Effects of Seed Physical Characteristics of Benin Soybean Germplasm on their Resistance to Callosobruchus maculatus Fabricius (Coleoptera: Bruchidae)
Research Article
Effects of Seed Physical Characteristics of Benin Soybean Germplasm on their Resistance to Callosobruchus maculatus Fabricius (Coleoptera: Bruchidae)
Yêyinou Laura Estelle Loko1*, Joelle Toffa1, Azize Orobiyi1, Gbèblonoudo Anicet Dassou2, Rolande Okpeicha1, Dieudonné Gavoedo1 and Alexandre Dansi2
1Laboratory of Applied Entomology (LEnA), Ecole Nationale Supérieure des Biosciences et Biotechnologies Appliquées (ENSBBA), Université Nationale des Sciences, Technologies, Ingénierie et Mathématiques (UNSTIM), BP 14 Dassa-Zoumé, Benin; 2Laboratory of Biotechnology, Genetic Resources and Plant and Animal Breeding (BIORAVE), ENSBBA, UNSTIM, BP 14 Dassa-Zoumé, Benin.
Abstract | The pulse beetle Callosobruchus maculatus Fabricius (Coleoptera: Bruchidae) is an important pest of stored soybean grains. It is imperative to screen out the prevailing genotypes of Beninese soybean cropping systems in order to find out the resistant ones against C. maculatus infestations. The effect of seven grain physical traits (testa thickness, colour, texture, hardness, length, breadth, and 100-grain mass) on the susceptibility of eight soybean varieties to C. maculatus were evaluated in the laboratory. The correlations and contributions of the studied traits were evaluated using correlation path coefficient analysis. The tested soybean varieties showed a variation in physical seed characteristics. A differential susceptibility of soybean varieties to C. maculatus was observed, with the white seeded variety Whéwhé having longest and largest grains was the most susceptible. Based on the Dobie susceptibility index, the Yovoton variety was proved to be resistant to C. maculatus attacks. While, Kecheke, Houeton, Adjaton and Vovoh varieties were classified as moderately resistant to C. maculatus. The correlation analysis indicated that 100-seed weight had significant positive correlation with F1 progeny (r = 0.439), seed consumption (r = 0.467), number of eggs laid (r = 0.295) and susceptibility index (r = 0.453). Path coefficient analysis showed that each seed physical character and its interactions with the others characters influenced soybean grains susceptibility to C. maculatus. Soybean seed thickness showed the higher direct positive effect on soybean susceptibility to C. maculatus indicating that breeding should be done based on this trait to improve soybean seed resistance.
Received | June 24, 2021; Accepted | July 26, 2022; Published | October 18, 2022
*Correspondence | Yêyinou Laura Estelle Loko, Laboratory of Applied Entomology (LEnA), Ecole Nationale Supérieure des Biosciences et Biotechnologies Appliquées (ENSBBA), Université Nationale des Sciences, Technologies, Ingénierie et Mathématiques (UNSTIM), BP 14 Dassa-Zoumé, Benin; Email: lokoestelle@yahoo.fr
Citation | Loko, Y.L.E., J. Toffa, A. Orobiyi, G.A. Dassou, R. Okpeicha, D. Gavoedo and A. Dansi. 2022. Effects of seed physical characteristics of benin soybean germplasm on their resistance to Callosobruchus maculatus fabricius (Coleoptera: Bruchidae). Sarhad Journal of Agriculture, 38(4): 1468-1477.
DOI | https://dx.doi.org/10.17582/journal.sja/2022/38.4.1468.1477
Keywords | Glycine max, Path analysis, Mediation analysis, Bruchids, Varietal resistance
Copyright: 2022 by the authors. Licensee ResearchersLinks Ltd, England, UK.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Introduction
The soybean (Glycine max (L.) Merr.) is a food crop of economic importance in Republic of Benin with an estimated production of 230000 tons in 2019 (FAO, 2019). This legume seed, with its high protein value, is an important pillar in the fight against malnutrition in rural areas and is mainly used in infant food (Wendland and Sills, 2008; Chadare et al., 2018). Beninese population consume soybean under various forms (flour, milk, cheese, etc.), and use it in animal feed due to its low cost and availability (Ayenan et al., 2017; Hounhouigan et al., 2020; Idrissou et al., 2020). However, soybean seeds are subject to enormous postharvest losses due to storage insect attacks with an estimated losses average 10% of produced soybean (Chelladurai et al., 2014). In addition, these storage insects lead to a rapid degradation of the soybean grain quality and a loss of germination viability (Ulemu et al., 2016).
The beetle Callosobruchus maculatus (Fabricius) (Coleoptera: Bruchidae) was found to be the main pest of stored soybean grains in the Republic of Benin (Loko et al., 2021). Farmers to protect stored soybeans from bruchid attacks mainly use synthetic chemical insecticides (Loko et al., 2021). While, the use of synthetic insecticides such as imidacloprid insecticide, not only have undesirable effects on human health, they also reduce the germination capacity (up to 22.6%) of soybean seeds (Pereira et al., 2020). Among the alternative control methods against bruchid pests, the use of resistant varieties is one of the cheapest control methods that seems to be more easily adopted by farmers (Msiska et al., 2018). Although varietal resistance of soybean to C. maculatus attacks has been demonstrated by several studies (Allotey et al., 2004; Sharma and Thakur, 2014a; Ulemu et al., 2016), no information is available on the resistance of Benin soybean germplasm to bruchid infestations. Whereas, the identification of soybean varieties resistant to C. maculatus could be directly useful for scientific research (varietal development and improvement) and development (varietal introduction and exchange).
A great diversity of soybean varieties is found in Beninese agriculture (Loko et al., 2021). However, it is known that physical characteristics of soybean seeds influence their resistance to bruchid pests (Ulemu et al., 2016). Therefore, it is important to identify the physical traits responsible of soybean resistance to C. maculatus. Indeed, a good knowledge of soybean resistance factors to bruchid infestations is necessary for the breeding of resistant varieties (Msiska et al., 2018). The objective of this study was to: (i) assess the resistance level of soybean genotypes grown in Republic of Benin against C. maculatus attacks; (ii) assess the influence of soybean physical grain characteristics on their susceptibility to C. maculatus.
Materials and Methods
Plant material
Eight soybean varieties presenting different morphological characteristics grown in the south and centre Benin were used for experiments (Loko et al., 2021). The soybean seeds were obtained from farmers in eight villages in the southern Benin (Figure 1). The soybean seeds were sorted using a binocular microscope to ensure that they were not damaged or infested. Sterilization of soybean seeds was done by drying them in an oven at a temperature of 30°C for 24 h (Msiska et al., 2018). Healthy seeds were conditioned at room temperature (25±2°C) and relative humidity of 65±5% in the laboratory for 2 weeks.
Physical characteristics of soybean varieties
Seven grain physical traits (testa thickness, colour, texture, hardness, length, breadth and 100-seed weight) were evaluated using various tools. Ten seeds of each soybean variety were randomly chosen to measure their testa thickness and size (length and width) using a Marathon electronic micrometer (measuring range 0-25 mm). The pigmentation and texture of the grains of the different soybean varieties were analysed by observation under a stereoscopic microscope coupled with a digital video camera. While the seed hardness was measured with a “Shore A” hardness tester and the one hundred seed weight was measured with an electronic scale. The data were taken from 10 randomly selected seeds. The seed moisture content was measured using a digital moisture meter lds-1g analyser with a Kohstar micro-control computer.
Bruchid rearing
Bruchids were collected from infested soybeans obtained from farmers in the village of Gangnigon in the district of Kpankou. These bruchids were kept in the laboratory of entomology of the International Institute of Tropical Agriculture (IITA-Benin). For this purpose, 50 C. maculatus adults (unsexed) were putted in plastic boxes (6 cm × 10 cm) containing 300 g of soybeans from a mixture of seeds of the different soybean varieties, previously sorted and sterilised. These boxes were covered with a muslin cloth to allow ventilation of the conservation medium and avoid insect escape. The plastic boxes were kept on shelves under laboratory conditions (70 ± 5% RH and 26±2°C). The adult insects were removed of experimental boxes after seven days of oviposition, and the boxes were kept until adult emergence. Progeny were used for experiments.
Screening of soybean varieties for resistance to Callosobruchus maculatus
The resistance of eight soybean varieties to C. maculatus was tested using the methodology described by Sharma and Thakur (2014a). The trials were conducted on soybeans containing 12-13% relative humidity (Table 1). For this purpose, 50 seeds of each previously sterilised soybean genotype were weighed using an electronic scale and placed in plastic boxes (3 cm × 4 cm). Four (2 males and 2 females) C. maculatus adults (1-3 days old) were placed in each plastic box. The sexing of C. maculatus was done base on the shape and size of the abdomens as described by Bandara and Saxena (1995). The experiments were conducted under laboratory conditions (24 ± 2°C, 75 ± 5%, and 12 h / 12 h) and deposited in a randomized block design with 4 replicates. Daily, dead insects were removed from the experimental boxes and replaced by live ones (Oigiangbe and Onigbinde, 1996). After seven days, the adult bruchids were removed from the soybean samples and the number of eggs laid on seeds of each variety was recorded. After 25 days, the experimental boxes were observed daily to count the number of adult insect emergence (Adebayo et al., 2016). The emerged adults were removed from the boxes after counting. Daily counting was stopped when no emergence was observed after 5 consecutive days (Lephale et al., 2012). The percentage of adults emerged was calculated following the formula (Sharma and Thakur, 2014a):
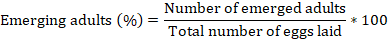
After the period of observation of adult emergence, the number of attacked grains (based on the emergence holes) and the final weight of the seeds in each experimental box were determined according to the formulas (Sharma and Thakur, 2014a):
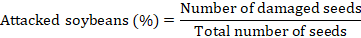
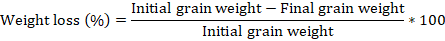
The Dobie susceptibility index was calculated according to the formula (Dobie, 1977):
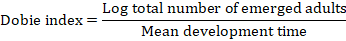
Where the mean development time is the time (days) from the middle of the oviposition period to the emergence of fifty percent of the F1 progeny (Dobie, 1977). The soybean varieties were classified using the following sensitive scale: 0-3 = resistant, 4-7 = moderately resistant, 8-10 = susceptible, and ≥ 11 = highly susceptible (Dobie, 1974).
Data analysis
The data expressed in percentage (mortality, weight loss and reproductive inhibition), and the number of F1 offspring emerged were arcsine (arscine√x) and log (log (x)) transformed respectively to homogenise their variance before subjected to ANOVA. The Student–Newman–Keuls (SNK) test with a probability of 5% was performed using IBM SPSS software version 25 to identify significant differences between the means. The correlation between the physical characteristics of soybeans and susceptibility to attack by C. maculatus was calculated using Pearson’s coefficient using Minitab 17 software. To identify the direct and indirect effects of the correlation coefficients, the path coefficient analysis and mediation analysis was done using SPSS AMOS software version 21 (Deway and Lu, 1959) with Dobie susceptibility index taken as the dependent variable while the seed physical characteristics were considered as the independent variables.
Table 1: Physical characteristics of seeds of the soybean cultivars from southern Benin screened for resistance to Callosobruchus maculatus.
|
Soybean varieties |
Seed coat features |
Seed dimensions (mm) |
Seed characteristics |
||||||
|
Colour |
Texture |
Thickness (mm) |
Length |
Width |
Hardness (Shore A) |
Moisture content (%) |
Weight of 100 seeds (g) |
||
|
Adjaton |
Yellowish white |
Smooth |
0.16±0.02abc |
5.37 ± 0.43a |
4.68 ± 0.33a |
95.70 ± 7.62ab |
12.41 ± 0.30a |
7.59 ± 0.32a |
|
|
Agliki |
Buff |
Smooth |
0.15 ± 0.09ab |
6.36 ± 0.79b |
5.42 ± 0.50b |
101.15±5.63bc |
12.60 ± 0.27a |
10.52 ± 0.29b |
|
|
Anoumèton |
Reddish brown |
Rough |
0.12 ± 0.01a |
7.34 ± 0.38cd |
5.32 ± 0.29b |
96.25 ± 4.43ab |
13.04 ± 0.41a |
10.85 ± 0.39b |
|
|
Houéton |
Buff |
Smooth |
0.15 ± 0.02ab |
7.35 ± 0.41cd |
5.32 ± 0.37b |
89.95 ± 5.13a |
12.53 ± 0.32a |
10.61 ± 0.54b |
|
|
Kèchèkè |
Buff |
Smooth |
0.21 ± 0.05c |
6.70 ± 0.88bc |
5.63 ± 0.55bc |
97.25 ± 9.28ab |
12.39 ± 0.27a |
10.97 ± 0.45b |
|
|
Vovoh |
Yellow |
Smooth |
0.13 ± 0.03a |
6.64 ± 0.70bc |
5.49 ± 0.53bc |
104.20 ± 1.73c |
12.31 ± 0.34a |
10.60 ± 0.20b |
|
|
Whéwhé |
White |
Smooth |
0.19 ± 0.03bc |
7.74 ± 0.57d |
5.92 ± 0.36c |
95.30 ± 3.37ab |
12.73 ± 0.31a |
14.24 ± 0.90d |
|
|
Yovoton |
Buff |
Smooth |
0.14 ± 0.03ab |
6.80 ± 0.49bc |
5.70 ± 0.38bc |
102.5 ± 6.06bc |
12.56 ± 0.34a |
12.32 ± 0.69c |
|
Mean in a column followed by the same letter(s) do not differ significantly at the 5% level by SNK test.
Table 2: Mean number of eggs laid, percent of adults emergence, number of adult progeny, median development time of Callosobruchus maculatus, seed damage, weight loss, and Dobie index susceptibility.
|
Soybean genotype |
Mean number of eggs laid |
Number of adult progeny |
Percent of adults emerged |
Median of development time (days) |
Seed damage (%) |
Weight loss (%) |
Dobie suscepti-bility index |
Resistance category |
|
Adjaton |
14.50± 4.20a |
1.75±1.25a |
12.62 ± 9.33a |
35.87±5.07a |
3.50 ± 2.51a |
2.04 ± 1.40abc |
5.42 |
Moderately resistant |
|
Agliki |
27.50± 17.07a |
6.00±3.16ab |
29.41± 21.32ab |
29.83±1.76a |
12.00± 6.32ab |
2.38 ± 0.88bc |
10.66 |
Susceptible |
|
Anoumèton |
18.00± 9.62a |
7.75±9.50ab |
37.79± 26.61ab |
33.50±1.69a |
15.50± 19.00ab |
3.25 ± 1.58c |
10.24 |
Susceptible |
|
Houéton |
14.00± 4.32a |
3.75±1.70ab |
27.79± 12.67ab |
34.25±3.52a |
7.50 ± 3.41a |
0.91 ± 0.48ab |
7.91 |
Moderately resistant |
|
Kèchèkè |
21.50± 15.52a |
3.00±1.15a |
21.59± 16.81a |
31.75±1.30a |
6.00 ± 2.30a |
0.72 ± 0.29ab |
7.82 |
Moderately resistant |
|
Vovoh |
14.25± 3.77a |
1.25±1.50a |
7.28± 8.46a |
35.12±5.26a |
2.50 ± 3.00a |
0.37 ± 0.15a |
4.58 |
Moderately resistant |
|
Whéwhé |
27.00± 23.50a |
14.50±9.46b |
75.29± 47.27b |
30.79±0.48a |
29.00 ± 18.93b |
7.76 ± 2.54d |
13.18 |
Very susceptible |
|
Yovoton |
20.00± 14.14a |
1.01±0.81a |
5.00 ± 4.08a |
35.33±5.43a |
2.00 ± 1.63a |
0.31 ± 0.01a |
3.92 |
Resistant |
Mean values ± standard error in a column followed by the same letter(s) do not differ significantly at the 5% level by SNK test.
Results and Discussion
Physical characteristics of the soybean seeds
The soybeans tested had a colour diversity and only the Anoumèton variety showed a rough texture (Table 1). The seed coat thickness of the different varieties varied between 0.01 to 0.21 mm. The Kèchèkè variety showed significantly (F = 4.760, df = 79, P ≤0.000) the thickest seed coat. While, the Adjaton variety significantly exhibited the shortest grain (F = 18.845, df = 79, P ≤ 0.000) and the narrowest (F = 7.692, df = 79, P ≤ 0.000). The hardness of the seeds varied from 89.95 to 104.2 Shore A. The Houeton variety exhibited significantly (F = 6.123, df = 79, P ≤ 0.000) the softer seeds. The moisture of the seeds of the different varieties varied between 12.31 and 13.04% (Table 2). The Whéwhé variety exhibited significantly the highest 100 seed weight (F = 153.363, df = 79, P ≤ 0.000) (Table 1).
Resistance of soybean varieties to C. maculatus attacks
The average number of eggs laid by C. maculatus on the different soybean varieties varied from 14.00 ± 4.32 (Houeton) to 27.00 ± 23.50 (Whèwhè) (Table 2). However, this difference was not significant (F = 0.481, df = 31, P = 0.839) between the different varieties. The number of hatched eggs varied from 1.01 ± 0.81 (Yovoton) to 14.50 ± 9.46 (Whéwhé). The number of eggs hatched on the Whéwhé variety was significantly (F = 5.627, df = 31, P = 0.001) different from that of the other varieties (Table 3). The percentage of emerged adults ranged from 5 to 75.29%. The lowest percentage of emerged adults was observed on the Yovoton variety (5.00 ± 4.08%) which differed significantly (F = 4.326, df = 31, P = 0.003) from those of the Vovoh (8.46 ± 7.28%), Adjaton (12.62 ± 9.33%) and Kèchèkè (21.59 ± 16.81%) varieties. The development time of C. maculatus on the different
Table 3: Correlation coefficients between seed physical characters of eight soybean cultivars and their susceptibility parameters to Callosobruchus maculatus.
|
Vari-ables |
COL |
TEX |
THI |
LEN |
WID |
HAR |
MOC |
WEI |
SI |
NEL |
PRO |
CON |
|
COL |
- |
|||||||||||
|
TEX |
0.192 |
- |
||||||||||
|
THI |
-0.208 |
-0.267* |
- |
|||||||||
|
LEN |
-0.263* |
0.234* |
0.074 |
- |
||||||||
|
WID |
-0.281* |
-0.082* |
0.251 |
0.578 *** |
- |
|||||||
|
HAR |
0.165 |
-0.083 |
-0.018 |
-0.064 |
0.181 |
- |
||||||
|
MOC |
-0.129 |
0.444*** |
-0.004 |
0.448 *** |
0.164 |
0.080* |
- |
|||||
|
WEI |
-0.396*** |
-0.022 |
0.115 |
0.620 *** |
0.597*** |
0.052 |
0.337 ** |
- |
||||
|
SI |
-0.318** |
0.283* |
0.159 |
0.418 *** |
0.220* |
-0.249* |
0.671 *** |
0.453 *** |
- |
|||
|
NEL |
-0.222* |
-0.034 |
-0.058 |
0.089 |
0.296** |
0.191 |
0.096 |
0.295 ** |
0.321 ** |
- |
||
|
PRO |
-0.116 |
0.208 |
-0.030 |
0.373 *** |
0.241* |
-0.097 |
0.372 *** |
0.439 *** |
0.654 *** |
0.561 *** |
- |
|
|
CON |
-0.017 |
0.194 |
0.091 |
0.353 *** |
0.220 |
-0.188 |
0.357 *** |
0.467 *** |
0.765 *** |
0.288 ** |
0.761 *** |
- |
Colour (COL), texture (TEX), thickness (THI), length (LEN), width (WID), hardness (HAR), moisture content (MOC) and weight of 100 seeds (WEI), susceptibility index (SI), number of eggs laid (NEL), F1 progeny (PRO), and seed consumption (CON). Significant correlations at *p < 0.05, **p < 0.01, ***p < 0.001; ns: not significant.
varieties was extended from 29 (Agliki) to 35 (Adjaton) days. There was no significant difference (F = 1.843, df = 31, P = 0.125) in the development time of C. maculatus on the different varieties tested. The percentage of damaged seed varied from 2.00 ± 1.63 (Yovoton) to 29 ± 18.93% (Whéwhé). A significant difference (F = 4.619, df = 31, P = 0.002) was observed between the different varieties in terms of damage. The weight loss of the different varieties due to the consumption of C. maculatus varied from 0.31 ± 0.01% (Yovoton) to 7.76 ± 2.54% (Whéwhé). The maximum weight loss by the Whéwhé variety was significantly (F = 19.244, df = 31, P = 0.000) different from the other varieties. The susceptibility index ranged from 3.92 to 13.18 with the variety Yovoton classified as resistant to attack by C. maculatus.
Correlation between the seed physical characteristics and the different observations
The correlation analysis showed a positive and significant correlation between 100-seed weight and F1 progeny (r= 0.439), seed consumption (r = 0.467), and number of eggs laid (r= 0.295), respectively (Table 3). A positive and significant correlation also exist between seed length and F1 progeny (r = 0.373), seed consumption (r= 0.353), respectively. It is the same with seed moisture content and F1 progeny (r = 0.372), seed consumption (r = 0.357), respectively. While, seed width was positively correlated with number of eggs laid (r = 0.296) and F1 progeny (r = 0.241), respectively. Only seed coat colour showed a significant and negative correlation (r = -0.222) with number of eggs laid by C. maculatus females.
The correlation analysis between the physical characteristics of soybeans and their resistance to attack by C. maculatus showed that there was a significant negative correlation (r = -0.32) between the colour of the seeds and the susceptibility index. Likewise, a significant and negative correlation (r = -0.25) was observed between the seed hardness and the susceptibility index. However, a significant positive correlation (r = 0.28) was noted between the seed texture and the susceptibility index. Seed measurements (length and width), moisture and 100-seed weight showed a significant positive correlation with the resistance of the tested soybean varieties to C. maculatus (Table 3).
Direct and indirect effects of the physical characteristics of soybeans on resistance to attack by C. maculatus
Direct and indirect effects of the seed physical characteristics on the resistance to C. maculatus attacks were evaluated (Figure 2). The root mean square error of approximation (RMSEA) was inferior to 0.05 indicating the good fit of the used model. The high value of the determination coefficient of path analysis estimated at 0.64, and the low effect of the residual variable (5.45) showed a strong relationship between the susceptibility to C. maculatus attacks and analysed variables. The seed coat thickness showed the highest positive direct effect (10.54) on soybean susceptibility to C. maculatus. Direct path coefficient values on soybean susceptibility to C. maculatus were also found for seed coat texture (3.02), 100-grain mass (0.65), and seed length (0.28). Seed width (-0.50), seed colour (-0.33), and seed hardness (-0.08) had negative direct effects on soybean susceptibility to C. maculatus. The interrelation between the evaluated seed physical characteristics also showed that each variable influenced the soybean susceptibility to C. maculatus by acting with the others variables (Table 4). The highest positive indirect effects of 3.982 on soybean susceptibility to C. maculatus was induced by seed coat thickness through 100 grain mass (Table 4). The indirect effect of seed colour and seed width via seed hardness were more important and masked its direct effect on susceptibility index of soybean to C. maculatus.
Among the alternative control methods against stored grain pests, the use of resistant varieties is one of the most economical means of control and the adoption of which by producers seems to be easier. Our study revealed a significant variability in the physical characteristics of Beninese soybean seeds and a differential susceptibility to infestation by C. maculatus. Indeed, several authors have demonstrated
Table 4: Estimates of direct and indirect effects of the seed physical characteristics on the susceptibility index of soybean genotypes from southern Benin to Callosobruchus maculatus.
|
Pathway |
Path analysis |
|
Seed colour and susceptibility index |
|
|
Direct effect |
-0.331 |
|
Indirect effect via seed length |
-0.160 |
|
Indirect effect via seed width |
-0.101 |
|
Indirect effect via seed coat thickness |
-0.007 |
|
Indirect effect via seed hardness |
0.786 |
|
Indirect effect via seed texture |
0.043 |
|
Indirect effect via 100 grain mass |
-0.486 |
|
Seed texture and susceptibility index |
|
|
Direct effect |
3.018 |
|
Indirect effect via seed length |
0.636 |
|
Indirect effect via seed width |
-0.132 |
|
Indirect effect via seed coat thickness |
-0.042 |
|
Indirect effect via seed hardness |
-1.764 |
|
Indirect effect via seed colour |
0.857 |
|
Indirect effect via 100 grain mass |
-0.123 |
|
Seed hardness and susceptibility index |
|
|
Direct effect |
-0.082 |
|
Indirect effect via seed length |
-0.008 |
|
Indirect effect via seed width |
0.014 |
|
Indirect effect via seed coat thickness |
0.000 |
|
Indirect effect via seed colour |
0.035 |
|
Indirect effect via seed texture |
-0.004 |
|
Indirect effect via 100 grain mass |
0.013 |
|
Seed length and susceptibility index |
|
|
Direct effect |
0.280 |
|
Indirect effect via seed colour |
-0.432 |
|
Indirect effect via seed width |
0.382 |
|
Indirect effect via seed coat thickness |
0.004 |
|
Indirect effect via seed hardness |
-0.498 |
|
Indirect effect via seed texture |
0.086 |
|
Indirect effect via 100 grain mass |
1.252 |
|
Seed width and susceptibility index |
|
|
Direct effect |
-0.500 |
|
Indirect effect via seed length |
0.977 |
|
Indirect effect via seed colour |
-0.782 |
|
Indirect effect via seed coat thickness |
0.025 |
|
Indirect effect via seed hardness |
2.398 |
|
Indirect effect via seed texture |
-0.051 |
|
Indirect effect via 100 grain mass |
2.039 |
|
Seed thickness and susceptibility index |
|
|
Direct effect |
10.539 |
|
Indirect effect via seed length |
1.275 |
|
Pathway |
Path analysis |
|
Indirect effect via seed width |
2.560 |
|
Indirect effect via seed colour |
-5.895 |
|
Indirect effect via seed hardness |
-2.448 |
|
Indirect effect via seed texture |
-1.688 |
|
Indirect effect via 100 grain mass |
3.982 |
|
100-grain mass and susceptibility index |
|
|
Direct effect |
0.646 |
|
Indirect effect via seed length |
0.307 |
|
Indirect effect via seed width |
0.175 |
|
Indirect effect via seed coat thickness |
0.003 |
|
Indirect effect via seed hardness |
0.200 |
|
Indirect effect via seed texture |
-0.004 |
|
Indirect effect via seed colour |
-0.322 |
the varietal resistance of soybean to attacks by C. maculatus (Allotey et al., 2004; Ulemu et al., 2016). The white seeded variety Whéwhé with significantly the longest and largest grain was most susceptible to attack by C. maculatus. This is not surprising because it is known that the small seed size is among the physical characteristics of tolerant soybean varieties to C. maculatus (Sharma and Thakur, 2014b) because larval growth is limited by low food availability and space. Indeed, the Adjaton variety with yellow white seed and having the smallest measurements was found to be moderately resistant to C. maculatus. However, the big size of soybean seed is among the varietal preferential criteria of Beninese farmers (Loko et al., 2021). Therefore, it is important to make in place a national breeding program involving Whéwhé variety and Yovoton variety as progenitors to meet farmer’s needs. Indeed, Yovoton variety, which exhibited average seed physical characteristics, was found to be resistant to C. maculatus attacks. The resistance of this variety may relate to the low adult bruchid emergence, low seed damage, and low seed weight loss due to the existence of physical and or chemical barriers in the seeds thus affecting larval penetration. Therefore, the Yovoton variety must be popularized and integrated into varietal creation programs in order to minimize losses recorded during soybean storage. However, the identification of the genes and biochemicals that are responsible of Yovoton variety resistance should be determine and taken into account in research programs.
Our results revealed that soybean seed physical characteristics did not influence the oviposition of C. maculatus females. Indeed, Sekender et al. (2020) reported that C. maculatus is able to lay eggs on any seed, even if the seed is not suitable for larval development. However, the number of eggs laid by C. maculatus females was negatively correlated to seed coat colour. This is in accordance with Baidoo et al. (2015), which showed that C. maculatus uses less Bambara groundnut-coloured seeds as oviposition site. In addition, Chen et al. (2019) demonstrated that the oviposition of C. maculatus females at high densities is affected by the seed coat colour. The fact that the oviposition of C. maculatus females, F1 progeny and seed consumption were positively correlated to soybean seed size is not surprising because it is known that larger seeds provide more surface area and nutrients for developing bruchids (Nwanze and Horber, 1975). Likewise, similarly to Kaur and Ramzan (2001), we reported the negative correlation between seed hardness and the susceptibility index of soybean variety to C. maculatus. Indeed, the seed hardness is known as a factor limiting the penetration of C. maculatus larvae through the soybean seed coat (Kosini and Nukenine, 2019). This could be explained the fact that Vovoh variety with the highest seed hardness was found to be moderately resistant. The significant positive correlation of seed texture, seed length, width, moisture content and 100-seed weight with the susceptibility index of soybean variety indicated that these characters are efficient in seed resistance determination to C. maculatus.
The path coefficient analysis revealed that the seed coat thickness exhibited the strongest positive effect on susceptibility index of soybean to C. maculatus. This indicated that seed coat thickness is a good predictor of soybean resistance to C. maculatus and must be taken in account in soybean resistance breeding to bruchid pests. Indeed, soybean seed coat acts as a physical (Msiska et al., 2018), and biochemical (Sharma and Thakur, 2004c; Silva et al., 2018) barrier against penetration by C. maculatus. The positive direct effects of seed coat texture, 100-grain mass, and seed length were expected because these seed physical characteristics were previously reported to be positively correlated to susceptibility of some pulses to C. maculatus (Dasbak et al., 2009; Tripathi et al., 2020). Therefore, soybean seed resistance to C. maculatus could be improved by selecting for seed coat thickness, seed coat texture, 100-grain mass and seed length. The negative direct effects of seed width, seed colour, and seed hardness on susceptibility index of soybean to C. maculatus suggest that only their positive indirect effects on other traits influence the seed resistance. Therefore, breeders should take into account the direct and indirect effects of the physical characteristics of the soybean seed on their susceptibility to C. maculatus for the breeding of resistant soybean varieties.
Conclusions and Recommendations
There is a large diversity of seed physical characteristics among the eight soybean varieties cultivated in the Republic of Benin. Only Yovoton variety was resistant to C. maculatus and could serve as progenitor in soybean breeding programs. Seed texture, seed length, seed width, seed moisture content and 100-seed weight showed a significant positive correlation with the resistance of the tested soybean varieties to C. maculatus. Positive direct effects of seed coat thickness, seed coat texture, 100-grain mass, and seed length on soybean resistance to C. maculatus suggest that their integration in a breeding program could improve soybean seed resistance against this pest.
Novelty Statement
Our study assessed for the first time, the susceptibility of soybean seeds of diverse varieties cultivated in south and central Benin to C. maculatus and identified the contribution of diffident physical seed characters to their resistance.
Author’s Contribution
Yêyinou Laura Estelle Loko: Designed the experimentation, analysed the data, and wrote the first draft.
Azize Orobiyi, Rolande Okpeicha and Dieudonné Gavoedo: Performed the entomological essays.
Joelle Toffa, Gbèblonoudo Anicet Dassou and Alexandre Dansi: Corrected the first draft of the manuscript.
All authors read and approved the final manuscript.
Conflict of interest
The authors have declared no conflict of interest.
References
Adebayo, A.M., S.A. Babarinde, G.O. Adesina and S.O. Oladoye. 2016. Evaluation of selected cowpea lines and cultivars for inherent resistance against cowpea seed beetle, Callosobruchus maculatus Fabricius (Coleoptera: Chrysomelidae: Bruchinae). J. Nat. Sci. Res., 6(10): 6-10.
Allotey, J. and E.O. Oyewo. 2004. Some aspects of the biology and control of Callosobruchus maculatus (F.) on some stored soyabean Glycine max (L.) Merr varieties. Afr. J. Food Agric. Nutr. Dev., 4(2): 1-11. https://doi.org/10.4314/ajfand.v4i2.19160
Ayenan, M.A.T., P.L. Sèwadé and S.M. Agboton. 2017. Towards effective soybean seed systems in Benin: Current situation and prospects for production and delivery of good quality seed. J. Crop Improv., 31(3): 379-399. https://doi.org/10.1080/15427528.2017.1304479
Baidoo, P., N. Kwansa and C. Annin. 2015. The role of seed coat and its pigmentation on the acceptance of Bambara groundnut (Vigna subterranea L. Verdc.) variety by the cowpea beetle, Callosobruchus maculatus (F.). Adv. Entomol., 3: 125-131. https://doi.org/10.4236/ae.2015.34015
Bandara, K.A.N.P. and R.C. Saxena. 1995. A technique for handling and sexing Callosobruchus maculatus (F.) adults (Coleoptera: Bruchidae). J. Stored Prod. Res., 31(1): 97–100. https://doi.org/10.1016/0022-474X(94)00030-W
Chadare, F.J., Y.E. Madode, N. Fanou-Fogny, J.M. Kindossi, J.O.G. Ayosso, S.H. Honfo, A.P.P. Kayodé, A.R. Linnemann and D.J. Hounhouigan. 2018. Indigenous food ingredients for complementary food formulations to combat infant malnutrition in Benin: A review. J. Sci. Food Agric., 98(2): 439-455. https://doi.org/10.1002/jsfa.8568
Chelladurai, V., K. Karuppiah, D.S. Jayas, P.G. Fields and N.D.G. White. 2014. Detection of Callosobruchus maculatus (F.) infestation in soybean using soft X-ray and NIR hyperspectral imaging techniques. J. Stored Prod. Res., 57: 43–48. https://doi.org/10.1016/j.jspr.2013.12.005
Chen, Q., J. Ma, H. Yang, J. Gong, X. Gong, and Q. Weng. 2019. Seed-coat colour affects oviposition in the bean beetle, Callosobruchus maculatus (Coleoptera: Chrysomelidae). Ann. Zool. Fenn., 56(1-6): 199-205. https://doi.org/10.5735/086.056.0116
Dasbak, M.A., B.C. Echezona and J.E. Asiegbu. 2009. Pigeon pea grain physical characteristics and resistance to attack by the bruchid storage pest. Int. Agrophys., 23: 19-26.
Dewey, D.R. and N.H. Lu. 1959. A correlation and path-coefficient analysis of components of crested wheat grass seed production. Agron. J., 51: 515-518. https://doi.org/10.2134/agronj1959.00021962005100090002x
Dobie, P., 1974. The laboratory assessment of the inherent susceptibility of maize varieties to post-harvest infestations by Sitophilus zeamais Motsch. (Coleoptera: Curculionidae). J. Stored Prod. Res., 10: 183-197. https://doi.org/10.1016/0022-474X(74)90006-X
Dobie, P., 1977. The contributions of tropical stored products centre to the study of insect resistance in stored maize. Trop. Stored Prod. Info., 3: 4-7.
FAO (Food and Agricultural Organization). 2019. Food and Agricultural Organization of the United Nations. http://www.faostat.org
Hounhouigan, M.H., K.M. Kounouewa, C. Ayesiga, and P. Ingenbleek. 2020. Sojagnon: shaping the Beninese soy system to meet the challenges of an emerging market. Int. Food Agribus. Manag. Rev., 23: 143-156. https://doi.org/10.22434/IFAMR2019.0026
Idrissou, Y., W.H.S. Sanni, A.A. Seidou, J.A. Ayena, B.G.C. Assogba and T.I. Alkoiret. 2020. Cotton seed cake replacement by soybean pulp in the diet of West African dwarf lambs in Benin: Zootechnical and economic performances. Rev. Elev. Med. Vet. Pays Trop., 73(2): 107-111. https://doi.org/10.19182/remvt.31875
Kaur, H. and M. Ramzan. 2001. Effect of physical characters of soybean on oviposition and development of Callosobruchus maculatus (Fabricius). J. Res., 38 (3-4): 207-211.
Kosini, D. and E. Nukenine. 2017. Susceptibility of three legume species to Callosobruchus maculatus (Coleoptera: Chrysomelidae) attack and impact of rearing medium on female oviposition host preference. Agric. Res. Tech. Open Access J., 8(1): 555728. https://doi.org/10.19080/ARTOAJ.2017.08.555728
Lephale, S., A. Addo-Bediako and V. Ayodele. 2012. Susceptibility of seven cowpea cultivars (Vigna unguiculatus) to cowpea beetle (Callosobruchus maculates). Agric. Sci. Res. J., 2(2): 65-69.
Loko, Y.L.E., M. Zandjanakou-Tachin, D. Montcho, J. Toffa, A. Agolo, R. Okpeicha, A. Orobiyi, D. Gavoedo and A. Dansi. 2021. On-farm management of soybean (Glycine max [L.] Merr.) varietal diversity in southern and central regions of Benin republic. Agric. Res., https://doi.org/10.1007/s40003-021-00576-6
Msiska, U.M., B. Miesho, M. Hailay, S. Kyamanywa, P.R. Rubaihayo, T. Odong, P. Tukamuhabw, E. Nuwamanya and D.L. Nabirye. 2018. Biochemicals associated with Callosobruchus chinensis resistance in soybean. Afr. J. Rural Dev., 3(3): 859-868.
Nwanze, K.F. and E. Horber. 1975. How seed size affects the occurrence of “active” and “miniature” forms of Callosobruchus maculatus in laboratory populations 1. Environ. Entomol., 4(5): 729–732. https://doi.org/10.1093/ee/4.5.729
Oigiangbe, N.O. and A.O. Onigbinde. 1996. The association between some physico-chemical characteristics and susceptibility of cowpea (Vigna unguiculata (L.) Walp) to Callosobruchus maculatus (F.). J. Stored Prod. Res., 32(1): 7-11. https://doi.org/10.1016/0022-474X(96)00001-X.
Pereira, R.C., M.F. Pelloso, L.V. Correia, T.C. Matera, R.F. dos Santos, A.L. Braccini, G.G. De Bastiani, C. Coppo and B.G. da Silva. 2020. Physiological quality of soybean seeds treated with imidacloprid before and after storage. Plant Soil Environ., 66: 513–518. https://doi.org/10.17221/364/2020-PSE
Sekender, S., S. Sultana, T. Akter and S. Begum. 2020. Susceptibility of different stored pulses infested by pulse beetle, Callosobruchus chinensis (Lin.). Dhaka Univ. J. Biol. Sci., 29(1): 19-25. https://doi.org/10.3329/dujbs.v29i1.46527
Sharma, S. and D.R. Thakur. 2014c. Biochemical basis for bruchid resistance in cowpea, chickpea and soybean genotypes. Am. J. Food Technol., 9(6): 318-324. https://doi.org/10.3923/ajft.2014.318.324
Sharma, S. and D.R. Thakur. 2014a. Comparative developmental compatibility of Callosobruchus maculatus on cowpea, chickpea and soybean genotypes. Asian J. Biol. Sci., 7: 270-276. https://doi.org/10.3923/ajbs.2014.270.276
Sharma, S. and D.R. Thakur. 2014b. Studies on the varietal preference of Callosobruchus maculatus on soybean genotypes. Asian J. Biol. Sci., 7: 233-237. https://doi.org/10.3923/ajbs.2014.233.237
Silva, N.C., J.G. Conceição, K.E. Ventury, L.F. De Sá, E.A. Oliveira, I.S. Santos, V.M. Gomes, M.N. Costa, A.T. Ferreira, J. Perales, J. Xavier-Filho, K.V. Fernandes and A.E. Oliveira. 2018. Soybean seed coat chitinase as a defense protein against the stored product pest Callosobruchus maculatus. Pest Manag. Sci., 74: 1449–1456. https://doi.org/10.1002/ps.4832
Tripathi, K., T.V. Prasad, R. Bhardwaj, S.K. Jha, D.P. Semwal, P.G. Gore and S. Bhalla. 2020. Evaluation of diverse germplasm of cowpea [Vigna unguiculata (L.) Walp.] against bruchid [Callosobruchus maculatus (Fab.)] and correlation with physical and biochemical parameters of seed. Plant Genet. Resour., pp. 1–10. https://doi.org/10.1017/S1479262120000180
Ulemu, M.M., S. Kyamanywa and P. Tukamuhabwa. 2016. Genetic sources of bruchid resistance in soybean: A review. Ruforum Work. Doc. Ser., 14 (3): 151-159.
Wendland, K.J. and O.E. Sills. 2008. Dissemination of food crops with nutritional benefits: Adoption and disadoption of soybeans in Togo and Benin. Nat. Resour. Forum, 32: 39–52. https://doi.org/10.1111/j.1477-8947.2008.00169.x
To share on other social networks, click on any share button. What are these?








