Effects of Carbon Sources and Growth Regulators on the Tissue Culture of Sugarcane
Research Article
Effects of Carbon Sources and Growth Regulators on the Tissue Culture of Sugarcane
Mazhar Ullah and Mohammad Sayyar Khan*
Genomics and Bioinformatics Division, Institute of Biotechnology and Genetic Engineering (IBGE), The University of Agriculture, Peshawar 25000 Khyber Pakhtunkhwa, Pakistan.
Abstract | Tissue culture protocols with enhanced efficacy are prerequisite for the effective micro-propagation of sugarcane. Various factors affecting tissue culture must be evaluated and optimized to produce maximum callus and regenerated plantlets. In this research, different auxins [Dichlorophenoxy Acetic acid (2,4-D), Nephthalene Acetic Acid (NAA)], cytokinin [Benzyl Amino Purine (BAP)] either alone or in combination with each other and carbon sources [sucrose, glucose and fructose] with varying concentrations (2, 4 and 6%) were evaluated for their effect on the tissue culture of sugarcane variety-CP 77/400. For callus induction 2,4-D as an auxin was found to be most effective as compared to NAA. BAP and NAA in combination resulted in high regeneration capacities. Amongst the carbon sources, sucrose was most effective both for callus induction and regeneration. ANOVA revealed significant differences (P ≤ 0.05) amongst the callusing media (CM). Maximum callus induction (48.55%) was achieved on CM-2 augmented with 2.5 mg L-1 2,4-D and 4% sucrose. All the carbon sources at 4% concentration in CM-2 showed maximum callus induction. However, the best results were shown by sucrose with 50.33% callus induction. Significant differences (P ≤ 0.05) were observed amongst the different shooting media (SM). Maximum regeneration (72.06%) was observed on SM-2 supplemented with BAP (2 mg L-1), NAA (0.25 mg L-1) and 6% sucrose. Different carbon sources at 6% concentration in SM-2 showed high regeneration however sucrose resulted in maximum shoot regeneration (77.98%). Roots were established on ½ MS having 1.5 mg L-1 NAA and regenerated plantlets were transferred to silt and clay soil (1:1) and successfully acclimatized to greenhouse condition.
Received | September 15, 2021; Accepted | November 09, 2021; Published | December 24, 2021
*Correspondence | Mohammad Sayyar Khan, Genomics and Bioinformatics Division, Institute of Biotechnology and Genetic Engineering (IBGE), The University of Agriculture, Peshawar 25000 Khyber Pakhtunkhwa, Pakistan; Email: sayyarkhankazi@aup.edu.pk
Citation | Ullah, M. and M.S. Khan. 2022. Effects of carbon sources and growth regulators on the tissue culture of sugarcane. Sarhad Journal of Agriculture, 38(1): 312-321.
DOI | https://dx.doi.org/10.17582/journal.sja/2022/38.1.312.321
Keywords | Saccharum officinarum, Carbon compounds, Micro-propagation, Auxins, Cytokinins
Introduction
Sugarcane, a perennial grass family, reproduces both sexually and asexually. Asexually it reproduces via sets (stem cutting with 2-3 buds) while in vitro propagation involves the use of shoot tip, axillary shoot, bud and immature leaf whorls (Belete, 2017) also reproduces sexually through seed propagation, which provides a base for breeding purposes. Due to the lack of conducive environmental conditions for sugarcane flowering, its improvement via conventional breeding is a tedious task (Ullah et al., 2016). Furthermore, high level of heterozygosity, and non-viable nature of sugarcane seeds due to high level of variability offers challenges for crop improvement in sugarcane (Khan et al., 2008; Belete, 2017). In addition, its narrow genetic background is also an important factor affecting sugarcane improvement (Jackson, 2005). All these factors collectively contribute to the difficulty of improving sugarcane varieties which therefore have compelled farmers to use the out-dated varieties of sugarcane. In order to propagate “disease free healthy sugarcane plants”, several methodologies such as treating with hot water, aerated steam and prior washing with fungicides are in practice (Jalaja et al., 2008). However, these treatments have shown adverse effects on germination ultimately affecting plant growth. Drawbacks of conventional breeding include low propagation rates and lengthy time span of 10-12 years for the development of a potential variety. Vegetative propagation of sugarcane via infected sets results in low productivity and decreased varietal vigour (Lakshmanan et al., 2006; Sani and Mustapha, 2010).
Plant tissue culture has emerged as an alternative approach for improvement and multiplication of disease-free sugarcane plant material (Sengar et al., 2011). Proliferation rate of sugarcane can be increased by 20-30 times via tissue culture techniques (Snyman et al., 2006). It can help in the production of thousands of safe plantlets with the same genetic makeup. In addition, it also helps in germplasm storage for a longer period of time (Khan et al., 2009). Conventional breeding is a time-consuming process while tissue culture in contrast helps in large-scale micro-propagation and reduces the time between selection and commercialization of new sugarcane varieties (Khan et al., 2009). Moreover, conventional techniques produce 4-5 shoots per bud while tissue culture is estimated to have a production rate of about 10,000 identical plants from a single bud in about 3-4 months (Lee, 1987). Through somaclonal variation, in vitro mutants with improved genetic background can be developed, thus providing a possible approach for the development of improved sugarcane varieties (Ali et al., 2012). In plants like sugarcane when clonal propagation is required, an intervening callus phase is skipped and directly the plantlet is regenerated (Ali et al., 2012). Apical meristem used as an explant can help in the production of virus free healthy stock due to the fact that the cells rapidly divide preventing the viral assembly. Somatic hybridization via protoplast fusion can help in overcoming the problem of species barrier in breeding programs which makes tissue culture as an indispensable science in crop improvement (Sharma et al., 2011). Moreover, the success of sugarcane transformation depends on callus induction which is transformed via agrobacterium mediated or biolistic transformation and is later regenerated into a transformed plantlet. Moreover, interspecific crosses can be made via tissue culture technologies (Ullah et al., 2016).
The present study was planned to optimize the various factors such as growth hormones and carbon sources which affect tissue culture of sugarcane. This study will provide efficient protocols for future sugarcane crop improvement via tissue culture.
Materials and Methods
The research was performed at Genomics and Bioinformatics Laboratory, Institute of Biotechnology and Genetic Engineering (IBGE), The University of Agriculture Peshawar, Pakistan.
Plant materials
Selected variety - CP 77/400 was generously provided by the Sugar Crop Research Institute (SCRI), Mardan and was cultivated in the fields at IBGE.
Preparation of callusing media
Callusing media was prepared in dH2O using MS medium (Murashige and Skoog, 1962) supplemented with different combination and concentration of growth regulators and carbon source as described in Table 1. Media pH was adjusted at 5.7 + 0.1. After autoclaving, media was poured in petri plates.
Table 1: Media composition for callus and shoot induction.
|
Callus induction media |
Carbon Sources (%) |
|
|
Control |
0 mg L-1 2.4-D + 0 mg L-1 NAA |
Sucrose (2, 4, 6) Glucose (2, 4, 6) Fructose (2, 4, 6) |
|
CM-1 |
2 mg L-1 2.4-D + 0 mg L-1 NAA |
|
|
CM-2 |
2.5 mg L-1 2.4-D + 0 mg L-1NAA |
|
|
CM-3 |
3 mg L-1 2.4-D + 1 mg L-1 NAA |
|
|
CM-4 |
0 mg L-1 2.4-D + 1 mg L-1 NAA |
|
|
CM-5 |
0 mg L-1 2.4-D + 3 mg L-1 NAA |
|
|
CM-6 |
2 mg L-1 2.4-D + 4 mg L-1 NAA |
|
|
CM-7 |
4 mg L-1 2.4-D + 0 mg L-1 NAA |
|
|
Shoot induction media |
Sucrose (2, 4, 6) Glucose (2, 4, 6) Fructose (2, 4, 6) |
|
|
Control |
0 mg L-1 NAA + 0 mg L-1 BAP |
|
|
SM-1 |
1 mg L-1 NAA+ l mg L-1 BAP |
|
|
SM-2 |
2 mg L-1 NAA+ 0.25 mg L-1 BAP |
|
|
SM-3 |
2 mg L-1 NAA+ 0.5 mg L-1 BAP |
|
|
SM-4 |
3 mg L-1 NAA+ 0.25 mg L-1 BAP |
|
Preparation and inoculation of explant
Immature leaf whorls collected from six-month-old field grown plants were used as explant. The explants were washed with tap water thoroughly for surface cleansing and were rinsed twice with sterilized water inside the Laminar Flow Unit (LFU). Explants were then washed with 70% ethanol for 30-60 sec followed by a wash with sterilized water. Finally, explants were washed with 15% bleach for 10 min followed by 2 washes with sterilized water. The explants were placed on sterilized filter paper for drying. The upper leaf whorls were removed and the innermost 3-4 immature whorls were sliced into 4 mm thickness. The leaf whorls were then inoculated on the CM and were incubated under 2000 lux light at 28 + 02 oC.
Callus induction
Callus initiation from the explants inoculated on plates was observed daily. After initiation, callus was exposed to 16hrs photoperiod. The experiment was repeated thrice and the callusing percentage was calculated via formula reported previously (Ahmad et al., 2016).
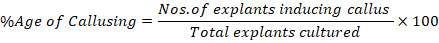
Shooting media preparation
Shooting media was prepared in dH2O using MS medium supplemented with different combination and concentration of growth regulators and carbon sources as described in Table 1. Media pH was adjusted at 5.7 + 0.1. After the autoclave, media was poured in the petri plates.
Plantlet regeneration
The induced calli were shifted to shooting media and were exposed to 16 h photoperiod. The plates were observed daily. The shooting frequencies were calculated via formula reported previously (Ahmad et al., 2016).
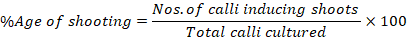
Preparation of rooting media
Root induction media was prepared in dH2O using ½ MS medium supplemented with 1.5 mg L-1 NAA. Media pH was adjusted and kept at 5.7 + 0.1. The sterilized media was poured in the jars.
Acclimatization
For acclimatization, the generated plantlets were sown in silt and clay soil in 2:1 combination in a polythene bag. Stepwise acclimatization of plantlets was performed.
Statistical analysis
Data (n = 3) were statistically analysed by “Statistix 8.1” to determine the significant effects of carbon sources and growth regulators on the tissue culture of sugarcane. Whereas, LSD test was carried out to determine if significant (P < 0.05) differences occurred between individual treatments.
Table 2: Effect of growth regulators and sucrose concentrations on callus induction in CP 77/400. Values are means ± SD.
|
Media |
Sucrose Concentrations |
Means |
||
|
2% |
4% |
6% |
||
|
Control |
0.00L ± 0.00 |
0.00L ± 0.00 |
0.00L ± 0.00 |
0.00g |
|
CM-1 |
40.77c ± 1.33 |
42.00c ± 1.34 |
38.44de ± 1.68 |
40.40b |
|
CM-2 |
48.44ab ± 0.88 |
50.22a ± 1.57 |
46.99b ± 0.58 |
48.55a |
|
CM-3 |
36.55e ± 1.83 |
40.00cd ± 1.20 |
36.77e ± 2.83 |
37.77c |
|
CM-4 |
31.55fg ±1.83 |
33.55f ± 1.83 |
29.66g ± 1.33 |
31.59d |
|
CM-5 |
30.11g ± 1.07 |
33.55f ± 0.50 |
29.44g ± 1.34 |
31.03d |
|
CM-6 |
20.33i ± 1.19 |
23.55h ± 1.01 |
19.22i ± 1.34 |
21.03e |
|
CM-7 |
10.33jk ± 2.3 |
12.33j ± 1.15 |
10.00k ± 1.73 |
10.89f |
|
Means |
27.26b |
29.40a |
26.32c |
|
Different letters in a row or column represent significant differences at P ≤ 0.05; LSD Value for Concentrations= 0.8179; LSD Value for Media= 1.3357; LSD Value for Concentrations X Media= 2.3135.
Results and Discussion
Callus Induction
Growth regulators with sucrose in media
Callus induction frequency on media having different combinations and concentrations of growth regulators along with varying sucrose concentrations is shown in Table 2. ANOVA showed significant differences (P ≤ 0.05) among callus induction in used media. Callus induction was decreased in the following order: CM-2 (48.55%) > CM-1 (40.40%) > CM-7 (10.89%) (Table 4). The data also showed significant differences (P ≤ 0.05) amongst the different sucrose concentrations used. Highest callus induction (29.40%) was observed when sucrose at 4% concentration was used while the lowest callus induction (26.32%) was observed on media having 6% sucrose (Table 2). Interaction between media and carbon sources showed non-significant differences. The maximum callus (50.22%) was produced on a CM-2 media with 4% sucrose followed by callus induction (48.44 %) on the same media having 2% sucrose (Figure 1B-F). Minimum callus induction was observed on CM-7 with 6% sucrose. On control media no callus induction was observed (Figure 1A).
Table 3: Effect of growth regulators and glucose concentrations on callus induction in CP 77/400. Values are means ± SD.
|
Media |
Glucose Concentrations |
Means |
||
|
2% |
4% |
6% |
||
|
Control |
0.000n ± 0.00 |
0.000n ± 0.00 |
0.000n ± 0.00 |
0.000h |
|
CM-1 |
31.61b ± 0.68 |
32.77b ± 0.77 |
28.95c ± 1.64 |
31.11b |
|
CM-2 |
36.33a ± 1.00 |
37.03a ± 1.01 |
35.33a ± 1.15 |
36.26a |
|
CM-3 |
23.11d ± 0.77 |
23.22d ± 1.54 |
21.22e ± 1.07 |
22.52c |
|
CM-4 |
19.11fg ± 0.77 |
19.45f ± 0.50 |
18.55fgh ± 1.71 |
19.03d |
|
CM-5 |
17.33hij ± 1.76 |
17.76ghi ± 1.20 |
15.11jk ± 1.71 |
16.70e |
|
CM-6 |
15.44k ± 0.50 |
16.86ijk ± 0.33 |
13.33l ± 0.88 |
15.15f |
|
CM-7 |
10.33m ± 1.15 |
11.17m ± 0.76 |
10.17m ± 0.76 |
10.56g |
|
Means |
19.16b |
20.22a |
17.83c |
|
Different letters in a row or column represent significant differences at P ≤ 0.05; LSD Value for Concentrations= 0.5883; LSD Value for Media= 0.9607; LSD Value for Concentrations X Media= 1.6640.
Growth regulators with glucose in media
Callus induction on media having different combinations and concentrations of growth regulators along with varying glucose concentrations is shown in Table 3. Significant differences (P ≤ 0.05) were observed among callus induction in used media. Callus induction was decreased in the following order: CM-2 (36.16%) > CM-1 (31.11%) > CM-7 (10.56%) (Table 3). ANOVA showed significant differences (P ≤ 0.05) of different glucose concentration on callus induction. Maximum callus induction (19.72%) was observed when media was augmented with 4% glucose. However, callus induction decreased at 6% glucose concentration. The data on interactions had statistically non-significant differences. CM-2 with 4% glucose showed optimum (36.83%) callus induction followed by the same media supplemented with 2% glucose (Figure 2B-F). Callus induction was minimum (10.17%) on CM-7 having 6% glucose. On control media, no callus induction was observed (Figure 2A).
Table 4: Effect of growth regulators and fructose concentrations on callus inductionin CP77/400.Values are Means ± SD.
|
Media |
Fructose Concentrations |
Means |
||
|
2% |
4% |
6% |
||
|
Control |
0.000k± 0.00 |
0.000k ± 0.00 |
0.000k ± 0.00 |
0.000g |
|
CM-1 |
28.77c ± 0.50 |
30.11c ± 0.69 |
27.06d ± 0.76 |
28.65b |
|
CM-2 |
33.50ab ± 0.69 |
34.33a ± 1.00 |
32.33b ± 0.58 |
33.39a |
|
CM-3 |
19.55e ± 1.68 |
20.77e ± 1.01 |
17.55f ± 0.39 |
19.29c |
|
CM-4 |
16.11fg ± 1.01 |
16.55fg ± 0.84 |
15.77g ± 1.17 |
16.14d |
|
CM-5 |
13.10h ± 1.76 |
14.10gh ± 0.88 |
13.66h ± 1.20 |
13.62e |
|
CM-6 |
13. 10h ± 0.67 |
13.10h ± 0.88 |
13.77h ± 1.34 |
13.32e |
|
CM-7 |
7.33ij ± 0.88 |
8.00i ± 1.00 |
6.33j ± 1.52 |
7.22f |
|
Means |
16.43b |
17.12a |
15.81c |
|
Different letters in a row or column represent significant differences at P ≤ 0.05; LSD Value for Concentrations= 0.5864; LSD Value for Media= 0.9576; LSD Value for Concentrations X Media= 1.6586.
Growth regulators with fructose in media
The data on callus induction on media with different combinations and concentrations of growth regulators along with varying fructose concentrations is shown in Table 4. ANOVA showed significant differences (P ≤ 0.05) among callus induction in used media. Callus induction was decreased in the following order: CM-2 (33.39%) > CM-1 (28.65%) > CM-7 (7.22%) (Table 4). Effect of different fructose concentration on callus induction was statistically significant (P ≤ 0.05). Callus induction was high (17.12%) in the case of media with 4% fructose, whereas high fructose concentration (6%) showed adverse effects on callus induction. CM-2 with 4% fructose showed optimum callus induction (34.33%) followed by the same media (33.50%) supplemented with 2% fructose (Figure 3B-F). Callus induction was minimum (6.33%) on CM-7 having 6% fructose. Control media showed no callus induction (Figure 3A).
Plant regeneration
Table 5: Effect of growth regulators and sucrose concentrations on shoot induction in CP 77/400. Values are means ± SD.
|
Media |
Sucrose Concentrations |
Means |
||
|
2% |
4% |
6% |
||
|
Control |
0.00j ± 0.00 |
0.00j ± 0.00 |
0.00j ± 0.00 |
0.00e |
|
SM-1 |
11.33i ± 1.53 |
26.88g ± 1.18 |
31.33f ± 0.58 |
23.18d |
|
SM-2 |
64.66c ± 1.50 |
73.55b ± 1.50 |
77.98a ± 3.95 |
72.06a |
|
SM-3 |
22.44h ± 1.18 |
33.55F ± 1.07 |
50.44d ± 1.73 |
35.48c |
|
SM-4 |
46.89e ± 1.17 |
52.22d ± 1.34 |
66.89c ± 0.96 |
55.33b |
|
Means |
29.06c |
37.24b |
45.33a |
|
Different letters in a row or column represent significant differences at P ≤ 0.05; LSD Value for Concentrations= 1.1596; LSD Value for Media= 1.4970; LSD Value for Concentrations X Media = 2.5929.
Growth regulators with sucrose in media
The data on shoot regeneration on media having different combinations and concentrations of growth regulators along with varying sucrose concentrations is shown in Table 5. ANOVA showed significant differences (P ≤ 0.05) among shoot induction in used media. Shoot regeneration frequencies decreased in the following order: SM-2 (72.06%) > SM-4 (55.33%) > SM-1 (23.18%). Shoot regeneration was high (45.33%) on media having 6% sucrose concentration, while 2% sucrose concentration was less effective for shoot regeneration. when SM-2 was supplemented with 6% sucrose maximum shoot regeneration (77.98%) was observed followed by shoot regeneration (73.55%) on the same media augmented with 4% sucrose (Figure 4A and B). Minimum shoot regeneration potential (6.33%) was observed on SM-1 media having 2% sucrose. On control media, no shoot induction was observed (Figure 4C).
Growth regulators with glucose in media
Shoot regeneration capacities on media having different combinations and concentrations of growth regulators along with varying glucose concentrations are given in Table 6. Significant differences (P ≤ 0.05) were present among shoot induction in used media. Shoot regeneration frequencies decreased in the following order: SM-2 (31.07%) > SM-4 (23.62%) > SM- (9.77%). Glucose concentration also significantly (P ≤ 0.05) affected shoot regeneration. Shoot regeneration was maximum (20.13%) on media supplemented with 6% glucose concentration while minimum (12.26%) in 2% sucrose concentration. SM-2 media augmented with 6% glucose resulted in maximum shoot regeneration (41.22%). In contrast, SM-1 media having 2% glucose resulted in minimum shoot regeneration (6.55%) (Figure 5A and B). On control media no shoot induction was observed (Figure 5C).
Growth regulators with fructose in media
The data on shoot regeneration on media having different combinations and concentrations of growth regulators along with varying fructose concentrations is shown in Table 7. ANOVA showed significant differences (P ≤ 0.05) among shoot induction in used media. Shoot regeneration frequencies decreased in the following order: SM-2 (21.69%) > SM-4 (17.33%) > SM-1 (9.03%). Significant differences (P ≤ 0.05) were observed among shoot regeneration in media with different fructose concentration. Maximum Shoot regeneration (15.10%) was observed on media having 6% fructose concentration. However, 2% sucrose concentration resulted in minimum shoot regeneration (8.66%). Supplementing SM-2 with 6% fructose resulted in maximum shoot regeneration (27.77%) followed by 22.22% on the same media with 4% fructose (Figure 6A and B). Shoot regeneration was minimum (6.99%) on SM-1 media supplemented with 2%fructose. On control media, no shoot induction was observed (Figure 6C).
Table 6: Effect of growth regulators and glucose concentrations on shoot induction in CP 77/400. Values are means ± SD.
|
Media |
Glucose Concentrations |
Means |
||
|
2% |
4% |
6% |
||
|
Control |
0.00i ± 0.00 |
0.00i ± 0.00 |
0.00i ± 0.00 |
0.00e |
|
SM-1 |
6.55h ± 2.22 |
10.21g ± 1.01 |
12.55fg ± 0.39 |
9.77d |
|
SM-2 |
23.55c ± 1.83 |
28.44b ± 1.00 |
41.22a ± 1.65 |
31.07a |
|
SM-3 |
12.77f ± 2.38 |
15.66e ± 1.84 |
18.44d ± 1.34 |
15.62c |
|
SM-4 |
18.44d ± 1.68 |
23.99c ± 1.15 |
28.44b ± 2.55 |
23.62b |
|
Means |
12.26 C |
15.66B |
20.13A |
|
Different letters in a row or column represent significant differences at P ≤ 0.05; LSD Value for Concentrations= 1.0880; LSD Value for Media= 1.4046; LSD Value for Concentrations X Media = 2.4328.
Table 7: Effect of growth regulators and fructose concentrations on shoot induction in CP 77/400. Values are means ± SD.
|
Media |
Fructose Concentrations |
Means |
||
|
2% |
4% |
6% |
||
|
Control |
0.00j ± 0.00 |
0.00j ± 0.00 |
0.00j ± 0.00 |
0.000e |
|
SM-1 |
6.99i ± 1.00 |
9.11h ± 1.01 |
10.99gh ± 0.58 |
9.0300d |
|
SM-2 |
15.07de ± 0.22 |
22.22b ± 1.08 |
27.77a ± 1.69 |
21.688a |
|
SM-3 |
9.14h ± 0.57 |
13.11ef ± 2.70 |
15.77d ± 0.83 |
12.673c |
|
SM-4 |
12.11fg ± 0.50 |
18.88c ± 2.41 |
20.99b ± 0.58 |
17.328b |
|
Means |
8.66c |
12.66b |
15.10a |
|
Different letters in a row or column represent significant differences at P ≤ 0.05; LSD Value for Concentrations= 0.8814; LSD Value for Media= 1.1379; LSD Value for Concentrations X Media = 1.9710.
Root induction and acclimatization
The efficient root induction was found after 7 weeks in media (½ MS + 1.5 mg L-1 NAA) (Figure 7). Decreased concentration of sucrose (2%) resulted in vigorous rooting. Furthermore, keeping the lower portion in continuous darkness also had a positive impact on the root induction. Successful acclimatization of the in vitro CP 77/400 plantlets to greenhouse condition was observed on mixture of silt and clay soil in 1:1 ratio. It is suggested that plant incubation for 7 days in growth room condition together with the application of ¼ MS instead of water improves the survival under greenhouse conditions.
Lack of conducive environmental conditions for flowering and heterogeneous nature of sugarcane is major constraints in sugarcane breeding programs (Nand and Singh, 1994). Propagation of perennial crops like sugarcane via stem cuttings is not a rapid enough approach to fulfil the demand of increasing population. Plant tissue culture is an alternative approach to rapidly propagate disease free sugarcane plant material (Gopitha et al., 2010). Therefore, reproducible tissue culture protocols are the prerequisite for sugarcane micro-propagation and improvement by genetic engineering.
Efficient tissue culture depends on the type and concentration of growth regulators, type of explant and carbon source. In present research, immature leaf whorls were observed to be an indispensable explant for callus induction. The high efficacy of Immature leaf whorls as explant have also been extensively reported in literature (Khan et al., 2009; Ali et al., 2012; Ullah et al., 2016; Khan et al., 2021). Immature leaf whorls have actively dividing cells with remarkable ability of dedifferentiation and re-differentiation. Jehangir et al. (2010) also used inner immature leaf whorls for sugarcane tissue culture which supports the findings of the present research. Frequent phenolic compound accumulation occurs while using more mature explants which hampers the callus formation (Siddiqui et al., 1994). The injured cells of the explant have high nutrients uptake capacity which results in rapid cell proliferation subsequently promoting callus formation. Therefore, immature leaf whorls for its healthy juvenile nature are recommended for the production of healthy embryogenic sugarcane calli. Alternatively, Bisht et al. (2011) and Wang and Juang (1971) have used eye buds for efficient callus induction which contradicts the present research.
Callus induction capability is highly affected by the type and optimum concentration of the growth regulators used. Auxins like NAA and 2,4-D involved in rapid elongation of cells (Kaur and Gosal, 2009), are frequently used to promote callus induction in monocots (Torres, 1989). The present research suggests 2,4-D as the best auxin for the establishment of callus cultures in sugarcane as compared to NAA. Previously, 2,4-D was reported as an indispensable auxin for callus induction in sugarcane; however, its recommended concentration i.e., 3 mg L-1 contradicts the findings of the present research (Jehangir et al., 2010; Yadav and Ahmad, 2012). Stress induced by 2,4-D has a positive impact on embryogenesis and embryogenic competence at early stages in monocots. However, beyond a limit of 2.5 mg L-1, accumulation of phenolic compounds in the media was observed which may be attributed to the herbicidal effect and growth retardant capacity of 2,4-D. Consequently, browning and ultimate death of the explants occurs due the blockage of nutrients uptake. Ullah et al. (2016) observed maximum callus induction using 5 mg L-1 2,4-D and 10% coconut water which contradicts the finding of the present research. However, the use of coconut water (rich in cytokinins and vitamins) probably neutralizes the negative impact of high 2,4-D concentration. NAA is regarded as a weak auxin as compared to 2,4-D and therefore, is mostly used for regeneration. In the present research, an increase in NAA concentration positively impacted callus induction; however, the overall production was less as compared to 2,4-D. The underwhelming effect of NAA on callus induction in sugarcane was also reported previously (Gopitha et al., 2010; Karim et al., 2002). In monocots, 2,4-D is often replaced with NAA, which at low concentration promotes regeneration (Torres, 1989).
The type and concentration of carbon source also affects the callus induction capabilities. Sucrose, glucose and fructose have been widely used in tissue culture media. Sugars, mostly used as carbon sources in media, act as osmoticum and thus induce osmotic stress in the medium (Ali and Iqbal, 2012). Sucrose as a carbon source in 4% concentration performed better in terms of callus induction as compared to glucose and fructose. These results are congruent to the results achieved previously by Ali and Iqbal (2012); however, they used 3% sucrose. Jawan et al. (2014) also proposed optimum callus induction at lower sucrose concentration. Osmotic stress plays an important role in callus development. For optimum callus growth, an optimum level of carbon sources is a prerequisite to induce osmotic stresses. High osmotic potential in the media induced by sucrose helps in the rapid uptake of nutrients, vital for cell growth (Jawan et al., 2014). On the contrary, Wani et al., (2014) obtained optimum callus in Costus pictus using glucose and suggested it as an effective carbon source. Fructose as a carbon source has been reported to be less efficient in terms of callus production (Wani et al., 2014).
In vitro regeneration process involves the perception of growth regulators by cells to develop their organogenic potential. This phenomenon requires an optimized level of cytokinins and auxins. Interaction between auxins and cytokinins are involved in developmental processes e.g the formation of meristem (shoot and root meristems) which leads to the establishment of the whole plant body (Su et al., 2011). The present research suggests 2.0 mg L-1 BAP + 0.25 mg L-1 NAA as an optimum cytokinin to auxin concentration for best shoot induction. The results of Ali et al. (2012) are in complete agreement with the present research; however, they used different concentrations of growth regulators. In monocots like sugarcane, regeneration is promoted by decreasing 2,4-D concentration or replacing it with a weak auxin such as NAA (Torres, 1989). Therefore, NAA is an important auxin for regeneration in monocots. Findings of the present research were supported by a previous study (Behera and Sahoo, 2009). Naz et al. (2008) and Jehangir et al. (2010) used 2,4-D or IAA instead of NAA as an auxin in combination with BAP for efficient shoot regeneration in sugarcane which contradicts the findings of the present research.
Effective carbon source is also an important parameter for in vitro regeneration of plantlets. Amongst the analysed carbon sources, sucrose efficiently promoted regeneration when used at 6% in the media. Gopitha et al. (2010) also observed maximum shoot regeneration in sugarcane at high sucrose concentration which supports the present results. However, Bisht et al. (2011) obtained maximum regeneration at 3% sucrose concentration contradicting the finding of the present research. Amutha et al. (2003) also suggested glucose, and fructose as less efficient for shoot regeneration. The efficiency of a sucrose as carbon source may be attributed to its high uptake capacity by cell across plasma membrane. Furthermore, sucrose being a major product of photosynthesis plays an important role in plant growth and development. It may also regulate gene expression either directly or indirectly (Winter and Huber, 2000). In addition, metabolism of specific compounds is also linked with the osmotic stresses. Many researchers have suggested glucose as an effective carbon source for regeneration (Maretzaki et al., 1972; Ahloowalia and Maretzaki, 1983; Amiri and Kazemitabar, 2011). High regeneration efficiency of glucose as carbon source might be due to the efficient metabolism of glucose in the presence of various hormonal combinations contradicting the present results (Wani et al., 2014). Fructose causes hyperhydricity in the media which affects different cellular phenomenon such as sugar metabolism, lowers cellulose content, less ethylene production, which can have adverse effects on regeneration (Bouza et al., 1992).
Roots were easily induced on ½ MS augmented with 1.5 mg L-1 NAA. Root induction on NAA containing media was also revealed by Bisht et al. (2011). The positive impact of low NAA concentration on rooting was reported by Sughra et al. (2014). These previous studies support the findings of the present research. On the contrary, Gopitha et al. (2010) proposed higher concentrations of NAA as vital for maximum root induction. Yadav and Ahmad (2013) observed vigorous rooting on media supplemented with BAP, Kinetin and NAA. After rooting, the plants were successfully acclimatized to a mixture of silt and clay soil (1:1). Roy and Kabir (2007) used soil and compost (2:1) for acclimatization. Keeping the plants in the growth room and application of ¼ MS instead of water during the first week prevented the plants from rapid yellowing and consequently the plants were healthy enough to survive the initial shock. This may be the best alternative approach for successful acclimatization of in vitro plants.
Conclusions and Recommendations
Plant growth regulators and carbon sources at their optimum concentrations highly affect the tissue culture of sugarcane. Sucrose was a favourable carbon source for the tissue culture of sugarcane. 2,4-D at optimum concentration was found as the best growth regulator. For efficient regeneration, optimum level of BAP and NAA is important. However, in sugarcane lower NAA concentration in combination with BAP highly induced shoot regeneration. Whereas, NAA at low concentration augmented in ½ MS promoted rapid root induction. Efficient acclimatization of regenerated plantlets was in silt and clay mixture (1:1) soil.
Acknowledgments
We highly acknowledge Pakistan Science Foundation for funding the present research. [Grant No./Project No. PSF/NSLP/KP-AU (281)].
Novelty Statement
Different growth regulators and carbon sources were evaluated for their impact on the tissue culture of sugarcane.
Author’s Contribution
Mazhar Ullah: Conducted experiments, data analysis and manuscript preparation.
Mohammad Sayyar Khan: Conceived and designed the experiment and manuscript write-up.
Conflict of interest
The authors declare no conflict of interest.
Refernces
Ahmad, K.A. Jalal, H. Rajab, M. Ullah and M.S. Khan. 2016. Screening of promising Brassica Napus varieties for callus induction and regeneration under in vitro conditions. Int. J. Biol. Biotech., 13 (2): 203-21.
Ali, S. and J. Iqbal. 2012. Influence of physical factors on callogenesis in Sugarcane (Saccharum officinarum L.). Sci. Int., 24(2): 167-170.
Ali, S., M.S. Khan and J. Iqbal 2012. In vitro direct plant regeneration from cultured young leaf segments of sugarcane (Saccharum officinarum L.). J. Anim. Plant Sci., 22(4): 1107-1112.
Amiri, S. and S.K. Kazemitabar. 2011. Enhancement of callus induction and regeneration efficiency from embryo cultures of Datura stramonium by adjusting carbon sources and concentrations. Afr. J. Biotechnol., 10(50): 10101-10107. https://doi.org/10.5897/AJB10.816
Amutha, S.A. Ganapathi and M. Muruganantham. 2003. In vitro organogenesis and plant formation in Vigna radiata (L.) Wilczek. Plant. Cell. Tiss. Org., 72: 203-207. https://doi.org/10.1023/A:1022266110750
Ahloowalia, B.S. and A. Maretzaki. 1983. Plant regeneration via somatic embryogenesis in sugarcane. Plant Cell Rep., 2: 21-25.
Bisht, S.S., A.K. Routray and R. Mishra. 2011. Rapid in vitro propagation technique for sugarcane variety 018. Int. J. Pharma Biol. Sc., 2(4): 1-8.
Behera, K.K. and S. Sahoo. 2009. Rapid In vitro micro propagation of sugarcane (Saccharum officinarum L. cv- Nayana) through callus culture. Nat. Sci., 7(4):1-10.
Belete, G. 2017. Review on in vitro propagation of sugarcane to advance the value of tissue culture. Agri. Res. Tech., 5(4): 555670. https://doi.org/10.19080/ARTOAJ.2017.05.555670
Bouza, L., M. Jaques, Y. Maziere and Y. Arnaud. 1992. In vitro propagation of Prunus tenella Batsch. cv. ‘Firehill’: Control of vitrification increase of the multiplication rate and growth by chilling. Scientia Hort., 52: 143-155. https://doi.org/10.1016/0304-4238(92)90016-6
Gopitha, K., A.L. Bhavani and J. Senthilmanickam. 2010. Effect of the different auxins and cytokinins in callus induction, shoot and root regeneration in sugarcane. Int. J. Pharma. Biol. Sc., 1(3): 1-7.
Jawan, J., G.J. Joeplik, H. Indan, M.M.B. George and J.A Gansau. 2014. Effect of basal media and carbon sources on callus culture maintenance of Vanda dearie. Borneo Sci., 34.
Jahangir, G.Z., I.A. Nasir, R.A. Sial, M.A. Javid and T. Husnain. 2010. Various hormonal supplementations activate sugarcane regeneration in-vitro. J. Agric. Sci., 2(4): 231-237. https://doi.org/10.5539/jas.v2n4p231
Jalaja, N., D. Neelamathi and T. Sreenivasan. 2008. Micro propagation for quality seed production in sugarcane in Asia and the Pacific. Food and Agric Organization of the UN, sugarcane pub, USA, pp. 13-60.
Jackson, P.A. 2005. Breeding for improved sugar content in sugarcane. Field Crops Res., 92: 277-290. https://doi.org/10.1016/j.fcr.2005.01.024
Khan, M.S., W. Ahmad, M. Ullah and A. Jan. 2021. Transformation of the sugarcane variety (CP 77/400) with the promoter of C3H52 gene. J. Anim. Plant Sci., 31(1): 156-165.
Khan, I., M. Dahot, N. Seema, A. Yasmeen and M. Naqvi. 2009. Direct regeneration of sugarcane plantlets: a tool to unravel genetic heterogeneity. Pak. J. Bot., 41(2): 797-814.
Kaur, A. and S.S. Gosal. 2009. Desiccation of callus enhances somatic embryogenesis and shoot regeneration in sugarcane. Indian J. Biotechnol., 8: 332-334.
Khan, S.A., H. Rashid, M.F. Chaudhary, Z. Chaudhry, Z. Fatima, S.U.D Siddiqui and M. Zia. 2009. Effect of cytokinins on shoot multiplication in three elite sugarcane varieties. Pak. J. Bot., 41(4): 1651-1658.
Khan, S.A., H. Rashid, F.M. Chaudhary, Z. Chaudhary and A. Afroz. 2008. Rapid micro propagation of three elite Sugarcane (Saccharum officinarum L.) varieties by shoot tip culture. Afr. J. Biotechnol., 7: 2174-2180.
Karim, M.Z., M.N. Amin, M.A. Hossain, S. Aslam, F. Hossain and R. Alam. 2002. Micropropagation of two Sacharrum officinarum varieties from callus culture. J. Biol. Sci., 2(10): 682-685. https://doi.org/10.3923/jbs.2002.682.685
Lakshmanan, P., R. Geijskes, L. Wang, G. Smith and A. Elliott. 2006. Developmental and hormonal regulation of direct shoot organogenesis and somatic embryogenesis in sugarcane (Saccharum spp. interspecific hybrids) leaf culture. Plant Cell Rep., 25(10): 1007-1015. https://doi.org/10.1007/s00299-006-0154-1
Lee, T.S.G. 1987. Micropropagation of sugarcane (Saccharum spp.). Plant Cell Tissue Org. Cult., 10: 47-55. https://doi.org/10.1007/BF00037496
Murashige, T. and F. Skoog. 1962. A revised media for rapid growth and bioassay with tobacco tissue culture. Physiol. Plant., 15: 473-497. https://doi.org/10.1111/j.1399-3054.1962.tb08052.x
Maretzki, A., M. Thom and L.G. Nickell. 1972. Influence of osmotic potentials on the growth and chemical composition of sugar cane cell culture. Hawaii Plant Res., 48: 183-199.
Naz, S., A. Ali and A. Siddique. 2008. Somatic embryogenesis and plantlet formation in different varieties of sugarcane (Sacchrum officinarumL.) HSF-243 and HSF-245. Sarhad J. Agric., 24(4): 593-598.
Nand, L. and H.N. Singh. 1994. Rapid clonal multiplication of sugarcane through tissue culture. Plant Tissue cult., 4: 1-7.
Roy, P.K. and M.H. Kabir. 2007. In vitro mass propagation of sugarcane (sacharrum officinarum L.) var. isd 32 shoot tips and folded leaf cultures. Biotechnol., 6(4): 588-592. https://doi.org/10.3923/biotech.2007.588.592
Sani, L.A. and Y. Mustapha. 2010. Effect of genotype and 2,4-D concentration on callogenesis in sugarcane (Saccharum spp. hybrids). Bayero J. Pure Appl. Sci., 3(1): 238-240. https://doi.org/10.4314/bajopas.v3i1.58800
Sharma, S., D. Sarkar, S, K. Pandey, P. Chandel and J.K. Tiwari. 2011. Stoloniferous shoot protoplast, an efficient cell system in potato for somatic cell genetic manipulations. Sci. Hortic., 128: 84-91. https://doi.org/10.1016/j.scienta.2011.01.007
Sughra, M.G. S.A. Altaf, R.M. Rafique, M.S. Muhammad, S.N. R. Balouch and D.M. Umar. 2014. In vitro regenerability of different sugarcane (saccharum officinarum L.) Varieties through shoot tip Culture. Pak. J. Biotechnol., 11(1): 13-23.
Su, Y.H., Y.B. Liu and X.S. Zhang. 2011. Auxin–Cytokinin interaction regulates meristem development. Mol. Plant., 4(4): 616-625. https://doi.org/10.1093/mp/ssr007
Snyman, S.J., T. Van-Antwerpen, V. Ramdeen, G.M. Meyer, J.M. Richards and R.S. Rutheford. 2006. Micropropagation by direct somatic embryogenesis: is disease elimination also a possibility? Proc. Int. Soc. Sugarcane Technol., 26: 943-946.
Siddiqui, S.H., A Khatri, M.A. Javed, N.A. Khan and G.S. Nazamani. 1994. In vtiro culture, a source of variability and an aid to sugarcane improvement. Pak. J. Agric. Res., 15: 127-133.
Sengar K., R. Sengar and S.K. Garg. 2011. The effect of in vitro environmental conditions on some sugarcane varieties for micro propagation. Afr. J. Biotechnol., 10(75): 17122-17126. https://doi.org/10.5897/AJB11.2195
Torres, K.C. 1989. Tissue Culture Techniques for Horticultural Crops. 1st edition, Van Nostrand Reinhold, New York. https://doi.org/10.1007/978-1-4615-9756-8_4
Ullah, M., H. Khan, M.S. Khan, A. Jan, K. Ahmad and A.W. Khan. 2016. In vitro Plant Regeneration of Sugarcane (Saccharum officinarum L.); The Influence of Variety, Explant, Explant position and Growth Regulators. ARPN J. Agric. Biol. Sci., 11 (7): 267-273.
Wani, S.J., I.A. Kagdi, P.S. Tamboli, V.S. Nirmalkar, S.N Patil and A.K. Sidhu. 2014. Optimization of MS media for callus and suspension culture of Costus pictus. J. Sci. Eng., 5(2): 390-394.
Winter, H. and S.C. Huber. 2000. Regulation of sucrose metabolism in higher plants: Localization and regulation of activity of key enzymes. Crit. Rev. Plant Sci., 19:31-67. https://doi.org/10.1080/07352680091139178
Wang, P.J. and S.M. Juang. 1971. Studies on tissue culture of sugarcane (Saccharum officinarum L.) CV. N. CO-310 bot. bull. Academia Sinica XII: 41-44.
Yadav, S. and A. Ahmad. 2012. Standardization of callus culture techniques for efficient sugarcane micropropagation. Cibtech J. Bio-Protocol., 2 (2): 29-32.
To share on other social networks, click on any share button. What are these?








