveira, C.M.G. Silva, N. Bilesky-José, M. Guilger, J. Troost, H.C. Oliveira, R. Stolf-Moreira, L.F. Fraeto and R. De Lima. 2017. Nanocapsules containing neem Azadirachta ndica oil: development, characterization and toxicity evaluation. Sci. Rep., 7(1): 1-12. https://doi.org/10.1038/s41598-017-06092-4
Radha, R., and P. Susheela. 2014. Efficacy of plant extracts on the toxicity, ovipositional deterrence and damage assessment of the cowpea weevil, Callosobruchus maculatus (Coleoptera: Bruchidae). J. Entomol. Zool. Stud., 2(3): 16-20.
Rahman, A., and F.A. Talukder. 2006. Bio-efficacy of some plant derivatives that protect seed against the pulse beetle, Callosobruchus maculatus. J. Insect. Sci., 6(1): 135-142. https://doi.org/10.1673/1536-2442(2006)6[1:BOSPDT]2.0.CO;2
Raja, N., 2014. Botanicals: Sources for eco-friendly biopesticides. J. Biofertil. Biopestic., 5(1): 1. https://doi.org/10.4172/2155-6202.1000e122
C., C. J.T. 2012. Essential oils in insect control: Low-risk products in a high-stakes world. Ann. Rev. Entomol., 57: 405–424. https://doi.org/10.1146/annurev-ento-120710-100554
Sarswati, N., R.B. Thapa, Y. Dhoj, S. Pokhrel, P. Prasad, S. Subedi and A. Upadhya. 2011. Efficacy of plant dusts, oils and indigenous materials against stored pulse beetle Callosobruchus maculatus L. Pak. J. Zoo, 43(4): 733-737.
Saxena, B. and R.Z. Sayyed. 2018. Botanical insecticides effectively control chickpea weevil, Callosobruchus maculatus. Environ. Sustain., 1: 295–301. https://doi.org/10.1007/s42398-018-00029-x
Schneider-Orelli, O., 1947. Entomologisches Praktikum. Sauerlander, Aarau, Switzerland.
Sharma, N., and M.A. Hanna. 1989. A microwave oven procedure for soybean moisture content determination. Cereal chemist, (USA).
Sharma, S., and D.R. Thakur. 2014. Comparative developmental compatibility of Callosobruchus maculatus on cowpea, chickpea and soybean genotypes. Asian J. Biol. Sci., 7: 270-276. https://doi.org/10.3923/ajbs.2014.270.276
Singal, P.K. and Z. Singh. 1990. Studies of plant oils as surface protectants against pulse beetle, Callosobruchus analis F. in chickpea, Cicer arietinum L. in India. Trop. Pest. Manage., 36(3): 314-316. https://doi.org/10.1080/09670879009371496
Singh, A., A. Khare and A.P. Singh. 2012. Use of vegetable oils as biopesticide in seed protection. A review. J. Biofertil. Biopestic., 3(2): 1-9
Singh, K.D., A.J. Mobolade, R. Bharali, D. Sahoo and Y. Rajashekar. 2021. Main plant volatiles as stored seed pest management approach: A review. J. Agric. Food Res., 4: 100127. https://doi.org/10.1016/j.jafr.2021.100127
Singh, R.P., 1993. Neem for the management of stored seed insects in developing countries. In: World Neem Conference Bangalore, India. Souvenir. pp. 69-80.
D., P.G. and A. . 2011. Contact toxicity, feeding reduction and repellency of essential oils from three plants from the ginger family (Zingiberaceae) and their major components against Sitophilus zeamais and Tribolium castaneum. J. Stored Prod. Res., 104(4): 445–1454. https://doi.org/10.1603/EC11050
Stejskal, V., J. Hubert and Z. Li. 2018. Human health problems and accidents associated with occurrence and control of storage arthropods and rodents. In: Recent advances in stored product protection. Springer, Berlin, Heidelberg. pp. 19-43. https://doi.org/10.1007/978-3-662-56125-6_2
Stejskal, V., T. Vendl, R. Aulicky, and C. Athanassiou. 2021. Synthetic and natural insecticides: Gas, liquid, gel and solid formulations for stored-product and food-industry pest control. Insects, 12(7): 590. https://doi.org/10.3390/insects12070590
Suthisut, D., P.G. Fields and A. Chandrapatya. 2011. Fumigant toxicity of essential oils from three (ai plants Zingiberaceae) and their major compounds against Sitophilus zeamais, Tribolium castaneum and two parasitoids. J. Stord. Prod. Res., 47(3): 222–230. https://doi.org/10.1016/j.jspr.2011.03.002
Tamgno, B.R., and T.S.L. Ngamo. 2014. Utilisation des produits derives du neem Azadirachta indica A. Juss comme alternatifs aux insecticides synthe- tiques pour la protection des semences de maıs et de sorgho dans la Vallee du Logone. Sci. Technol. Dev., 15: 1-8.
Tariq, A., S. Afsheen, M. Hussain, A. Zia, S.S. Shah, S. Afzal and I. Khan. 2017. Bio-efficacy of plant powders against Callosobruchus chinensis L. (Coleoptera: Bruchidae) in infested chickpea seeds. A. J. Agric. Biol., 12(3): 119-125.
Tripathi, A.K., V. Prajapati, S.P.S. Khanuja and S. Kumar. 2003. Effect of d-limonene on three stored-product beetles. J. Ecol. Entomol., 96(3): 990-995. https://doi.org/10.1093/jee/96.3.990
Tripathi, P., N.K. Dubey, R. Banerji and J.P.N. Chansouria. 2004. Evaluation of some essential oils as botanical fungi-toxicants in management of post-harvest rotting of citrus fruits. W. J. Microbiol. Bio-tech., 20(3): 317-321. https://doi.org/10.1023/B:WIBI.0000023844.80464.59
Tsao, R., C.J. Peterson and J. R. Coats. 2002. Glucosinolate breakdown products as insect fumigants and their effect on carbon dioxide emission of insects. BMC Ecol., 2: 1e7. https://doi.org/10.1186/1472-6785-2-5
Upadhyay, R.K., and S. Ahmad. 2011. Management strategies for control of stored grain insect pests in farmer stores and public warehouses. J. Agric. Sci., 7(5): 527-549.
Varma, S., and P. Anandhi. 2010. Biology of pulse beetle Callosobruchus chinensis L. (Coleoptera: Bruchidae) and their management through botanicals on stored mung seeds in Allahabad region. Leg. Research: Int. J., 33(1).
Varshney, C., R.K. Song, S. Saxena Azam, S. Yu, A.G. Sharpe and T. Millan. 2013. Draft genome sequence of chickpea (Cicer arietinum) provides a resource for trait improvement. Nat. Biotech. 31(3): 240. https://doi.org/10.1038/nbt.2491
Zia, S., M. Sagheer, R. Abdul, A. Mahboob, K. Mehmood and Z. Haider. 2013. Comparative bio-efficacy of different Citrus peel extracts as grain protectant against Callosobruchus chinensis, Trogoder magranarium and Tribolium castaneum. World Appl. Sci. J., 21(12): 1760-1769.
To share on other social networks, click on any share button. What are these?







