Effectiveness of Butylated Hydroxytoluene in Maintaining the Quality of Gaga Chicken Sperm in Liquid Storage for 72 Hours
Effectiveness of Butylated Hydroxytoluene in Maintaining the Quality of Gaga Chicken Sperm in Liquid Storage for 72 Hours
Khaeruddin1, Gatot Ciptadi2, Muhammad Yusuf3, Iswati4, Setya Budhi Udrayana4, Sri Wahjuningsih2*
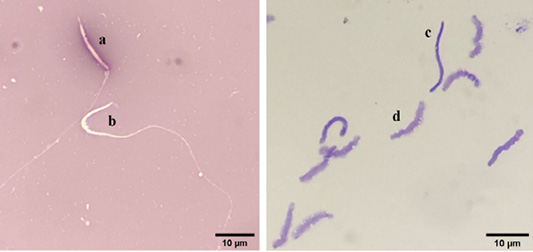
Evaluation of chicken sperm viability using eosin-nigrosin staining. a: dead sperm, b: alive sperm) (left), and evaluation of DNA damage in chicken sperm using toluidine blue staining (c: damaged DNA, d: intact DNA) (right).
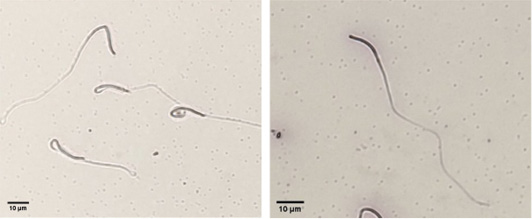
Evaluation of plasma membrane integrity of chicken sperm using HOST (left: intact plasma membrane, right: damaged plasma membrane).
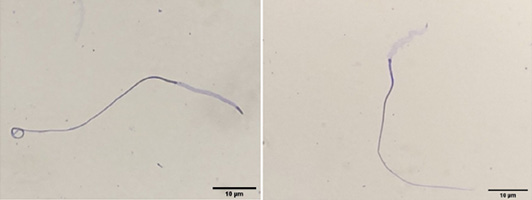
Evaluation of acrosome integrity of chicken sperm using coomassie brilliant blue staining (left: intact acrosome, right: damaged acrosome).
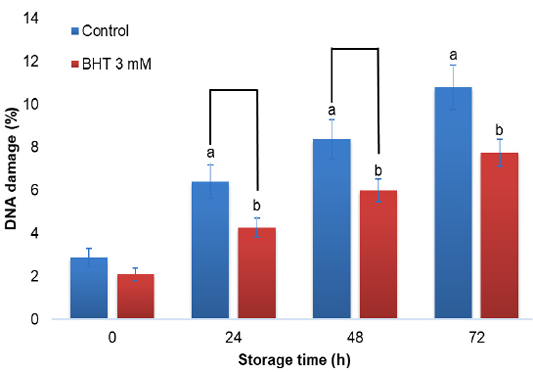
DNA damage of Gaga chicken sperm with the addition of BHT 3 mM in diluent during cold storage (%) (n=10).
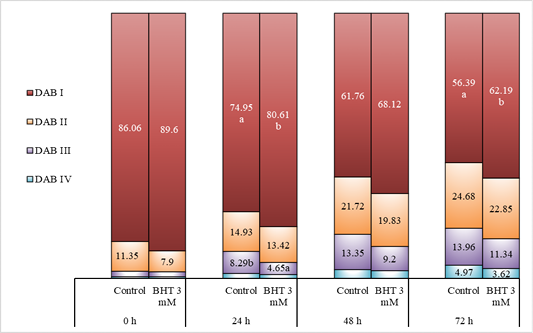
Mitochondrial activity of Gaga chicken sperm with the addition of BHT 3 mM in diluent during cold storage (%) (n=10).
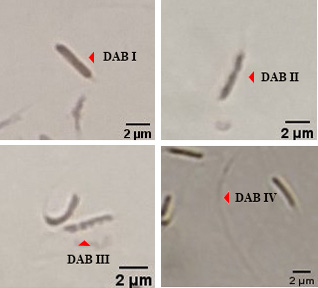
Evaluation of mitochondrial activity of chicken sperm using DAB assay.
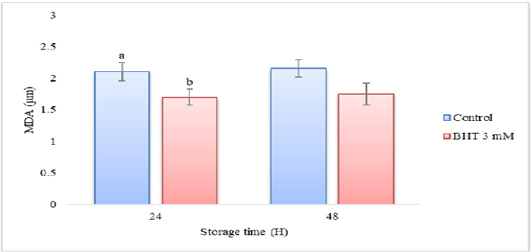
MDA levels of Gaga chicken semn with the addition of BHT 3 mM in diluent during cold storage (n=10).
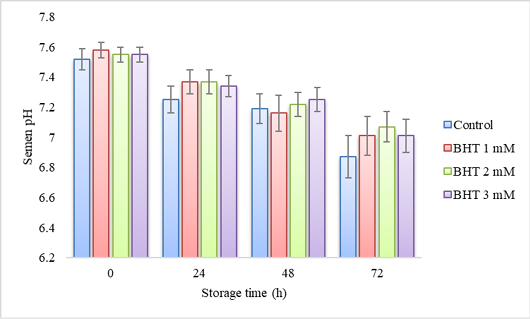
pH of Gaga chicken semen with the addition of in diluent during cold storage (n=10).