Effect of in Ova Injection of Lysophospholipid in Hatching Traits, Chick’s Quality, and Chicks Physical Traits of Broiler (Ross 308)
Research Article
Effect of in Ova Injection of Lysophospholipid in Hatching Traits, Chick’s Quality, and Chicks Physical Traits of Broiler (Ross 308)
Majeed Ajafar1, Hashim Hadi Al-Jebory1*, Mohammed Khalil Ibrahim Al-Saeedi2
1Department of Animal Production, College of Agriculture, Al-Qasim Green University, Babylon Province, Iraq; 2Department of Environment, College of Environmental Science, Al-Qasim Green University, Babylon Province, Iraq.
Abstract | This study was conducted in two experiments at the Al-Anwar Poultry Company hatchery from November 11, 2023, to December 2, 2023, to demonstrate the effect of Lysophospholipid (LPL) injection on hatching eggs on the quality of hatched chicks. A total of nine hundred eggs were used in this study. All the eggs were set in an automatic incubator at the same time. On the 12th day of embryonic age, half of the eggs (450 eggs) were divided into 6 groups (75 eggs per group). The first egg group was kept in the incubator without treatment and was a negative control (NC). The second egg group was injected with 0.3 ml of NACL solution and served as a positive control (PC).In contrast, the 3rd, 4th, 5th, and 6th egg groups were injected with phospholipids at a concentration of 1, 2, 3, and 4% (0.3 ml/egg), respectively. On the 18th day of embryonic age, the second half of the egg was divided and treated similarly to the first half of the egg. The results showed that injecting at 12 days of embryo age resulted in a significant superiority (P < 0.05) for groups G1, G4, and G5 regarding the hatching rate. The embryonic mortality rate was significantly reduced in groups G1 and G5. The percentage of pipped eggs and dead pipped chicks was significantly increased in the G2 and G3 groups, respectively. Chick activity increased significantly in all treatments compared to treatment T2. When injected at 18 days of age of the embryos, there was a significant (P < 0.01) improvement for G6 groups in the hatching rate and embryonic mortality rate. Additionally, all LPL injection groups showed significant improvements in the weight of hatched chicks, activity, and leg length compared with the control groups. In the chicks’ length, chick activity increased significantly (P < 0.01) in the G4, G5, and G6 groups. Wing length also increased significantly in the G4 and G5 groups, and tonic immobility improved significantly (P < 0.05) in the G3 and G6 groups.
Keywords | Lysophospholipid, Hatching traits, Chick’s quality, Chick’s physical traits, Broiler, Energy
Received | March 19, 2024; Accepted | April 12, 2024; Published | May 18, 2024
*Correspondence | Hashim Hadi Al-Jebory, Department of Animal Production, College of Agriculture, Al-Qasim Green University, Babylon Province, Iraq; Email: hashimhadi@agre.uoqasim.edu.iq
Citation | Ajafar M, Al-Jebory HH, Al-Saeedi MKI (2024). Effect of in Ova injection of lysophospholipid in hatching traits, chick’s quality, and chicks physical traits of broiler (Ross 308). Adv. Anim. Vet. Sci., 12(7):1206-1213.
DOI | https://dx.doi.org/10.17582/journal.aavs/2024/12.7.1206.1213
ISSN (Online) | 2307-8316
Copyright: 2024 by the authors. Licensee ResearchersLinks Ltd, England, UK.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Introduction
Optimizing dietary fat consumption in broiler farming is essential for achieving cost-effective output. Bile salts act as an emulsifier when dietary fat enters the digestive system, facilitating fat breakdown and improving the lipase enzyme’s interaction with fat (Khonyoung et al., 2015). However, due to the insufficient production of bile salts in young chicks, the utilization of rapidly degrading phospholipids serves as a source of energy and enhances the absorption of yolk fats (Noy and Sklan, 1998; Al-Marzooqi and Leeson, 1999). Phospholipase A2 cleaves a hydrophobic fatty acid from phospholipids to produce rapidly hydrolyzable phospholipids (LPL), also known as hydrolyzed lecithin, enzymatically modified lecithin, and lysolecithin (Joshi et al., 2006). When compared to other forms of lipids, LPL exhibits superior hydrophilic characteristics and reaches an equilibrium value upon hydrolysis, unlike typical emulsifiers such as lecithin and bile salts (Joshi et al., 2006; Hasenhuettl and Hartel, 2008). Consequently, it increases lipid bioavailability in birds and decreases the size of lipid droplets, stabilizing micelles in the small intestine and improving the efficiency of lipid digestion (Schwarzer and Adams, 1996).
Moreover, LPL changes cell membrane permeability and expands membranous pores in intestinal cell membranes, causing a greater flux of small and large molecules of digested nutrients across the cell membrane (Lundbaek et al., 2010; Arouri and Mouritsen, 2013) and phospholipids also contribute it is rapidly degraded as part of the energy requirements of intestinal cells and reduces their damage, therefore expanding the crypts’ depth as well as villi length and width, which improves intestinal health (Skoura and Hla, 2009; Boontiam et al., 2017). Solbi et al. (2021) and Lu et al. (2022) noted that adding rapidly decomposing phosphorous fats to the broiler diet improved the productive performance of birds. The current study sought to illustrate the impact of early feeding at varying concentrations of LPL on the phenotypic and physical traits of the hatched chicks, as well as the hatching characteristics.
Materials and Methods
This study was carried out at the hatchery of Al-Anwar Poultry Company, Al-Muradiyah area/Babil Governorate. from November 11, 2023, to December 2, 2023.
Source of experimental eggs
Acquired broiler hatching eggs (ROSS 308) from the hatchery of Al-Anwar Poultry firm and imported from Bábolna Brojler, a Hungarian firm. Clean eggs, free of dirt and weighing 65 ± 1 g, were chosen; filthy eggs or defects were not included.
Preparation of a solution of LPL
The rapidly decomposing phospholipids were obtained from the Iranian company behin_daroo, after which the concentrations were dissolved in a NaCl solution for the experiment, according to the abovementioned two parameters.
Experimental treatments
Nine hundred eggs were divided into two groups, each containing 450 eggs, injected at 12 and 18 days of the embryos’ lifespan. The hatching egg treatments were divided into six treatments according to the following Figure 1.
Studied characteristics
Hatching rate
The hatching percentage and percentage of embryonic mortality were calculated according to (Zaki and Al-Jebory, 2021).
Chicks quality
Percentage of pipped eggs
After the end of the hatching process, all unhatched eggs were broken individually. The eggs containing complete embryos that could not peck the eggshell were recorded, and the percentage was calculated according to (Tona et al., 2003; Al-Jaryan et al., 2023).
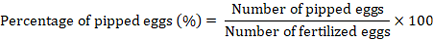
Percentage of live pipped chicks
It represents the number of chicks that could peck the eggshell incompletely and are still alive inside. Its percentage was calculated using the following equation (Tona et al., 2003; Al-Jaryan et al., 2023).
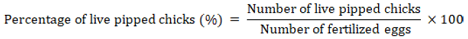
Percentage of dead pipped chicks
It represents the number of chicks that could peck the eggshell incompletely and perish inside it, and its percentage was calculated according to the following equation (Tona et al., 2003; Al-Jaryan et al., 2023).
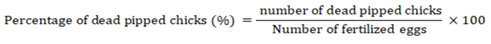
Percentage of abnormal chicks
The number of deformed and weak chicks characterized by congenital disabilities, such as deformities of the beak, feet, legs, wings, etc., was calculated according to the following equation (Tona et al., 2003; Al-Jaryan et al., 2023).
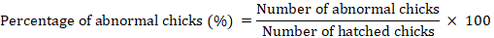
Physical characteristics of hatched chicks
- Weight of the chick upon hatching: The hatched chicks for each replicate were weighed by the hatchery treatment using an empty box and individually placed in an empty box after subtracting its weight from the weight of the box.
- Chick length was measured according to (Willemsen et al., 2008).
- Wing length was measured by (Osaiyuwu et al., 2009) method.
- Leg length was measured according to (Willemsen et al., 2008).
- Tonic immobility was calculated according to (Geidam et al., 2007).
- The appearance of hatched chicks was calculated according to Tona et al. (2003).
Statistical analysis
The impact of the investigated groups on the different attributes was examined using a completely randomized design (CRD) in the data analysis. The Duncan (1955) multinomial test assessed any significant differences between the means. It has carried out in statistical analysis by the following mathematical model using the SAS (2012) program:
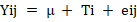
Results and Discussion
First trail
Hatching traits
The effect of the study on hatching traits (ova injection at 12 days) is presented in Tables 1 and 2. The hatching rate showed a significant decrease (P < 0.05) for the G2 and G3 groups compared to the G1, G4, and G5 groups. Simultaneously, the embryonic mortality rate significantly increased in G2 compared with G1, G4 and G5 groups, with no differences among the other groups. Also, the groups had no significant difference in the weight of hatched chicks.
Table 1: Effect of in ova injection at 12 days of embryonic age with LPL in some hatching traits.
|
Groups |
Mean± standard error |
||
|
Hatching rate % |
Embryonic mortality rate % |
Hatching chick's weight (gm) |
|
|
G1 |
80.00±0.01 a |
20.00±0.01 b |
41.00±1.00 |
|
G2 |
62.00±2.00 b |
38.00±2.00 a |
41.50±0.50 |
|
G3 |
64.00±2.00 b |
36.00±4.00 a |
42.14±0.01 |
|
G4 |
74.00±2.00 a |
26.00±1.00 b |
42.72±0.16 |
|
G5 |
74.00±4.00 a |
26.00±4.00 b |
42.46±0.31 |
|
G6 |
71.00±1.00 ab |
29.00±1.00 ab |
42.51±0.49 |
|
Significate |
* |
* |
NS |
N.S: Not significant * (P≤0.05). a, b, and c differ at (P≤0.05). Type the meaning of the abbreviations (G1 to G6) here.
In terms of piped egg rate (Table 2), there was a significant (P < 0.05) increase for the G2 group compared to the G1 group. No significant differences were observed between the G3, G4, G5, and G6 groups regarding live, dead, and abnormal chicks rates. However, there was a significant difference (P<0.05) in hatchability for the G3 group compared to the G1, G5, and G6 groups.
Chicks quality traits
There was no significant difference among groups in chick length, wing length, leg length, and tonic immobility (Table 3). Table 4 shows that the highest chick activity was observed in the G3 group. In contrast, the lowest chick activity was observed in the G2 group compared to the other groups, as so not significant in appearance and feather condition, case of yolk retracted inside the abdomen, eye’s appearance, leg appearance, navel case, and residual yolk.
Table 2: Effect of in ova injection at 12 days of embryonic age with LPL in piped egg rate, live piped chicks rate, dead piped chicks rate, and abnormal chicks rate.
|
Groups |
Mean± standard error |
|||
|
Piped egg rate % |
Live piped chicks rate % |
Dead piped chicks rate % |
Abnormal chicks rate % |
|
|
G1 |
17.00±3.00b |
1.00±0.50 |
2.00±1.00 b |
2.00±0.01 |
|
G2 |
30.00±0.01a |
2.00±0.01 |
6.00±2.00 ab |
4.00±2.00 |
|
G3 |
26.00±6.00ab |
1.00±0.50 |
9.00±1.00 a |
2.00±0.01 |
|
G4 |
19.00±1.00 ab |
3.00±1.00 |
5.00±1.00 ab |
3.00±1.00 |
|
G5 |
23.00±5.00 ab |
0.00±0.00 |
3.00±1.00 b |
1.00±0.50 |
|
G6 |
25.00±1.00 ab |
2.00±1.00 |
2.00±1.00 b |
1.00±0.50 |
|
Significate |
* |
NS |
* |
NS |
N.S: Not significant * (P≤0.05). a, b, and c differ at (P≤0.05). Type the meaning of the abbreviations (G1 to G6) here.
Table 3: Effect of in ova injection at 12 days of embryonic age with LPL in chick length, wing length, leg length, and tonic immobility.
|
Groups |
Mean± standard error |
|||
|
Chick length (mm) |
Wing length (mm) |
Leg length (mm) |
Tonic immobility (mint) |
|
|
G1 |
18.26±0.81 |
3.53±0.03 |
4.16±0.08 |
2.54±0.06 |
|
G2 |
17.83±0.44 |
3.80±0.06 |
4.30±0.25 |
2.63±0.16 |
|
G3 |
18.00±0.57 |
3.63±0.08 |
4.60±0.21 |
2.06±0.08 |
|
G4 |
18.46±0.31 |
3.70±0.15 |
4.46±0.08 |
2.72±0.42 |
|
G5 |
18.06±0.58 |
3.73±0.08 |
4.50±0.21 |
1.73±0.26 |
|
G6 |
18.83±0.16 |
3.77±0.03 |
4.53±0.03 |
2.13±0.68 |
|
Significate |
NS |
NS |
NS |
NS |
NS: not significant.
Table 4: Effect of in ova injection at 12 days of embryonic age with LPL in chick physical traits.
|
Groups |
Mean± standard error |
||||||
|
Activity |
Appearance and feather condition |
Case of yolk retracted inside the abdomen |
Eye’s appearance |
Leg appearance |
Navel case |
Residual sac |
|
|
G1 |
4.00±0.01 b |
9.33±0.67 |
10.00±0.00 |
12.00±0.00 |
13.33±2.67 |
12.00±0.00 |
8.00±0.00 |
|
G2 |
3.33±0.33 c |
8.00±0.01 |
6.67±3.33 |
12.00±0.00 |
10.00±2.67 |
12.00±0.00 |
8.00±0.00 |
|
G3 |
5.00±0.01 a |
8.67±0.67 |
10.00±0.00 |
12.00±0.00 |
16.00±0.00 |
12.00±0.00 |
8.00±0.00 |
|
G4 |
4.00±0.01 b |
8.67±0.67 |
10.67±0.67 |
12.00±0.00 |
16.00±0.00 |
12.00±0.00 |
8.00±0.00 |
|
G5 |
4.00±0.0 b |
8.70±0.64 |
10.00±0.00 |
12.00±0.00 |
16.00±0.00 |
12.00±0.00 |
8.00±0.00 |
|
G6 |
4.00±0.0 b |
8.65±0.65 |
10.00±0.01 |
12.00±0.00 |
13.33±2.67 |
12.00±0.00 |
8.00±0.00 |
|
Significate |
** |
NS |
NS |
NS |
NS |
NS |
NS |
NS: Not significant. ** (P≤0.01). a, b, and c differ at (P≤0.01).
Weight of residual yolk and weight of chick without yolk
Table 5 shows the effect of treatments on the weight of residual yolk and the weight of chick without yolk. the highest (P<0.05) weight of residual yolk was observed in G3 followed by G1 and G2, while the lowest weight of residual yolk was observed in G4, G5, and G6 groups. The highest chick weight without yolk was observed in the G4, G5, and G6 groups, followed by the G2 and G3 groups, and the lowest value was observed in the control group, G1. There was no difference between G4, G5, and G6.
Table 5: Effect of in ova injection at 12 days of embryonic age with LPL in weight of residual yolk and weight of chick without yolk.
|
Groups |
Mean± standard error |
|
|
Weight of residual yolk (gm) |
Weight of chick without yolk (gm) |
|
|
G1 |
5.33±0.05 b |
35.67±0.59 c |
|
G2 |
5.39±0.08 b |
36.11±0.58 b |
|
G3 |
5.47±0.04 a |
36.67±0.07 b |
|
G4 |
5.12±0.03 c |
37.60±0.37 a |
|
G5 |
5.09±0.01 c |
37.37±0.04 ab |
|
G6 |
5.10±0.02 c |
37.41±0.19 ab |
|
Significate |
* |
* |
* (P≤0.05). a, b, and c differ at (P≤0.05).
Table 6: Effect of in ova injection at 18 days of embryonic age with LPL in some hatching traits.
|
Groups |
Mean± standard error |
||
|
Hatching rate % |
Embryonic mortality rate % |
Hatching chick's weight (gm) |
|
|
G1 |
79.00±1.00 b |
21.00±1.00 b |
41.33±0.16 c |
|
G2 |
69.00±1.00 c |
31.00±1.00a |
41.17±0.07 c |
|
G3 |
85.00±1.00 ab |
15.00±1.00 bc |
42.16±0.10 b |
|
G4 |
80.00±0.01 b |
20.00±0.01 b |
42.66±0.13 a |
|
G5 |
84.00±4.00 ab |
16.00±4.00 bc |
42.62±0.02 a |
|
G6 |
88.00±2.00 a |
12.00±2.00 c |
42.44±0.03 ab |
|
Significate |
** |
** |
** |
** (P≤0.01). a, b, and c differ at (P≤0.01).
Second trial
Hatching traits
The effect of the study on hatching traits (ova injection at 18 days) is presented in Tables 6 and 7, a significant (P<0.05) increase in hatching rate for the G6 group compared to G1, G2, and G4 groups and significantly for the G1, and G4 groups on G2 group. While the G6 group was the least significant in embryonic mortality rate compared to G1, G2, and G4 groups, and significant improvement for the G1, G3, G4, and G5 groups comparative G2 group, in hatching chicks weight was significant (P<0.05) increase for the G4 and G5 groups on the G1, G2, and G3 groups at the same time significant increase for the G3, and G6 groups compared to G1 and G2 groups.
Table 7: Effect of in ova injection at 18 days of embryonic age with LPL in piped egg rate, live piped chicks rate, dead piped chicks rate, and abnormal chicks rate.
|
Groups |
Mean± Standard error |
|||
|
Piped egg rate % |
Live piped chicks rate % |
Dead piped chicks rate % |
Abnormal chicks rate % |
|
|
G1 |
16.00±2.00ab |
2.00±1.00 |
3.00±1.00 |
4.00±2.00 |
|
G2 |
21.00±1.00a |
3.00±1.50 |
7.00±3.00 |
3.00±1.50 |
|
G3 |
12.00±2.00bc |
2.00±1.00 |
1.00±0.50 |
1.00±0.50 |
|
G4 |
14.00±2.00bc |
4.00±2.00 |
3.00±1.00 |
0.00±0.00 |
|
G5 |
14.00±2.00bc |
0.00±0.00 |
2.00±1.00 |
2.00±1.00 |
|
G6 |
9.00±1.00c |
0.00±0.00 |
3.00±1.00 |
0.00±0.00 |
|
Significate |
* |
NS |
NS |
NS |
NS: Not significant. * (P≤0.05). a, b, and c differ at (P≤0.05).
In terms of piped egg rate, the G2 was the significant (P<0.05) height compared to all in ova injection groups, while there was no significant difference in live piped chicks rate, dead piped chicks rate, and abnormal chicks rate.
Chicks quality traits
The effect of LPL injection in ova at 18 days of embryo age on chick quality traits is presented in Table 8. In chicks, a significant increase in length was observed for the G5 and G6 groups compared to the G1 and G2 groups. Additionally, there was a significant increase in chick wing length for the G4 and G5 groups compared to the G3 group. Moreover, all ovo injection LPL groups significantly increased compared to the control groups. The LPL level also resulted in a significant improvement in tonic immobility compared to the G1 group.
Table 9 showed significant improvement (P<0.05) for the G3, G4, G5, and G6 in chicks activity compared to G1 and G2 groups but insignificant in appearance and feather condition, case of yolk retracted inside the abdomen, eye’s appearance, leg appearance, navel case, and residual yolk.
Weight of residual yolk and weight of chick without yolk
As shown in Table 10, the weight of residual yolk decreased significantly (P<0.01) in the G1, G2, and G4 groups on other groups. The G3 group decreased significantly compared to the G6 group, while the weight of chicks without yolk significantly improved (P<0.ent for all LPL in ova injection groups compared to control groups.
Table 8: Effect of in ova injection at 18 days of embryonic age with LPL in chick length, wing length, leg length, and tonic immobility.
|
Groups |
Mean± standard error |
|||
|
Chick length (mm) |
Wing length (mm) |
Leg length (mm) |
Tonic immobility (mint) |
|
|
G1 |
17.50±0.28b |
3.50±0.00ab |
3.67±0.16b |
1.65±0.12a |
|
G2 |
17.10±0.06b |
3.67±0.12ab |
3.67±0.33b |
1.31±0.18ab |
|
G3 |
17.90±0.46ab |
3.30±0.10b |
4.93±0.13a |
0.68±0.02c |
|
G4 |
18.76±0.14ab |
4.00±0.11a |
5.10±0.21a |
0.85±0.06bc |
|
G5 |
18.43±0.21a |
4.00±0.28a |
4.83±0.20a |
1.03±0.29bc |
|
G6 |
18.60±0.36a |
3.76±0.26ab |
5.01±0.28a |
0.65±0.17c |
|
Significate |
** |
* |
** |
** |
* (P≤0.05), ** (P≤0.01). a, b, and c differ at (P≤0.05) and (P≤0.01).
Results of the study from the two experiments showed a significant improvement in the hatching traits and characteristics of the hatched chicks in the second experiment (injection at 18 days of embryo age) compared to the first experiment. Treatment G1 was notably superior in the first experiment compared to the other treatments. This difference may be attributed to the injection process at this embryo development stage, which could be unsuitable for embryonic growth and development. Most studies have
Table 9: Effect of in ova injection at 18 days of embryonic age with LPL in chick physical traits.
|
Groups |
Mean± Standard error |
||||||
|
Activity |
Appearance and feather condition |
Case of yolk retracted inside the abdomen |
Eye’s appearance |
Leg appearance |
Navel case |
Residual case |
|
|
G1 |
4.00±0.00 c |
9.33±0.67 |
12.00±0.00 |
14.67±0.67 |
13.33±2.66 |
8.67±1.33 |
10.00±0.00 |
|
G2 |
4.00±0.00 c |
9.33±0.67 |
12.00±0.00 |
12.67±2.33 |
13.33±2.67 |
9.00±1.52 |
10.00±1.15 |
|
G3 |
5.33±0.33 ab |
10.00±0.00 |
12.00±0.00 |
14.67±0.33 |
16.00±0.00 |
10.67±0.67 |
11.00±0.57 |
|
G4 |
5.67±0.33 a |
10.00±0.00 |
12.00±0.00 |
15.67±0.33 |
16.00±0.00 |
11.33±0.67 |
11.00±0.00 |
|
G5 |
5.00±0.00 b |
10.00±0.00 |
12.00±0.00 |
15.33±0.67 |
13.33±2.67 |
11.00±0.57 |
10.67±0.67 |
|
G6 |
5.00±0.00 b |
10.00±0.00 |
12.00±0.00 |
15.67±0.33 |
16.00±0.00 |
11.33±0.33 |
11.00±0.57 |
|
Significate |
* |
NS |
NS |
NS |
NS |
NS |
NS |
NS: Not significant * (P≤0.05). a, b, and c differ at (P≤0.05).
Table 10: Effect of in ova injection at 18 days of embryonic age with LPL in weight of residual yolk and weight of chick without yolk.
|
Groups |
Mean± Standard error |
|
|
Weight of residual yolk (gm) |
Weight of chick without yolk (gm) |
|
|
G1 |
6.09±0.05 c |
34.24±0.31 c |
|
G2 |
6.17±0.01 c |
35.01±0.06 c |
|
G3 |
6.32±0.03 b |
35.84±0.17 ab |
|
G4 |
5.97±0.06 c |
36.69±0.28 a |
|
G5 |
6.38±0.01 ab |
36.24±0.03 a |
|
G6 |
6.51±0.05 a |
35.93±0.02 ab |
|
Significate |
** |
** |
** (P≤0.01). a, b, and c differ at (P≤0.01).
suggested that the optimal time for early feeding in the egg is 17.5-18 days of embryo age (Das et al., 2021) despite the significant superiority of fat injection treatments. Rapidly degrading phospholipids in the hatching characteristics and physical characteristics of hatched chicks compared to the positive control treatment, as LPL is considered very important for the needs of living organisms’ cells, as it is an essential component of cells in addition to its main role in meeting the energy needs of the epithelial cells of the intestines of poultry (Al-Jebory et al., 2023), the amount of energy consumed has a significant impact on the bird’s growth and body composition (Wiseman and Lewis, 1998). Previous studies have also shown that any defect in the birds’ access to the energy necessary for growth during the birds’ early life stages affects the live body weight and the birds’ feed conversion factor. Later (Zhao and Kim, 2017; Papadopoulos et al., 2018), which is in line with the research we are doing now, the weight of the hatched chicks and the hatching rate were shown to be greatly improved when the embryos were given energy levels using LPL egg injections, particularly when the injections were given at 18 days of embryo age. Polycarpo et al. (2016), and Zampiga et al. (2016). Zhao and Kim (2017) revealed that during the early feeding stage, LPL increased live body weight and feed conversion factor; this may be because birds secrete insufficient amounts of bile salts and lipase, which results in a reduced ability to digest and absorb fat, LPL functions primarily as an emulsifier in food supplements, which helps broiler chickens perform better by facilitating the digestion and absorption of fats (Boontiam et al., 2017).
Additionally, improved growth performance is partly attributable to the fact that When LPL is injected into hatching eggs, it binds to the intestinal cell membrane, changing the permeability and fluidity of membrane bilayers as well as the activities of protein channels (Maingret et al., 2000), ultimately increasing the transport of elements across the cell membrane and improving the absorption of yolk sac contents and nutrients in the final stage of development. Embryonic development and the presence of high amounts of energy may be reflected in increased growth and formation of body cells and an increase in the percentage of abdominal fat (Zaman et al., 2008; Zhao and Kim, 2017); this may positively impact the weight of hatched chicks, the small intestine’s shape reflects the health and capacity of the intestine when it comes to nutrient absorption, an increase in the depth and height of the villi indicates an increase in the intestinal area for absorption, which in turn leads to improved digestion and absorption of nutrients (Hosoyamada and Sakai, 2007). This in turn improves the health of the intestine and the bird, which is reflected in the characteristics of the hatched chicks, Khonyoung et al. (2015) noted that LPL can increase the proliferation of intestinal epithelial cells, leading to increased height of the duodenal mucosa in broilers, and LPL also causes the stimulation of gene expression of Claudin-3, a protein useful for maintaining tight sealing function, LPL It caused an increase in Ca and P contents in the bones only during the early stage of chick growth, this can be explained by the effects of LPL on the broilers of cells and by increasing the number and size of membrane pores and thus increasing the absorption of mineral elements, this explains the improvement in the physical traits of the hatched chicks, the decrease in the weight of the remaining yolk in the chicks of the first experiment in the fourth, fifth, and sixth groups, as well as the chicks of the third and fourth groups in the second experiment, returned to the role of LPL, which improved yolk absorption, at the same time, the weight of the hatched chicks increased, the weight of the hatched chicks of the fifth and sixth groups also improved in the second experiment, despite the increase in bag weight, the yolk may be due to the high level of energy available to the embryo, which increased the weight of the hatched chicks.
Conclusions and Recommendations
The study concluded that early feeding with egg injections at concentrations of 2%, 3%, and 4% improved yolk absorption and the weight of hatched chicks, especially with injections at 12 days of embryo age. This indicates that the benefits of LPL appear in the long term and simultaneously improve the characteristics of the hatching chicks. Additionally, an injection at 18 days old also positively affected the hatching and physical traits of the chicks, which is linked to the subsequent growth and performance of the hatched chicks and the increase in the weights of the broilers at marketing.
Acknowledgements
The authors would like to express their special thanks and gratitude to the Department of Animal Production/College of Agriculture/ Al-Qasim Green University and Al-Anwar company for all their kind and helpful support during the study period.
Novelty Statement
This study is the first one using Lysophospholipids in the Iraq/ Babylon government to improve chick growth.
Author’s Contribution
All authors contributed equally in the manuscript.
Ethics statement
All animals in this study were handled and cared for according to the appropriate biosecurity procedures. The study was performed by the rules of the ‘Guide for the Care and Use of Laboratory Animals and Broiler ROSS 308 guide that were approved by the Ethics Committee of the College of Agriculture, Al-Qasim Green University, Iraq (Number 20 AP on 13/10/2023) before starting this study.
Conflict of interest
The authors have declared no conflict of interest.
References
Al-Jaryan IL, Al-Jebory HH, Al-Saeedi MKI (2023). Effect of early feeding with different levels of anthocyanins in hatching, phenotypical, and physical traits of hatching broiler chicks (Ross 308). Res. J. Agric. Biol. Sci., 15(1): 7-13.
Al-Jebory HH, Qotbi AAA, Al-Saeedi MKI, Al-Khfaji FR, Ajafar M, Safaei A (2023). Biological activity of Lysophospholipids in poultry and ruminants: A review. Int. J. Multidiscip. Res. Growth Evaluat., 4(2): 504-511.
Al-Marzooqi W, Leeson S (1999). Evaluation of dietary supplements of lipase, detergent, and crude porcine pancreas on fat utilization by young broiler chicks. Poult. Sci., 78: 1561–1566. https://doi.org/10.1093/ps/78.11.1561
Arouri A, Mouritsen OG (2013). Membrane-perturbing effect of fatty acids and lysolipids. Prog. Lipid Res., 52: 130–140. https://doi.org/10.1016/j.plipres.2012.09.002
Boontiam W, Jung B, Kim YY (2017). Effects of lysophospholipid supplementation to lower nutrient diets on growth performance, intestinal morphology, and blood metabolites in broiler chickens. Poult. Sci., 96: 593–601. https://doi.org/10.3382/ps/pew269
Das R, Mishra P, Jha R (2021). In ovo feeding as a tool for improving performance and gut health of poultry: A review. Front. Vet. Sci., 8: 754246. https://doi.org/10.3389/fvets.2021.754246
Duncan DB (1955). Multiple range and multiple tests. Biometrics, 11: 1- 42. https://doi.org/10.2307/3001478
Geidam YA, Ibrahim UI, Bukar MM, Gambo HI, Ojo O (2007). Quality assessment of broiler day-old chicks supplied to Maiduguri. North-Eastern Nigeria. Int. J. Poult. Sci., 6: 107-110. https://doi.org/10.3923/ijps.2007.107.110
Hasenhuettl GL, Hartel RW (2008). Food emulsifiers and their applications. Vol. 19. New York: Springer. https://doi.org/10.1007/978-0-387-75284-6
Hosoyamada Y, Sakai T (2007). Mechanical components of rat intestinal villi as revealed by ultrastructural analysis with special reference to the axial smooth muscle cells in the villi. Arch. Histol. Cytol., 70: 107–116. https://doi.org/10.1679/aohc.70.107
Joshi A, Swaroopa GP, Bhaskar N, Thorat N (2006). Modification of lecithin by physical, chemical, and enzymatic methods. Eur. J. Lipid Sci. Technol., 108: 363–373. https://doi.org/10.1002/ejlt.200600016
Khonyoung D, Yamauchi K, Suzuki K (2015). Influence of dietary fat sources and lysolecithin on growth performance, visceral organ size, and histological intestinal alteration in broiler chickens. Livest. Sci., 176: 111–120. https://doi.org/10.1016/j.livsci.2015.03.011
Lu Z, Yao C, Tan B, Dong X, Yang Q, Liu H, Zhang S, Chi S (2022). Effects of lysophospholipid supplementation in feed with low protein or lipid on growth performance, lipid metabolism, and intestinal flora of largemouth bass (Micropterus salmoides). Aquacult. Nutr., 4347466: 12. https://doi.org/10.1155/2022/4347466
Lundbaek JA, Collingwood SA, Ing’olfsson HI, Kapoor R, Andersen OS (2010). Lipid bilayer regulation of membrane protein function: gramicidin channels as molecular force probes. J. R. Soc. Interface, 7: 373–395. https://doi.org/10.1098/rsif.2009.0443
Maingret F, Patel AJ, Lesage F, Lazdunski M, Honor´e E (2000). Lysophospholipids open the two-pore domain mechanogated K+ channels TREK-1 and TRAAK. J. Biol. Chem., 275: 10128–10133. https://doi.org/10.1074/jbc.275.14.10128
Noy Y, Sklan D (1998). Metabolic responses to early nutrition. J. Appl. Poult. Res., 7: 437–451. https://doi.org/10.1093/japr/7.4.437
Osaiyuwu OH, Salako AE, Adurogbangban O (2009). Body dimensions of Fulani and Yoruba ecotype chickens under intensive systems of management. J. Agric. For. Soc. Sci., 7(2): 195-201. https://doi.org/10.4314/joafss.v7i2.64358
Papadopoulos GA, Poutahidis T, Chalvatzi S, Di Benedetto M, Hardas A, Tsiouris V, Georgopoulou I, Arsenos, G, Fortomaris PD (2018). Effects of lysolecithin supplementation in lowenergy diets on growth performance, nutrient digestibility, viscosity and intestinal morphology of broilers. Br. Poult. Sci., 59: 232–239. https://doi.org/10.1080/00071668.2018.1423676
Polycarpo GV, Burbarelli MF, CarAo AC, Merseguel CE, Dadalt JC, Maganha SR, Sousa RL, CruzPolycarpo VC, Albuquerque R (2016). Effects of lipid sources, lysophospholipids and organic acids in maize-based broiler diets on nutrient balance, liver concentration of fat-soluble vitamins, jejunal microbiota and performance. Br. Poult. Sci., 57: 788– 798. https://doi.org/10.1080/00071668.2016.1219019
SAS, 2012. SAS/STAT user’s guide for personal computers. Release 9.1 SAS Institute Inc., Cary, NC, USA.
Schwarzer K, and Adams CA (1996). The influence of specific phospholipids as absorption enhancers in animal nutrition. Eur. J. Lipid Sci. Technol., 98: 304–308. https://doi.org/10.1002/lipi.19960980905
Skoura A, Hla T (2009). Lysophospholipid receptors in vertebrate development, physiology, and pathology. J. Lipid Res., 50: S293–S298. https://doi.org/10.1194/jlr.R800047-JLR200
Solbi A, Rezaeipour V, Abdullahpour T, Gharahveysi S (2021). Efficacy of lysophospholipids on growth performance, carcase, intestinal morphology, microbial population and nutrient digestibility in broiler chickens fed different dietary oil sources. Ital. J. Anim. Sci., 20(1): 1612-1619. https://doi.org/10.1080/1828051X.2021.1973599
Tona K, Bamelis F, De Ketelaere B, Bruggeman V, Moraes VMB, Buyse J, Onagbesan O, Decuypere E (2003). Effects of egg storage time on spread of hatch, chick quality, and chick juvenile growth. Poult. Sci., 82: 736–741. https://doi.org/10.1093/ps/82.5.736
Willemsen H, Everaert N, Witters A, De Smit L, Debonne M, Verschuere F, Garain P, Berckmans D, Decuypere E, Bruggeman V (2008). Critical assessment of chick quality measurements as an indicator of posthatch performance. Poult. Sci., 87: 2358–2366. https://doi.org/10.3382/ps.2008-00095
Wiseman J, Lewis C (1998). Influence of dietary energy and nutrient concentration on the growth of body weight and of carcass components of broiler chickens. J. Agric. Sci., 131: 361–371. https://doi.org/10.1017/S0021859698005851
Zaki AN, Al-Jebory HHD (2021). Effect of early feeding with zinc-methionine on improving growth performance and some biochemical characteristics of broilers. 1st Int. Virtual Conf. Environ. Sci. IOP Conf. Ser. Earth Environ. Sci., pp. 722. https://doi.org/10.1088/1755-1315/722/1/012035
Zaman Q, Mushtaq T, Nawaz H, Mirza M, Mahmood S, Ahmad T, Babar M, Mushtaq M (2008). Effect of varying dietary energy and protein on broiler performance in hot climate. Anim. Feed Sci. Technol., 146: 302–312. https://doi.org/10.1016/j.anifeedsci.2008.01.006
Zampiga M, Meluzzi A, Sirri F (2016). Effect of dietary supplementation of lysophospholipids on productive performance, nutrient digestibility and carcass quality traits of broiler chickens. Ital. J. Anim. Sci., 15: 521–528. https://doi.org/10.1080/1828051X.2016.1192965
Zhao PY, Kim IH (2017). Effect of diets with different energy and lysophospholipids levels on performance, nutrient metabolism, and body composition in broilers. Poult. Sci., 96: 1341–1347. https://doi.org/10.3382/ps/pew469
To share on other social networks, click on any share button. What are these?






