Effect of Fiber Degrading Enzymes Added in Soybean Hulls on the Production Performance, Hematology, Serum Biochemistry and Economics During Early Peak Production Period in Laying Hens
Effect of Fiber Degrading Enzymes Added in Soybean Hulls on the Production Performance, Hematology, Serum Biochemistry and Economics During Early Peak Production Period in Laying Hens
Muhammad Shuaib1*, Abdul Hafeez1, Naila Chand1 and Muhammad Tahir2
1Department of Poultry Science, Faculty of Animal Husbandry and Veterinary Sciences, The University of Agriculture Peshawar, Pakistan
2Department of Animal Nutrition, Faculty of Animal Husbandry and Veterinary Sciences, The University of Agriculture Peshawar, Pakistan
ABSTRACT
The present study aimed to investigate the combined effect of fiber degrading enzymes (β-Mannanase) and soybean hulls on the production performance, hematological parameters, and economics in the laying hens during the early (29 to 32 weeks) peak production period. Two hundred golden brown (RIR×Fayoumi) layer birds of age 28 weeks were used for the experimental purpose and were distributed in five groups control (CON), and treatment groups (T1, T2, T3, and T4), each had 4 replicates with 10 birds per replicate. The CON group had a corn-soybean basal diet while the T1 group contained 3%SH+20mg/kg enzyme, T2 3%SH+30mg/kg enzyme, T3 9%SH+20mg/kg enzyme, and T4 group 9%SH+30mg/kg enzyme in the feed. Overall feed intake, weight gain, and water intake were calculated (P<0.05) in the T2 diet group. Egg production, FCR, HDEP, and mortality was not effaced (P>0.05) in all weeks. Total revenue was calculated higher in the T2 group while the profit and cost-benefit ratio had a higher (P<0.05) value in the CON and T1 diet groups. The total cholesterol and low-density lipoprotein (LDL) were calculated (P<0.05) higher in the CON group while all other hematological and serum biochemistry parameters were not affected and were in the normal range. It is concluded that the replacement of soybean meal in the diet of laying hens by 3% SH in combination with enzyme (β-Mannanase) at the level of 20mg/kg feed has a positive effect on the overall performance of laying hens during the early peak egg production period.
Article Information
Received 23 April 2022
Revised 20 January 2023
Accepted 02 February 2023
Available online 01 May 2023
(early access)
Published 01 July 2024
Authors’ Contribution
MS study design animal trial, laboratory experiment, statistical analysis, and writing. AH study design, feed formulation, data evaluation, manuscript review. NC data analysis, manuscript review. MT study design, data analysis, manuscript review.
Key words
Soybean hull, β-mannanase, FCR, Economics, Hematology
DOI: https://dx.doi.org/10.17582/journal.pjz/20220423190447
* Corresponding author: shoaibwzr@gmail.com
0030-9923/2024/0005-2019 $ 9.00/0
Copyright 2024 by the authors. Licensee Zoological Society of Pakistan.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
INTRODUCTION
In general, fibrous feed stuffs can be introduced to the diet at a rate of 3 to 5% without affecting nutrient digestibility or growth performance in many poultry species (Jimenez-Moreno et al., 2009). For the last 5 decades, enzymes have played a vital role in the poultry feed industry by augmenting the nutritive worth of the feed ingredients and they are incorporated into poultry diets to reduce feed costs without compromising weight gain and feed efficiency (Walters, 2019). Exogenous enzyme studies have become increasingly popular, owing to their unique properties. According to Lima et al. (2007), the addition of exogenous enzymes to animal feed has goals like the elimination or hydrolysis of anti-nutritional components, NSP breakdown, enhanced nutrient digestibility, and supplementing of endogenous enzymes. Thus, exogenous enzymes in addition to enabling feed efficiency utilization can increase the use of low-cost ingredients for animal feed because the viscosity of the digesta reduces with use, potentiating the activity of endogenous enzymes on specific substrates (Ribeiro et al., 2011). Soybean hulls are the by-product of soybean seed when used for the extraction of oil and their chemical composition may vary due to the efficiency of the de-hulling process (Rojas et al., 2014), hence, the soybean hulls might contain varying quantities of celluloses (29-51%), hemicelluloses (10-25%), proteins (11-15%), lignin (1-4%) and pectin (4-8%) (Mielenz et al., 2009; Shuaib et al., 2022). Soybean hulls, therefore are, mainly lignocellulose physical entities and poultry birds cannot produce enzymes for the breakdown of non-starch polysaccharides (NSPs) existing in the cell wall of the grains and are kept un-hydrolyzed, resulting in low feed efficacy. Studies in recent years have recommended that the unwanted influences of NSPs can be overcome by nutritive modifications which include supplementation of appropriate synthetic enzymes (cellulase and hemicellulase) provisions in the diet of birds. These enzymes break down the NSPs and reduce intestinal adhesiveness and eventually improve the digestibility of nutrients by improving gut health and performance (Creswell, 1994). Furthermore, enzyme hydrolysis products may indirectly restrict the growth of certain pathogenic species in the lower gut by encouraging the growth of lactic acid bacteria (Meng and Slominski, 2005). It was therefore assumed that the addition of enzyme β-mannanase (HemicellTD) in a soybean hulls-based diet may compensate for the negative effect of the soybean hulls-based diet. Therefore, this study was designed to determine the effect of dietary inclusion of soybean hull supplemented with enzyme (β-mannanase) on the production performance, hematological and serum biochemistry, and economics during the early peak production period in laying hens.
MATERIALS AND METHODS
Housing and experimental environment
The study was conducted at the University of Agriculture Peshawar poultry farm.
Two hundred (200) golden brown (RIR × Fayoumi) layer birds of age 28 weeks were used for the experimental purpose and were randomly distributed into five groups of 40 birds each. Every group was further subdivided into four experimental replicates of 10 birds each and randomly assigned to one of the 5 treatments. The experimental diets were formulated in the Sadiq Brother (SB) feed mill (Rawalpindi). The CON group had a basal diet (Corn-soybean meal) while the T1 group contained 3%SH+20mg/kg enzyme, T2 3%SH+30mg/kg enzyme, T3 9%SH+20mg/kg enzyme, and the T4 group 9%SH+30mg/kg enzyme (β-mannanase (Hemicell™), USA) in the feed. Uniform environmental and managementmental conditions were provided to all the birds in the experimental house. The room temperature was kept at 75°F and was equipped with sufficient light (17 h/day). The flock was provided with a routine vaccination schedule. The composition of experimental diets is shown in Table I.
Production performance parameters
Feed intake was recorded on daily basis by subtracting total feed intake from total feed offered while egg production was recorded on daily basis. Hen day production (HD was calculated by the formula; the total number of eggs laid by the flock in a given period divided by the product of the number of days and the number of alive hens on each of these days. The body weight gain was recorded on weekly basis by subtracting the initial weight from the final weight of the body. Mortality was recorded on daily basis along with its possible cause of death after postmortem examination. Daily water intake was recorded by subtracting the total water consumed from the total water provided. FCR was recorded by the formula;
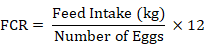
|
Nutrient % |
CON |
Diet |
|||
|
T1 |
T2 |
T3 |
T4 |
||
|
Corn |
53.1 |
52.1 |
52.1 |
50.5 |
50.5 |
|
Canola meal (34%) |
4.15 |
3.85 |
3.67 |
2.16 |
2.14 |
|
Soybean meal (44%) |
24.3 |
23.6 |
23.6 |
22.2 |
22.2 |
|
Guar meal |
1.00 |
0.00 |
0.00 |
0.00 |
0.00 |
|
Soybean hull |
0.00 |
3.00 |
3.00 |
9.00 |
9.00 |
|
β-mannanase (Hemicell) |
0.00 |
2.00 |
3.00 |
2.00 |
3.00 |
|
PBM Hi fat |
2.00 |
1.02 |
0.34 |
0.00 |
0.00 |
|
Poultry oil |
2.79 |
2.79 |
2.71 |
2.67 |
2.67 |
|
Salt |
0.32 |
0.32 |
0.28 |
0.26 |
0.26 |
|
Sodium bicarbonate |
0.10 |
0.10 |
0.10 |
0.10 |
0.10 |
|
Limestone/Chips |
11.1 |
10.1 |
10.1 |
10.0 |
9.16 |
|
DCP |
0.77 |
0.77 |
0.75 |
0.77 |
0.62 |
|
DLM |
0.08 |
0.08 |
0.08 |
0.07 |
0.08 |
|
Choline chloride (70 %) |
0.10 |
0.10 |
0.10 |
0.10 |
0.10 |
|
Vitamin premix broiler |
0.07 |
0.07 |
0.07 |
0.07 |
0.07 |
|
Mineral premix |
0.06 |
0.06 |
0.06 |
0.06 |
0.06 |
|
Phytase |
0.01 |
0.01 |
0.01 |
0.01 |
0.01 |
|
Enramycin |
0.02 |
0.02 |
0.02 |
0.02 |
0.02 |
|
Ethoxyquin/antioxidant |
0.01 |
0.01 |
0.01 |
0.01 |
0.01 |
|
NSPs |
0.02 |
0.00 |
0.00 |
0.00 |
0.00 |
|
Total |
100 |
100 |
100 |
100 |
100 |
To provide one kg of diet: Retinyl acetate, 4400 IU; DL-α-tocopheryl acetate 12 IU; Cholecalciferol 118µg; Thiamine 2.5mg; Menadione sodium bisulphite 2.40 mg; Niacin 30mg; Vit.B2 4.8 mg; D-pantothenic acid 10 mg; Vit. B6 5mg; Vit. B7 130 µg; Cyanocobalamine 19 µg; Vit.B9 2.5 mg; Mn 85 mg; Zinc 75 mg; Fe 80 mg; Iodine 1 mg; Selenium 130 µg; Copper 6 mg. CON, Control; T1, 3%SH+20mg/kg enzyme β-Mannanase; T2, 3%SH+30mg/kg enzyme β-Mannanase; T3, 9%SH+20mg/kg enzyme β-Mannanase; T4, 9%SH+30mg/kg enzyme β-Mannanase (Hemicell).
Hematology, serum biochemistry, and economics
The blood samples were collected from four birds of each replicate on the last day of each phase and examined for the evaluation of hematological and serum biochemistry parameters as explained by (Shuaib et al., 2022). The wright and giemsa stain fixed monolayer blood films were used for the estimation of differential white blood cells (WBCs) and a hemocytometer was used for the manual calculation of total WBCs and total red blood cells (RBCs) (Campbell, 1995). The packed cell volume (PCV) was measured by a standard manual technique using microhematocrit capillary tubes and centrifuged at 2500 rpm for 5 min. The cyanmethemoglobin methodology was implemented for the estimation of hemoglobin (HB) concentration in the blood. Erythrocytes indices such as mean corpuscular volume (MCV) and mean corpuscular hemoglobin concentrations (MCHC) were determined from total red blood cells (TRBC), PCV, and HB (Ritchie et al., 1994). Other blood parameters such as heterophile, lymphocyte, and heterophile to lymphocyte ratio (H:L) were also studied. Commercially available diagnostic kits (Lab Kit, Barcelona, Spain) were used for the determination of total blood protein and serum attributes such as total cholesterol (TC), high-density lipoprotein (HDL), and low-density lipoprotein (LDL). The equation (VLDL= TC-HDL-LDL) was used for the calculation of very low density lipoprotein (VLDP). Economics parameters like total revenue (TR), profit, and cost benefit ratio (CBR) were calculated by the formula e.g., TR (Rs) = Total number of eggs × price per egg.
Profit (Rs) = Total revenue - Total cost, CBR, Total revenue/total cost.
Statistical analysis
The data on performance, egg quality, hematological parameters, gut morphology, and nutrient digestibility were subjected to the analysis of variance (ANOVA) technique using a completely randomized design (CRD). The general linear model (GLM) procedure (Steel et al., 1997) of SPSS 21.0 was used to analyze the data statistically. Tukey’s test was applied to compare the significance of mean differences at a 5 percent level of significance.
RESULTS
The results regarding the use of soybean hulls and enzyme (β-mannanase) on the feed intake (FI), weight gain (WG), egg production (EP), and feed conversion ratio (FCR) in the laying hens are shown in Table II. Feed intake at all weeks and overall was recorded higher (P<0.05) in the T2 group than in all other groups. Weight gain during all weeks was recorded as non-significant (P>0.05) among all groups however overall had a higher (P<0.05) WG in the T2 group as compared to the remaining groups. The weekly, as well as overall EP and FCR, remained non-significant (P>0.05) among all the groups. The results related to water intake (WI), hen day egg production (HDEP), and mortality are shown in Table III. Water intake at week 29 was recorded as significantly higher (P<0.05) in the T2 treatment group than in the T1, CON, and T3 groups but at weeks 30 and 31 in the T2 group as compared to the T4, T3, and CON groups. During week 32 significantly higher (P<0.05) WI was calculated in the T2 group as compared to the T3, T1, and CON groups but the overall WI was calculated higher (P<0.05) in the T2 group than in all other groups. HDEP and mortality during all weeks and overall were calculated as non-significant (P>0.05) among all the groups. Table IV shows that the hematological and serum biochemistry parameters were not affected (P>0.05) except TC and LDL which were calculated significantly higher in the control group than in all other groups. The TR was recorded as higher (P<0.05) in the T2 group than in the T4, T3, and CON groups, while the profit and CBR in the CON group than in the T2, T3, and T4 groups accordingly.
Table II. Effect of dietary inclusion of soybean hull and enzyme on the feed intake, egg production, FCR and HDEP in the laying hens.
|
Parameters |
Weeks |
CON |
Treatments |
SEM |
P value |
|||
|
T1 |
T2 |
T3 |
T4 |
|||||
|
Feed intake (g) |
29 |
722d |
745b |
751a |
733c |
745b |
0.63 |
0.003 |
|
30 |
738e |
760c |
776a |
746d |
769b |
0.96 |
0.012 |
|
|
31 |
715d |
730c |
756a |
727c |
745b |
0.70 |
0.008 |
|
|
32 |
742d |
753c |
781a |
741d |
773b |
0.91 |
0.001 |
|
|
Overall |
2919e |
2989c |
3065a |
2949d |
3033b |
7.60 |
0.020 |
|
|
Egg production/bird |
29 |
5.13 |
5.32 |
5.40 |
5.24 |
5.27 |
0.75 |
0.497 |
|
30 |
5.04 |
5.29 |
5.45 |
5.18 |
5.25 |
0.65 |
0.369 |
|
|
31 |
5.08 |
5.34 |
5.58 |
5.27 |
5.32 |
0.59 |
0.565 |
|
|
32 |
5.11 |
5.32 |
5.54 |
5.27 |
5.32 |
0.67 |
0.537 |
|
|
Overall |
20.3 |
21.2 |
21.9 |
20.9 |
21.1 |
0.75 |
0.497 |
|
|
FCR |
29 |
1.68 |
1.67 |
1.66 |
1.67 |
1.69 |
0.01 |
0.141 |
|
30 |
1.74 |
1.72 |
1.71 |
1.72 |
1.74 |
0.03 |
0.248 |
|
|
31 |
1.68 |
1.65 |
1.64 |
1.66 |
1.67 |
0.01 |
0.133 |
|
|
32 |
1.72 |
1.70 |
1.69 |
1.70 |
1.72 |
0.02 |
0.241 |
|
|
Overall |
1.71 |
1.69 |
1.68 |
1.70 |
1.71 |
0.01 |
0.233 |
|
|
HDEP (%) |
29 |
74.4 |
76.0 |
77.1 |
74.8 |
75.2 |
2.05 |
0.041 |
|
30 |
73.8 |
75.4 |
76.4 |
74.0 |
75.0 |
1.34 |
0.032 |
|
|
31 |
75.7 |
77.2 |
78.2 |
76.2 |
76.0 |
2.05 |
0.041 |
|
|
32 |
81.1 |
83.4 |
80.7 |
82.6 |
83.2 |
1.09 |
0.027 |
|
|
Overall |
82.2 |
84.4 |
81.6 |
83.1 |
83.9 |
2.39 |
0.022 |
|
Means in the same row with different superscripts are significantly different (P<0.05). See Table I for details of treatment group.
Table III. Effect of dietary inclusion of soybean hull and enzyme on the weight gain, Water intake and mortality in the laying hens.
|
Parameters |
Weeks |
CON |
Treatments |
SEM |
P value |
|||
|
T1 |
T2 |
T3 |
T4 |
|||||
|
Weight gain (g) |
29 |
7.00 |
7.25 |
8.25 |
7.00 |
8.00 |
1.08 |
0.884 |
|
30 |
6.28 |
6.40 |
8.00 |
6.32 |
7.00 |
0.65 |
0.166 |
|
|
31 |
5.42 |
6.25 |
7.25 |
6.00 |
6.45 |
0.62 |
0.127 |
|
|
32 |
7.36 |
8.00 |
9.00 |
7.45 |
8.41 |
0.91 |
0.607 |
|
|
Overall |
26b |
27.5b |
32.2a |
26.2b |
30a |
1.74 |
0.049 |
|
|
Water intake (L/bird) |
29 |
1.09b |
1.11c |
1.15a |
1.09b |
1.14a |
1.37 |
0.001 |
|
30 |
1.12c |
1.16a |
1.16a |
1.13bc |
1.14b |
1.79 |
0.020 |
|
|
31 |
1.13c |
1.17a |
1.17a |
1.15b |
1.16b |
3.46 |
0.024 |
|
|
32 |
1.16c |
1.18b |
1.20a |
1.17b |
1.19a |
1.80 |
0.038 |
|
|
Overall |
4.52c |
4.64b |
4.70a |
4.55c |
4.64b |
1.53 |
0.021 |
|
|
Mortality (%) |
29 |
0.00 |
0.00 |
0.00 |
0.00 |
0.00 |
0.00 |
--- |
|
30 |
0.00 |
0.00 |
0.00 |
0.00 |
0.00 |
0.00 |
--- |
|
|
31 |
0.00 |
0.00 |
0.00 |
0.00 |
0.00 |
0.00 |
--- |
|
|
32 |
0.25 |
0.25 |
0.00 |
0.25 |
0.25 |
0.20 |
0.835 |
|
|
Overall |
0.25 |
0.25 |
00.00 |
0.25 |
0.25 |
0.25 |
0.835 |
|
Means in the same row with different superscripts are significantly different (P<0.05). See Table I for details of treatment group.
Table IV. Effect of dietary inclusion of soybean hull and enzyme on the hematology and serum biochemistry and economics during phase-1.
|
Parameters |
CON |
Treatments |
SEM |
P value |
|||
|
T1 |
T2 |
T3 |
T4 |
||||
|
RBCs (106μl) |
3.18 |
3.23 |
3.25 |
3.20 |
3.21 |
0.01 |
0.061 |
|
WBCs (103μl) |
3.08 |
3.02 |
3.00 |
3.06 |
3.04 |
0.02 |
0.084 |
|
PCV (%) |
27.2 |
27.4 |
28.4 |
27.2 |
27.3 |
0.40 |
0.437 |
|
HB (g/dl) |
9.85 |
10.0 |
10.0 |
9.89 |
10.0 |
0.02 |
0.061 |
|
MCHC (g/dl) |
36.0 |
36.4 |
35.2 |
36.3 |
37.0 |
0.56 |
0.312 |
|
MCV (fL) |
85.5 |
84.8 |
86.4 |
85.0 |
85.0 |
1.28 |
0.104 |
|
TC (mg/dl) |
134a |
131b |
130b |
127c |
126c |
1.38 |
0.014 |
|
HDL (mg/dl) |
63.4 |
64.0 |
64.2 |
65.1 |
66.0 |
1.03 |
0.073 |
|
LDL (mg/dl) |
45.0a |
42.0b |
41.3bc |
40.0c |
37.1d |
1.07 |
0.007 |
|
VLDL (mg/dl) |
25.4 |
25.0 |
24.0 |
23.2 |
23.4 |
2.00 |
0.447 |
|
Heterophile (%) |
21.0 |
23.0 |
23.4 |
21.0 |
22 |
1.15 |
0.525 |
|
Lymphocyte (%) |
56.0 |
57.0 |
56.5 |
58.0 |
57.4 |
1.25 |
0.702 |
|
H:L |
0.37 |
0.39 |
0.41 |
0.35 |
0.37 |
0.01 |
0.438 |
|
TP (mg/dl) |
5.07 |
5.10 |
5.12 |
5.08 |
5.09 |
0.01 |
0.063 |
|
Total revenue(Rs) |
348c |
354ab |
356a |
349bc |
352b |
2.38 |
0.252 |
|
Profit (Rs) |
151a |
150a |
147b |
143c |
140d |
2.58 |
0.056 |
|
Cost benefit ratio |
1.76a |
1.73ab |
1.70bc |
1.69c |
1.66d |
0.04 |
0.002 |
Means in the same row with different superscripts are significantly different (P < 0.05). See Table I for details of treatment group. RBC, red blood cells; WBCs, white blood cells; PCV, packed cell volume; HB, hemoglobin, MCHC, mean corpuscular hemoglobin concentrations; MCV, mean corpuscular volume; TC, total cholesterol; HDL, high-density lipoprotein; LDL, low-density lipoprotein; VLDL, very-low-density lipoprotein; H:L, heterophile to lymphocytes ratio; TP, total protein.
DISCUSSION
The effect of fiber degrading enzymes β-mannanase and soybean hulls in different concentrations on the production performance, hematological and serum biochemistry parameters, and economics in the golden brown (RIR × Fayoumi) laying hens were determined at the early peak egg production period. The overall feed intake, weight gain, and water intake were calculated (P<0.05) in the T2 group while the FCR, egg production, HDEP, and mortality were not affected although numerically (P<0.05) higher egg production and better FCR was recorded in the T2 group (3% SH+30mg/kg enzyme). Our results are in agreement with the findings of Esonu et al. (2006) who recorded significantly higher feed intake in broilers when fed 20% soya bean hulls meal and 1% enzyme (Safzyme) in the feed. A study by Abreu et al. (2018) indicated higher feed intake for the hens receiving 100g/ton enzyme complex as compared with ration without the addition of enzyme. Similarly, Danang and Tintin (2016) reported higher feed intake in the laying hens for the group receiving enzymes between 0.1-0.5 percent in the feed. The combination of the distiller’s dried grains with solubles (DDGS) levels and enzymes had a significant impact on feed efficiency (Javer et al., 2015). Our result is also in agreement with the finding of Esonu et al. (2005) in the laying hens and presented higher weight gain for 10, 20, and 30% soybean hull and 2% cellulitic enzyme in the diet as compared to a diet having only 10, 20 and 30% soybean hull without the enzyme addition. The result is also in agreement with the findings of Abreu et al. (2018) who described the beneficial effect of 100 g/t of the enzyme complex (xylanase, ß-glucanase, and phytase-based) for feed formulations to maximize performance and improved eggs production in laying hens. Similarly, Mathlouthi et al. (2003) also stated that enzyme supplementation did not affect egg production and egg weight however; numerically 11% increased egg production was calculated in the NSP enzymes supplemented group. Javer et al. (2015) described increased egg production in laying hens with the addition of 500 g/ton protease enzymes in the feed additive. Similarly, additions of real enzymes improved and enhanced the performance of the production of duck eggs during egg production than the control group (Danang and Tintin, 2016). Similarly, Silversides et al. (2006) reported no change in egg production with supplementation of xylanase and phytase individually or in combination with wheat-based laying hen diets with low levels of phosphorus. Similarly, Jalal and Scheideler (2001) observed no significant difference in egg production on enzyme (phytase) supplementation to corn soya-based layer diets. Similar to the findings of the present study, Sousa et al. (2019) recorded no significant effect on FCR (kg/dozen) when using fiber source and enzyme (xylanase) in a laying hens diet. In agreement with the results of the present study, the more efficient feed conversion ratio (from 2.15 to 2.03) was recorded by Danang and Tintin (2016) when the enzyme between 0.1-0.5% was provided in the feed to the treatment groups. To support our present study results, improved FCR from 2.11 to 1.99 on administering the enzyme Quatrazyme (20 mg/kg) in feed was reported by Javer et al. (2015). Similar to our result, Esonu et al. (2006) recorded a non-significant effect on the mortality of broilers when 10 and 20% soybean hulls meal and 1% enzyme (Safzyme) were used in the feed. The better feed intake in the T2 diet group is due to the beneficial effect of the enzyme (β-mannanase) on the gastrointestinal tract and its ability to break down the cell wall of the soybean hull into easily digestible components and similarly Almirall et al. (1993) had concluded that an increase in the feed intake occurred only after the enzyme supplementation decreased viscosity by degrading NSP components of the diet. The higher body weight gain and rate of egg-laying shown by the T2 group is due to the improved feed intake which supplies sufficient nutrients required to maintain higher egg production although there are no statistical differences among the groups. The better FCR in the T2 group is due to the relatively higher egg production in this group. Whether the water intake increases or decreases depends on the nature of the dietary fiber, however, factors such as environmental temperature, feed composition, and the physicochemical properties of the different ingredients and components of the diet might affect this relationship (Carre et al., 2013). Water intake is highly correlated with feed intake (Jiménez-Moreno et al., 2016) which is similar to the present study results and the increased feed intake in the T2 group (3%SH+30mg/kg enzyme) has resulted in increased water intake. During the experiment birds mortality was calculated (P>0.05) which was due to the accidental trapping of birds’ heads and wings in the nest wires.
TC and LDL values were higher (P<0.05) in the control group and lower in the soybean hulls and enzyme treatment groups which are similar to the results of Abdel et al. (2018) who reported decreased total cholesterol and low-density lipoprotein cholesterol serum levels in broiler diets from 1 to 42 days in all treatment groups having potato peels (PP) and sugar beet pulp (SBP) at the rate of 15% and 7.5% with added an enzyme mixture. Similar to the current study results, Bojarpou (2020) observed no significant effect on the blood parameters (P>0.05) in broiler chickens from the age of 1 to 42 days when used different levels of soybean hulls (2.5, 5, and 7.5%) in the diet. Soybean hulls contain both soluble and insoluble fiber portions and the soluble portion of fiber lowers the cholesterol by binding to it in the small intestine and preventing it from entering the blood stream and exiting the body through the feces. The values obtained in the present study for hematology and serum biochemistry parameters fell within the normal ranges as reported by Morton et al. (1994). According to Esonu et al. (2010), animals’ physiological responsiveness to their internal and external environments serves as a useful tool for tracking their health and it was described that the inclusion of 30% soybean hull (with/ without enzyme addition) had no significant effect on the internal physiology of the layers. Similarly in the present study, it followed that up to 9% inclusion of soybean hull in the feed with enzyme (β-mannanase) had no adverse effect on the internal physiology of the laying hens. The total revenue was calculated higher in the T2 group while the profit and cost-benefit ratio had a higher value in the CON group. Similarly, Esonu et al. (2006) also recorded negative feed cost savings and higher feed costs when using 10 and 20% soybean hull meal and cellulitic enzyme (Safzyme) 0.1% in the broiler finisher diet. Sousa et al. (2019) also recorded higher feed cost per egg carton when using different fiber sources (soybean hulls and coffee husks) and enzyme (xylanase) 0.075 g/kg in the laying hens feed. The amount of improvement attained by including enzymes in the diet depends on a variety of factors, including the type and quantity of cereal in the diet, the amount of a potentially variable anti-nutritive ingredient in a particular cereal, the range and quantity of the enzymes used, the kind of animal, the animal’s age, the bird’s physiology and the kind of gut microflora that is present (Bedford, 1996). The higher total revenue in the T2 group is due to the higher egg production. The control group had comparatively higher profit which is due to the lower feed intake in the CON group than all other treatment groups. The use of dietary soybean hull and enzyme in layer feed depends specifically on the market prices of the bird, egg, enzyme, and feed at the relevant time.
CONCLUSIONS
The findings of the present study showed overall better production performance and economics in a diet containing 3% soybean hull along with β-mannanase at 20mg/kg in comparison with other soybean hull and enzyme-treated groups. Therefore, 3% SH along with β-mannanase at 20mg/kg in feed is recommended for laying hens at the early peak production period.
Acknowledgement
Authors acknowledge the staff of the Department of Poultry Science and Faculty of Animal Husbandry and Veterinary Science (FAHVS), The University of Agriculture, Peshawar, Pakistan, and Sadiq Brother (SB) (Rawalpindi, Punjab) who provided technical and laboratory facilities.
Animal welfare statement
This study was approved by the animal welfare and care committee of the Faculty of Animal Husbandry and Veterinary Sciences, The University of Agriculture, Peshawar, Pakistan, and all the measures and tools was considered to minimize the pain and discomfort of birds during the conduction of this experiment.
Funding
This study was financially supported by the Higher Education Commission (HEC) of Pakistan through (HEC Indigenous Scholarship) grant.
IRB approval
The study was approved by the Advanced Studies and Research Board (ASRB), The University of Agriculture, Peshawar (No.1145/ASRB/UAP) dated 22/07/2020.
Ethical statement
The study was approved by the Ethical Committee of the Faculty of Animal Husbandry and Veterinary Sciences (FAHVS), The University of Agriculture Peshawar, before the conduction of this experiment.
Statement of conflict of interest
The authors have declared no conflict of interest.
REFERENCES
Abdel-Hafeez, H.M., Saleh, E.S.E., Tawfeek, S.S., Youssef, I.M.I. and Abdel-Daim, A.S.A., 2018. Utilization of potato peels and sugar beet pulp with and without enzyme supplementation in broiler chicken diets: Effects on performance, serum biochemical indices and carcass traits. J. Anim. Physiol. Anim. Nutr., 102: 56-66. https://doi.org/10.1111/jpn.12656
Abreu, M.T., Fassani, E.J., Silveira, M.M.B.M. and Viveiros, M.P., 2018. Enzymatic complex based on xylanase, beta-glucanase and phytase on feeds for light commercial layers at peak production. Anim. Ind. Bull., 75: 17-24. https://doi.org/10.17523/bia.v75n1p17
Almirall, M., Brufau, J., and Esteve-Garcia, E., 1993. Effects of intestinal viscosity on digestive enzyme activities at different ages supplemented with β-glucanases. In: Proceedings of the 1st symposium of enzymes in animal nutrition. Kartause Ittingen, Switzerland, pp. 69-72.
Bedford, M.R., 1996. The effect of enzymes on digestion. J. appl. Poult. Res., 5: 370-378. https://doi.org/10.1093/japr/5.4.370
Bojarpou, M., 2020. The effect of different levels of soybean hull on performance, carcass characteristics and blood parameters in broiler chickens. Res. Anim. Prod., 11: 1-9. http://rap.sanru.ac.ir/article-1-1013-en.htm.
Campbell, T.W., 1995. Avian hematology and cytology (No. Ed. 2). Lowa State University Press. ISBN: 9780813829708.
Carre, B., Lessire. M. and Juin, H., 2013. Prediction of metabolisable energy value of broiler diets and water excretion from dietary chemical analyses. Animal, 7: 1246-1258. https://doi.org/10.1017/S1751731113000359
Creswell, D.C., 1994. Upgrading the nutritional value of grains with the use of enzymes. Technical bulletin American Soybean Association. 341 Orchard Road No. 11-03 Liat Towers, Singapore. Viana.
Danang, B. and Rostini, T., 2016. The effect of protease enzyme supplementation to productivity eggs of alabio duck. Int. J. Biosci., 8: 203-208. https://repo-dosen.ulm.ac.id//handle/123456789/9816, https://doi.org/10.12692/ijb/8.2.202-208
Esonu, B.O., Iheshiulor, O., Chukwuka, O., Omede., A. and Ogbuewu, I., 2010. Performance characteristics and haematology of laying birds fed Safzyme supplemented soybean hull diet. Rep. Opin., 2: 16-21.
Esonu, B.O., Izukanne, R.O. and Inyang, O.A., 2005. Evaluation of cellulolytic enzyme supplementation on production indices and nutrient utilization of laying hens fed soybean hull-based diets. Int. J. Poult. Sci., 4: 213-216. https://doi.org/10.3923/ijps.2005.213.216
Esonu, B.O., Izukanne, R., Emenalom, O.O., Etuk, E.B., Samuel, S., Ezeoke, F. and Inyang, O.A., 2006. Evaluation and economics of enzyme supplementation on the performance of broiler finishers fed soybean hull meal-based diets. Niger. J. Anim. Prod., 33: 216-221. https://doi.org/10.51791/njap.v33i2.930
Jalal, M.A. and Scheideler, S.E., 2001. Effect of supplementation of two different sources of phytase on egg production parameters in laying hens and nutrient digestibility. Poult. Sci., 80: 1463-1471. https://doi.org/10.1093/ps/80.10.1463
Javer Alves, V.F., Geraldo, A., Machado, L.C., Brito, J.A.D., Bertechini, A.G. and Murakami, E.S.F., 2015. Effect of protease supplementation on the production performance of laying hens. Anim. Sci., 37: 29-33. https://doi.org/10.4025/actascianimsci.v37i1.22830
Jiménez-Moreno, E., Coca-Sinova, A.D. and González-Alvarado, J., 2016. Inclusion of insoluble fiber sources in mash or pellet diets for young broilers. Effects on growth performance and water intake. Poult. Sci., 95: 41-52. https://doi.org/10.3382/ps/pev309
Jiménez-Moreno, E., González-Alvarado, J.M., González-Serrano, A., Lázaro, R. and Mateos, G.G., 2009. Effect of dietary fiber and fat on performance and digestive traits of broilers from one to twenty-one days of age. Poult. Sci., 88: 2562-2574. https://doi.org/10.3382/ps.2009-00179
Lima, M.R., Silva, J.H.V., Araujo, J.A., Lima, C.B. and Oliveira, E.R.A., 2007. Enzimas exógenas na alimentação de aves. Acta Vet. Brasil., 1: 99-110.
Mathlouthi, N., Mohammed, M.A. and Larbier, M., 2003. Effect of enzyme preparation containing xylanase and β-glucanase on the performance of laying hens fed wheat/ barley or maize/ soybean meal-based diets. Br. Poult. Sci., 44: 60-66. https://doi.org/10.1080/0007166031000085374
Meng, X. and Slominski, B.A., 2005. Nutritive values of corn, soybean meal, canola meal, and peas for broiler chickens as affected by a multi carbohydrase preparation of cell wall degrading enzymes. Poult. Sci., 84: 1242–1251. https://doi.org/10.1093/ps/84.8.1242
Mielenz, J.R., Bardsley, J.S. and. Wyman, C.E., 2009. Fermentation of soybean hulls to ethanol while preserving protein value. BioResour. Technol., 100: 3532-3539. https://doi.org/10.1016/j.biortech.2009.02.044
Morton, D.B. and Jennings, M., 1994. Removal of blood from laboratory mammals and birds-reply. Lab. Anim., 28: 179-179. https://doi.org/10.1258/002367794780745317
Ribeiro, T., Lordelo, M.M.S., Ponte, P.I.P., Maçãs, B., Prates, J.A.M., Aguiar, F.M., Falcão, L., Freire, J.P.B., Ferreira, L.M.A. and Fontes, C.M.G.A., 2011. Levels of endogenous β-glucanase activity in barley affect the efficacy of exogenous enzymes used to supplement barley-based diets for poultry. Poult. Sci., 90: 1245-1256. https://doi.org/10.3382/ps.2010-01218
Ritchie, B., Harrison, J. and Harrison, R., 1994. Avian medicine. Winger’s Pul. Inc., Lake Worth, FL, USA.
Rojas, M.J., Siqueira, P.F., Miranda, L.C., Tardioli, P.W. and Giordano, R.L., 2014. Sequential proteolysis and cellulolytic hydrolysis of soybean hulls for oligopeptides and ethanol production. Ind. Crops Prod., 61: 202-210. https://doi.org/10.1016/j.indcrop.2014.07.002
Shuaib, M., Hafeez, A., Chand, N. and Tahir, M., 2022. Effect of dietary inclusion of soybean hull on production performance and nutrient digestibility during peak egg production period with different phases in laying hens. Pakistan J. Zool., 55: 397-405. https://doi.org/10.17582/journal.pjz/20211105091115
Silversides, F.G., Scott, T.A., Korver, D.R., Afsharmanesh, M. and Hruby, M., 2006. A study on the interaction of xylanase and phytase enzymes in wheat-based diets fed to commercial white and brown egg-laying hens. Poult. Sci., 85: 297-305. https://doi.org/10.1093/ps/85.2.297
Sousa, L.S.D., Carvalho, T.S.M., Nogueira, F.A., Saldanha, M.M., Vaz, D.P., Bertechini, A.G., Lara, L.J.C., 2019. Fiber source and xylanase on performance, egg quality, and gastrointestinal tract of laying hens. Rev. Brasil. Zoot., 48: 2019. https://doi.org/10.1590/rbz4820170286
Steel, R.G.D., Torrie, J.H. and Dickey, D.A., 1997. Principles and procedures of statistics, a biometrical approach. 3rd ed. McGraw Hill Book Press, New York. pp. 666.
Walters, H.G., 2019. Evaluating the efficacy of exogenous enzymes on nutrient digestibility and broiler performance (Doctoral dissertation), Texas A&M University, College Station, TX 77843, United States. https://hdl.handle.net/1969.1/186359.
To share on other social networks, click on any share button. What are these?










