Effect of Adding an Aqueous Extract of Aloe Vera (Aeav) to Tris Extender on some Characteristics of Goat Epididymal Spermatozoa at Different Cooling Times
Research Article
Effect of Adding an Aqueous Extract of Aloe Vera (Aeav) to Tris Extender on some Characteristics of Goat Epididymal Spermatozoa at Different Cooling Times
1Department of Animal Production Techniques, Technical Institute of Kufa, Al-Furat Al-Awsat Technical University, Iraq; 2University of Al-Muthanna, Faculty of Agriculture, Department of Animal Production, Al-Muthanna, Iraq.
Abstract | Plant extracts have been used as alternative additives for the semen preservation process in different farm animal species to improve semen properties such as viability, motility, and membrane integrity, which thereby increase sperm fertility. The current study was conducted to investigate the effect of adding an aqueous extract of Aloe vera leaf gel (AEAV) to the epididymal spermatozoa of local goats during the cooling process for different periods. The AEAV was prepared and stored in the refrigerator until the time of use. Epididymal spermatozoa were diluted by a Tris extender and divided into three treatments: T1 with a Tris extender only (control group), T2: 0.02 AEAV, and T3: 0.03 AEAV. Epididymal sperm were cooled at 5 ºC for 0, 24, 48, 72, 96, and 120 hours. The results show a significant effect (P≤0.01) of AEAV on sperm motility, abnormal sperm percentage, dead sperm percentage, and membrane integrity. The overall means were 51.11 %, 65.55 %, and 60.55%, for motility, 4.00 %, 4.33 %, and 1.5%, for abnormal sperm, 19.80 %, 17.64 %, and 19.33%, for dead sperm, and 74.25%, 78.63%, and 78.33%, for membrane integrity, respectively, for T1, T2, and T3. No significant differences were observed for dead sperm among 0.03 AEAV and the control group, abnormal sperm among 0.02 AEAV and the control, and also for HOST, between 0.02 and 0.03 AEAV. The best interaction between AEAV levels and cooling periods was reported for 0.03 of AEAV in fresh semen at 0 hrs. for all parameters of this study, except the HOST which did not differ at 0 hrs. of cooling among all AEAV levels. On the other hand, the results of the present study indicated significant differences (P≤0.01) for all six cooling periods (hrs.), except for abnormal sperm among 72, 96, and 120 hrs. post-cooling. It could be concluded that adding two levels (2% and 3%) of an aqueous extract of Aloe vera gel has improved some characteristics of goat epididymal sperm preserved under cooling conditions for different periods.
Keywords | Aloe vera, AEAV, Semen characteristics, Local buck, Cooling period
Received | April 05, 2024; Accepted | July 18, 2024; Published | August 15, 2024
*Correspondence | Safaa Sabbar Atiyah, Department of Animal Production Techniques, Technical Institute of Kufa, Al-Furat Al-Awsat Technical University, Iraq; Email: safaa.sabbar.iku@atu.edu.iq
Citation | Atiyah SS, Al-Sadoon AAZ, Jieish SK (2024). Effect of adding an aqueous extract of aloe vera (aeav) to tris extender on some characteristics of goat epididymal spermatozoa at different cooling times. Adv. Anim. Vet. Sci. 12(9): 1810-1817.
DOI | https://dx.doi.org/10.17582/journal.aavs/2024/12.9.1810.1817
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright: 2024 by the authors. Licensee ResearchersLinks Ltd, England, UK.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
INTRODUCTION
Semen preservation by chilling is considered a fundamental step of artificial insemination (AI) (Rahman et al., 2014) of farm animals. The harmful effects of sperm preservation in goats are due to the integrity of the cell membrane, as the cell membrane contains polyunsaturated fatty acids that are quickly oxidized, and the number of antioxidants in sperm cells is lower than in other cells (El-Hadary et al., 2023) thus, the susceptibility to the effect of oxidative stress is greater on sperm cells (Khalique et al., 2023). Preservation of semen using alternative additives such as plant extracts have been used in different farm animal species to improve semen quality, which thereby increases sperm fertility. Aloe vera is a medicinal plant that contains specific bioactive ingredients, including proteins, lipids, amino acids, alkaloids, saponins, flavonoids, enzymes, organic and inorganic compounds (Hamman, 2008; Akaberi et al., 2016, Lopez-Cervantes et al., 2018), vitamins C, B (1, 2, and 6), A, and E (Daniel, 2024), and mineral salts like sodium, calcium, iron, potassium, chloride, manganese, copper, and zinc (Sánchez et al., 2020; Ebrahim et al., 2020; Darzi et al., 2021; Saleem et al., 2022; Kamble et al., 2022). The physical parameters of a fresh Aloe vera leaf, including length, width, and gel weight, were conducted by Talukdar et al., (2023). The protective effect of Aloe vera on cooling semen has been determined in goats (Aguiar et al., 2012), cattle (Farias et al., 2019), dogs (Melo et al., 2014), and peccaries (Souza et al., 2016). Mona et al. (2023) indicated that adding Aloe vera raw gel extract at 10μg/ml to bovine spermatozoa showed increased sperm hyper-motility and acrosome reaction and produced the highest percentage of fertilized oocytes. According to Agbaye et al. (2023), adding 7.00 g/L of Aloe vera leaf gel to extended goat semen affected fertilizing capability and maintained spermatozoa motility and plasma membrane integrity by more than 60% for 72 hours. By enhancing sperm parameters and promoting spermatogenesis, Aloe vera showed strong spermatogenic activity. aloe vera seems to be an alternate plant-based agent for using in the preservation of sperm, particularly because of its rich compound and the existence of polysaccharides derived from it that have cryo-protective properties (Chauhan et al., 2014; Zhang et al., 2018), as well as a superior antibiotic that can enhance reproductive performance and animal development (Daniel, 2024). There are not many authors discussing how leaf extracts affect sperm properties. The aim of this study is to investigate the effect of adding aqueous extract of Aloe vera gel with two concentrations (2 and 3%) in goat epididymal spermatozoa cooled at 5 ºC and preserved for six storage periods (24, 48, 72, 96, and 120 hrs.) on percentages of sperm motility, morphological abnormal sperm, dead sperm, and hypo-osmotic swelling test (HOST).
MATERIALS AND METHODS
The current study was conducted in the graduate laboratories of the College of Agriculture, Al-Muthanna University, Department of Animal Production, from January 2021 until May 2022. This study was conducted in accordance with the instructions and procedures required in the postgraduate laboratory and approved by the Ethics Committee of Agriculture College, University of Al-Muthanna, ethical approval number 154LBI04006.
Collection Testes and Semen Preparation
The testes were collected from adult bucks immediately after slaughter, where they were cut together with the scrotum and then transported to the laboratory using a container containing ice cubes. The testes were removed and the epididymis was dissected. By flushing, the sperm were extracted from the cauda (Martinez-Pastor et al., 2006). After that, 100 ml of media was prepared, adding 63.3 g of Tris, 1.99 g of Citric Acid, 5 g of sucrose, and small percentages of antibiotics (Pwnicillin and Streptomycin). All of these materials are added to 80 ml of distilled water. After that, the medium is filtered in two batches using a Melbourne filter (22 and 45 mm), after which the pH of the medium is adjusted between 7.2 and 7.4.
Preparation of Aqueous Extract of Aloe Vera (AEAV)
Fresh Aloe vera leaves were harvested and washed; the outer layers were carefully peeled with forceps to prevent staining of the colorless, viscous inner gel. The gel was carefully scooped with a spatula into a beaker, homogenized, covered with foil paper, and kept at a temperature of 37 °C in a water bath. After that, 100 grams of raw gel were collected and mixed with 25 ml of distilled water using an electric mixer for one minute. The mixture was left for 12 hours with constant stirring using an electric shaker, then placed in the incubator at 37 °C until it was completely dry and stored for use in the refrigerator. After preparing the Tris medium in the manner mentioned above, two concentrations of AEAV, 0.02 and 0.03, were added to 4 ml of Tris solution and 1 ml of egg yolk, and the pH of the medium was measured. Then the medium was left in the incubator to warm, and then the sperm were added and examined.
Semen Evaluation
Sperm Motility: The semen was diluted using Tris diluent, and individual motility was estimated according to what was stated by Chemineau et al. (1991) by taking a drop of the diluted semen, placing on a glass slide, and placing the cover of the slide on the sample at a temperature of 37 °C. It was examined under a microscope at 40x magnification, and the motility was calculated based on the ratio. The percentage of sperm with forward movement and the strength and speed of their movement
Table 1: Effect of aloe vera concentration, Cooling Periods (hrs.) and their interaction on the individual motility of sperms (%).
|
AEAV Con.(%) |
Cooling Periods (hrs.) Mean± Stander Error |
Mean |
Sig. |
|||||
|
0 |
24 |
48 |
72 |
96 |
120 |
|||
|
0 |
76.66±1.66 A b |
70.0±2.88 B c |
61.66±4.40 C c |
46.66±4.40D c |
31.66±4.40 E c |
20.00±2.88 F c |
51.11 c |
** |
|
0.02 |
86.66±1.66 A a |
81.66±1.66 A a |
73.33±1.66 B a |
63.33±1.66 C a |
50.00±0.0 D a |
38.33±1.66 E a |
65.55 a |
|
|
0.03 |
86.66±1.66 A a |
76.66±1.66 B b |
66.66±1.66 C b |
56.66±1.66 D b |
45.00±0.00 E b |
31.66±1.66 F b |
60.55 b |
|
|
Mean |
83.33 a |
76.11 b |
67.22 c |
55.55 d |
42.22 e |
30.00 f |
||
|
Sig. |
** |
|||||||
Averages with small letters within one column indicate a comparison between transactions, and averages with capital letters within one row indicate a comparison between different periods within one transaction. ** : P≤0.01.
Abnormal Sperm Percentage: The percentage of morphological abnormal sperm was estimated based on Hancock (1951) using the same slide for estimating the percentage of dead sperm, and it was examined with a microscope at 40x magnification according to the following equation:
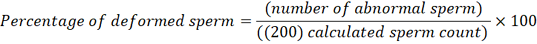
Dead Sperm Percentage: The percentage of dead sperm was estimated based on what was stated by Swanson and Beardon (1951) by taking a drop of fresh semen and placing it on a clean glass slide at a temperature of 37 °C. Then one drop of a mixture of eosin dye and necrocin (2:1) was added, and the two drops were mixed well and gently using a glass slide, made a smear on another glass slide at an angle of 45 degrees and examined it under a microscope with strong magnification (400x).
Hypo-osmotic Swelling Test (HOST): The percentage of plasma membrane integrity was estimated based on what was stated by Lomeo and Giambersio (1991) by taking 10µl of semen and 1 ml of distilled water (mOsm 0 /l, pH 7.02) and incubating the sample at a temperature of 37 °C for five minutes. Then, immediately post-incubation, we took a drop of semen and placed it on a clean glass slide at a temperature of 37 °C with a cover slide on the sample and examined under a microscope (400X).
Statistical Analysis
The data was analyzed using the Statistical Analysis System (SAS, 2012) to investigate the impact of various factors on the examined characteristics according to a factorial experiment (3×6) applied in a complete random design (CRD) according to the mathematical model below, and the significant differences between the means were compared with a polynomial test Duncan (Duncan, 1955) with a 0.01 probability of error. mathematical model:
yij = Ai + Bj + AB + eij
RESULTS AND DISCUSSION
Sperm Motility
Table 1 shows the effects of AEAV, storage time, and the interaction between them on the individual motility of spermatozoa in goat semen chilled at 5 °C. Results indicate a significant effect (P≤0.01); the highest rate was noticed in the first concentration of AV. Namely, 65.55% compared with the lowest rate that was noticed in the control group namely, 51.11%. The storage period affected individual motility, the least rate was reported in the 6th period (30.0%) compared with the highest rate reported in fresh semen (83.33%). The results indicated that the best interaction was reported in fresh semen at 0.02 and 0.03 of AV. namely 86.66%, while the lowest rate was reported in sperm motility at control at the 6th storage period, about 20%. The enhancement of sperm motility continues at 48, 72, 96, and 120 hours of storage by adding 0.02 and 0.03 AV compared with the control group.
Abnormal Sperm Percentage
The research results demonstrated that 3% AEAV had a significant effect (P<0.01) on the morphological abnormal sperm percentage. While there were no significant differences among AEAV at 3% and the control group (Table 2), the overall means were 4.00%, 4.33%, and 1.50% for T1, T2, and T3, respectively. Storage time was differed significantly (P<0.01) on abnormal sperm percentage, as shown in Table 2. Throughout all storage intervals, the mean percentages of abnormal sperm varied and declined with longer storage times.. There were no significant differences in mean percentages 72, 96, and 120 hours post-chilling, they were 4.72%, 4.94%, and 4.83%, respectively. The abnormal sperm (%) was significantly (P<0.01) impacted by the interaction between AEAV and storage time, except after 48 and 72 hours. after storage, where it showed similar percentages between the control and 3% AEAV (Table 2).
Dead Sperm Percentage
Results show a significant effect of AEAV concentrations on dead sperm percentage (%); the highest rate was noticed for T1 (control) and T3 (0.03), compared with the lowest
Table 2: Effect of aloe Vera concentration, Cooling Periods (hrs.), and their interaction on the Abnormal sperm percentage (%).
|
AEAV Con.(%) |
Cooling Periods (hrs.) Mean± Stander Error |
Mean |
Sig. |
|||||
|
0 |
24 |
48 |
72 |
96 |
120 |
|||
|
0 |
2.50±1.80 D b |
4.33±1.09 B a |
4.00±1.04 B a |
3.33±0.16 C c |
4.83± 0.16 A b |
5.00±1.06 A b |
4.00 a |
** |
|
0.02 |
2.66±1.16 D a |
2.66±1.16 D b |
3.66±1.01 C b |
5.00±1.32 B b |
6.50±2.17 A a |
5.50±1.25 B a |
4.33 a |
|
|
0.03 |
1.50±1.25 E c |
2.33±0.88 D c |
4.00±0.28 B a |
5.83±1.20 A a |
3.50±0.57 C c |
4.00±1.25 B c |
1.50 c |
|
|
Mean |
2.22 b |
3.11 ab |
3.88 ab |
4.72 a |
4.94 a |
4.83 a |
||
|
Sig. |
** |
|||||||
Averages with small letters within one column indicate a comparison between transactions, and averages with capital letters within one row indicate a comparison between different periods within one transaction. ** : P≤0.01.
Table 3: Effect of aloe Vera concentration, Cooling Periods (hrs.), and their interaction on the Dead sperm percentage (%).
|
AEAV Con.(%) |
Cooling Periods (hrs.) Mean± Stander Error |
Mean |
Sig. |
|||||
|
0 |
24 |
48 |
72 |
96 |
120 |
|||
|
0 |
2.00±1.15 F b |
6.16±0.92 E a |
11.66±0.60 D b |
21.33±0.72 C a |
33.66±0.44 B a |
44.00±2.64 A a |
19.80 a |
** |
|
0.02 |
1.33±0.88 F c |
4.66±0.72 E b |
11.16±0.83 D b |
18.83±2.18 C b |
29.00±1.50 B b |
40.83±1.20 A b |
17.64 b |
|
|
0.03 |
2.16±1.01 E a |
6.16±0.88 D a |
12.33±1.16 C a |
22.16±1.66 B a |
29.00±0.50 B b |
40.66±1.20 A b |
19.33 a |
|
|
Mean |
1.83 f |
5.66 e |
11.72 d |
20.77 c |
31.72 b |
41.83 a |
||
|
Sig. |
** |
|||||||
Averages with small letters within one column indicate a comparison between transactions, and averages with capital letters within one row indicate a comparison between different periods within one transaction. ** : P≤0.01.
Table 4: Effect of aloe Vera concentration, Cooling Periods (hr.), and their interaction on the HOST (%).
|
AEAV Con.(%) |
Cooling Periods (hrs.) Mean± Stander Error |
Mean |
Sig. |
|||||
|
0 |
24 |
48 |
72 |
96 |
120 |
|||
|
0 |
95.00±0.28 A a |
88.00±0.57 B b |
79.66±0.88 C b |
73.00±1.04 C b |
60.66±1.36 D b |
49.16±0.72 E b |
74.25 b |
** |
|
0.02 |
95.50±0.28 A a |
89.83±0.16 B ab |
84.00±0.28 B a |
75.83±1.01 C ab |
67.83±1.76 D a |
58.83±1.30 E a |
78.63 a |
|
|
0.03 |
96.66±0.44 A a |
90.00±0.57 B a |
84.33±0.92 C a |
76.33±1.69 D a |
68.16±0.88 E a |
57.17±1.58 F a |
78.33 a |
|
|
Mean |
95.72 a |
89.27 b |
82.66 c |
75.05 d |
65.55 e |
55.05 f |
||
|
Sig. |
** |
|||||||
Averages with small letters within one column indicate a comparison between transactions, and averages with capital letters within one row indicate a comparison between different periods within one transaction. ** : P≤0.01.
rate for T2 (0.02) (Table 3). Storage period had a significant effect on dead sperm percentage, least rate was reported in fresh semen (1.83%), compared with the highest rate reported in the 6th period (41.83%).There was a significant effect of interaction between AEAV concentrations and storage period, results indicated that the best interaction was reported in fresh semen at T3 (2.16±1.01) compared to the rate reported in semen with T1 concentration and 6th storage period (44.00±2.64). The percentage of dead sperm was significantly (P<0.01) influenced by the interaction between AEAV and storage intervals, except after 72 hrs. among T1, and T3, and also after 96 and 120 hrs. among all treatments, where it showed similar percentages (Table 3).
Hypo Osmotic Swelling Test (Host)
The HOST percentage results are shown in Table 4. Adding AEAV to Tris diluent was significantly increasing (P≤0.01) on the HOST test at T2 and T3 as compared to the control treatment, mean was 78.63%, 78.33%, and 74.25% for T2, T3, and T1, respectively. Storage time had significant effects (P<0.01) on HOST test percentages in all six periods of storage, with the best and highest rate noticed at zero time (95.72%), and then gradual decreasing up to 120 hrs. (55.05%), as obtained in Table 2. The HOST test was significantly affected (P<0.01) by the interaction between AEAV and storage times, except after 48, 72, 96, and 120 hours after storage, where it showed similar percentages between the two concentrations of AEAV (Table 2). There were no significant differences in mean percentages 72, 96, and 120 hrs. post- chilling, which were 4.72%, 4.94%, and 4.83%, respectively. Similar results were also observed for all treatments at zero time.
The current investigation assessed the effects of two distinct Aloe vera leaf gel concentrations (0% (control), 2%, and 3%) in Tris-egg yolk extender on local buck semen stored at 5 °C for 120 hours. The results of this study showed a significant difference (P<0.01) in the sperm motility, abnormal sperm percentage, dead sperm percentage, and HOST test from 24 throughout the 120 hrs. post cooling process. Many studies provide evidence of the importance of adding plant extracts to semen during different times of storage (Zhao et al., 2009; Neamah and Houbi, 2020; Neamah, 2023), including Aloe vera extract gel for chilling epididymal spermatozoa (Farias et al., 2019; Zareie et al.,2021; Seshoeni, 2023). Another study showed adding 2, 4, and 6% of Salvia Rosmarinus extract to the goat semen at cooling conditions enhanced integrity of the plasma membrane and sperm motility, viability, and reduction of the MDA level by 2 % (Zanganeh et al., 2013). In agreement with our findings, Singh et al. (2020) observed a significant difference between the Aloe vera and control group in terms of post-thaw% progressive motility, live spermatozoa, acrosomal integrity, sperm abnormalities, and HOST test. Also, our findings support the outcomes of Fakhrildin and Sudani (2014), who found that Aloe vera positively affects sperm parameters, including a highly significant increase in sperm motility, viability, HOST test, and morphological abnormal sperm. In contrast, our results did not agree with Olugbenga et al. (2011) that adding Aloe vera extract as a supplement (3 and 4% Aloe vera extract solution) increases dead sperm percentage abnormalities and decreases sperm motility and live sperm percentage in the buck semen while having no effect of Aloe vera extract on the semen color. The majority of plant species, including Aloe vera, have high levels of antioxidants, which can reduce the negative impacts of oxidative stress on sperm function by acting as ROS scavengers (Santaella and Pintus, 2021). Antioxidants are frequently added to semen extenders and are used for both cooling and cryopreservation storage of sperm (Mona et al., 2023). This could explain the high significant percentages in all parameters obtained in this study. Our research results are in agreement with Zareie et al. (2021), which showed that using 20 μg/ml of Aloe vera extract in the extender significantly improved membrane integrity, progressive motility, and total motility when compared to the control treatment. On the other hand, the results conflict with those of (Meena et al., 2019), who found that sperm motility is inhibited by Aloe vera leaf extract. there could be a difference in the experimental circumstances causing this disagreement. According to Melo et al. (2014), sperm motility may be considerably increased when cat spermatozoa are incubated with an extender containing AEAV for longer than an hour. Therefore, dilution of spermatozoa with AV. need a longer time for adaptation, which may be attributed to the mucilaginous property found in Aloe vera gel. In the present study, we did not observe this case, and the reason may be attributed to the species of animal, the individual differences in the sperm, or the physiological characteristics of the sperm from one type to another, or the reason may be due to the conditions of planting and irrigation of the plant, which may affect the mucilage property of Aloe vera. Results of the present study do not agree with Farias et al. (2017), who postulated that the use of AV extract at intermediate concentrations (above 10% but less than 20%) might have a better effect on sperm cells due to the plant extract’s high viscosity, which can limit sperm movement in viable cells. According to Mona et al. (2023), a high IVF rate in Holstein bulls was produced by adding 10 µg/ml of Aloe vera extract to bull spermatozoa, which was found to be the most effective concentration for improving sperm capacitation. High significant (p≤0.01) differences were found on the sperm abnormality percentage at various cooling periods within each group (Table 2). There were high (p≤0.01) significant variation between storage periods for all groups. The results indicated a significant decrease (p≤0.01) in the abnormal and dead sperm percentages, and the reason that may be attributed to using the aqueous extract of the Aloe vera plant is that the leaves do not only contain carbohydrates found in the wall of the ornament, such as cellulose and hemicellulose, but they also contain stored carbohydrates, such as acetyl manna (Ni et al., 2004). The presence of abnormal sperm in this work is consistent with the report of Jones, (2004) that several abnormal sperm are normally in all ejaculates. The decrease in semen properties observed in the present study during storage at 5°C may be due to a combination of factors, such as partially irreversible damage to the epididymal sperm membranes that leads to loss of vital cell components and thus a reduced metabolic rate of sperm. (Holt, 2000 ; De Pauw et al., 2003; Lima et al., 2013). Zareie et al., (2021) suggested adding the extract of Aloe vera improved the goat sperm motility during storage, and the antioxidant activity of Aloe vera led to a decrease in the malondialdehyde level. Yong et al. (2017) recorded that fish semen treated with Aloe vera extract enhanced sperm motility post-cryopreservation. Also, Aloe vera as a supplement increases the rat’s fertility (Jasem and Nasim, 2011). These studies show that Aloe vera plays a role in stopping sperm oxidation and can save sperm motility during storage. According to the reports, this plant contains zinc and the acid folic, which act as antioxidants and can enhance sperm quality by reducing semen apoptosis (Zareie et al., 2021). A study by Almasry et al., (2023) showed that adding Aloe vera (2 ml) to 1000 ml of drinking water enhances the semen quality. This effect is attributed to the components of Aloe vera, which are rich in antioxidants, as they work to neutralize the free radicals formed, as well as the fact that Aloe vera contains a special polysaccharide compound consisting mainly of mannose sugar, as it interacts with cell surface receptors, stimulating them and activating their faster growth and reproduction (Chandan et al., 2007). Buck spermatozoa are negatively affected by preservation because of the integrity of their cell membrane, which contains highly polyunsaturated fatty acids that quickly oxidize. In comparison to other cells, sperm cells have fewer antioxidants, thus, oxidative stress can more easily damage sperm cells (Khalique et al., 2023). There were significant differences among concentration of AEAV and the control group regarding conservation time for the sperm characteristics under study. The impact of various extenders at 4 ºC on sperm properties in ejaculated sperm from various species is widely recognized (Azawi et al., 1993; Mara et al., 2007). The composition of the extender has been linked to the differences in this investigation. The current findings clearly show that an extender based on aloe vera provides a better suitable medium to maintain the viability of epididymal sperm storage at 5 °C, as it may preserve sperm properties for up to 120 hours after chilling, The AV. composition could explains these best results. Goat semen extenders typically contain egg yolk and AEAV (Campos et al., 2003; 2004). Because of the low density lipoprotein portion (Moussa et al., 2002), egg yolk is well known for protecting sperm membranes against heat shock during storage at 5 ºC (Watson and Martin, 1975; De Leeuw et al., 1993). One of the limitations of this study is to determine the effectiveness of using Aloe vera extract in goat epididymal sperm cryopreservation, as well as the ideal viscosity of the extract. We also recommend conducting further studies on the use of Aloe vera extract in in-vitro fertilization and artificial insemination.
CONCLUSIONS AND RECOMMENDATIONS
The current study indicates that adding an aqueous extract of Aloe vera gel with two different concentrations has improved some characteristics of goat epididymal sperm preserved under cooling conditions for different periods. Aloe vera can be an alternative extender of plant origin for the preservation of goat semen, as it protects the sperm membranes, which are necessary for their viability and applicability in artificial insemination programs. According to the experiment’s findings, the use of AEAV in the extender of goat epididymal spermatozoa has a positive effect on cell protection during the cooling process. However, new studies need to be carried out in order to evaluate the addition of AEAV during cryopreservation and also after the thawing process.
ACKNOWLEDGMENTS
All thanks and gratitude to the staff of the Animal Production Department and Postgraduate Studies Laboratory at the College of Agriculture at Al-Muthanna University for providing all facilities to complete this experiment. Also, the authors introduce all their thanks and appreciation to the management and staff of the slaughterhouse that belongs to the veterinary hospital in Muthanna Governorate for their cooperation in completing the collection of testes.
NOVELTY STATEMENT
Based on our knowledge, we believe that there are few or no studies that have examined the effect of adding an aqueous extract of Aloe vera to Tris diluent on some characteristics of local epididymal sperm in goats for different cooling periods (0, 24, 48, 72, 96, and 120 hours.) at low concentrations of AEAV (2 and 3%).
AUTHOR’S CONTRIBUTIONs
All authors contributed equally.
Conflict of Interest
The authors declare that there is no conflict of interest.
REFRENCES
Agbaye FP, Alaba O, Sokunbi OA (2023). Effects of raw Aloe barbadensis leaf gel on quality and fertilising potential of extended semen from Red Sokoto bucks. Nige. J. Anim. Prod., 50(1): 152-160. https://doi.org/10.51791/njap.v50i1.3912
Aguiar GV, Santos BMB, Cavalcante JMM, Rodrigues FRN, Salgueiro CCM, Nunes JF (2012). Addition of Aloe vera to powder coconut water solution (ACP-101®) like cryoprotector of the goat semen cooled at 4°C. Ciênc Anim., 22: 292-295.
Akaberi M, Sobhani Z, Javadi B, Sahebkar A, Emami SA (2016). Therapeutic effects of Aloe spp. in traditional and modern medicine: a review. Biomed. Pharma., 31: 759-772. https://doi.org/10.1016/j.biopha.2016.09.096
Almasry S, Elsebai A, Elghalid O, El-Hady A, Mohamed A (2023). evaluation of semen quality, seminal biochemical traits, and histological features of aloe vera extract administrated to rabbit bucks. Egypt. Poult. Sci. J., 1;43(4):617-33. https://doi.org/10.21608/epsj.2023.332146
Azawi OI, Al-Dahash SY, Juma FT (1993). Effect of different diluents on Shami goat semen. Small Ruminant Res., 9(4):347-52. https://doi.org/10.1016/0921-4488(93)90012-7
Campos AC, Nunes JF, Monteiro AW, Figueiredo EL, Pinheiro JH, Ferreira MA, Araújo AA (2004). Viabilidade do sêmen caprino lavado e não lavado diluído em água de coco, resfriado e armazenado a 4ºC. Rev. Bras. Ciência Vet., 11(3):178-182. https://doi.org/10.4322/rbcv.2014.363
Campos AC, Nunes JF, Monteiro AW, Pinheiro JH, Ferreira MA, Araújo AA, Cruz JF (2003). Conservação do sêmen caprino a 4ºC durante o período seco e chuvoso no Nordeste do Brasil. Rev. Bras. Reprod. Anim., 27:620-4.
Chandan BK, Saxena AK, Shukla S, Sharma N, Gupta DK, Suri KA, Suri J, Bhadauria M, Singh B (2007). Hepatoprotective potential of Aloe barbadensis Mill. against carbon tetrachloride induced hepatotoxicity. J. Ethnopharmacol., 111(3):560-6. https://doi.org/10.1016/j.jep.2007.01.008
Chauhan SC, Gupta KC, Mukesh Agrawal MA (2014). Application of biodegradable Aloe vera gel to control post-harvest decay and longer the shelf life of Grapes, Int. J. Curr. Microbiol. Appl. Sci; 3 :632–642.
Chemineau P, Cagnie Y, Guerin Y, Orgeur P, Vallet JC (1991). Training Manual on Artificial Insemination in Sheep and Goat. F. A. O. Anim. Prod. Health, Paper No: 83.
Daniel CP (2024). Potential Health Benefits of Aloe vera in Livestock: A Review. J. Appl. Vet. Sci., 9(1): 94-104.
Darzi S, Paul K, Leitan S, Werkmeister JA, Mukherjee S (2021). Immunobiology and application of Aloe vera-based scaffolds in tissue engineering. Int. j. Mol. Sci., 22(4):1708. https://doi.org/10.3390/ijms22041708
De Leeuw FE, De Leeuw AM, Den Daas JH, Colenbrander B, Verkleij AJ (1993). Effects of various cryoprotective agents and membranestabilizing compounds on bull sperm membrane integrity after cooling and freezing. Cryobiology, 30: 32-44. https://doi.org/10.1006/cryo.1993.1005
De Pauw IM, Van Soom A, Mintiens K, Verberckmoes S, de Kruif A (2003). In vitro survival of bovine spermatozoa stored at room temperature under epididymal conditions. Theriogenology, 59: 1093-1107. https://doi.org/10.1016/S0093-691X(02)01207-4
Ebrahim AA, Elnesr SS, Abdel-Mageed MA, Aly MM (2020). Nutritional significance of aloe vera (Aloe barbadensis Miller) and its beneficial impact on poultry. World’s Poult. Sci. J., 76(4):803-14. https://doi.org/10.1080/00439339.2020.1830010
El-Hadary AE, El-Bawab IE, Metwaly KK, El-Rheem A, Samia M (2023). The Effect of Adding Antioxidants on Cooled Zarabi Buck Semen During Different Seasons. Alex. J. Vet. Sci., 79(1). https://doi.org/10.5455/ajvs.164073
Fakhrildin MB, Sodani IJ (1014). Effect of Aloe vera extracts on in vitro human sperm parameters for asthenozoospermic patients. J. Thi-Qar. Sci., 5: 8-13.
Farias CF, Tork AL, Rique AS, Queirós AF, Silva SV (2019). Estudo da eficácia da Aloe vera como crioprotetor vegetal na refrigeração de espermatozoides epididimários de bovinos. Rev. bras. reprod. Anim., 787-94.
Farías-Cervantes VS, Chávez-Rodríguez A, Delgado-Licon E, Aguilar J, Medrano-Roldan H, Andrade-González I (2017). Effect of spray drying of agave fructans, nopal mucilage and aloe vera juice. J. Food Process. Preserv., 41(4): e13027. https://doi.org/10.1111/jfpp.13027
Hamman JH (2008). Composition and applications of Aloe vera leaf gel. Molecules, 13(8), pp.1599-1616. https://doi.org/10.3390/molecules13081599
Hancock JL (1951). The morphology of bull spermatozoa. J. Exp. Biol., 29: 445-553. https://doi.org/10.1242/jeb.29.3.445
Holt WV (2000). Basic aspects of frozen storage of semen. Anim. Reprod. Sci., 62: 3-22. https://doi.org/10.1016/S0378-4320(00)00152-4
Jasem E, Nasim J (2011). Spermatogenic activity Aloe vera in adult male rat. Pharmacology online, 2: 886-889.
Jones R (2004). Sperm survival versus degradation in the mammalian epididymis: a hypothesis. Biol. Reprod., 71(5): 1405-11. https://doi.org/10.1095/biolreprod.104.031252
Kamble SD, Gatade AA, Sahoo AK, Sharma AK (2022). Physico-chemical composition and mineral content of Aloe vera (Aloe barbadensis miller) gel. Int. J. Multi. Educ. Res., 10;11(4):73-9.
Khalique MA, Rehman H, Andrabi SM, Majeed KA, Ahmad N, Fayyaz MH, Haider MS, Naz SS, Qureshi IZ, Sulaiman S (2023). Antioxidant effects of zinc-oxide nanoparticles on post-thaw quality and in vivo fertility of Beetal buck spermatozoa. Small Ruminant Res., 225: p.107012. https://doi.org/10.1016/j.smallrumres.2023.107012
Lima IC, Andrade IR, Aguiar GV, Silva MM, Catunda AG, Martins GA, Gadelha CR, Campos AC (2013). In vitro evaluation of goat cauda epididymal sperm, cooled in different extenders at 4ºC. Arch. zootecnia, 62(239): pp.429-437. https://doi.org/10.4321/S0004-05922013000300011
Lomeo AM, Giambersio AM (1991). Water-test: a simple method to assess sperm-membrane integrity. Int. J. Androl., 14: 278-282. https://doi.org/10.1111/j.1365-2605.1991.tb01093.x
López-Cervantes J, Sánchez-Machado DI, Cruz-Flores P, Mariscal-Domínguez MF, de la Mora-López GS, Campas-Baypoli ON (2018). Antioxidan capacity, proximate composition, and lipid constituents of Aloe vera flowers. J. Appl. Res. Med. Arom. Plan., 10: 93-98. https://doi.org/10.1016/j.jarmap.2018.02.004
Mara L, Dattena M, Pilichi S, Sanna D, Branca A, Cappai P (2007). Effect of different diluents on goat semen fertility. Anim. Reprod. Sci.,102(1-2):152-7. https://doi.org/10.1016/j.anireprosci.2007.02.007
Martinez-Pastor F, Garcia-Macias V, Alvarez M, Chamorro C, Herraez P, de Paz P, Anel L (2006). Comparison of two methods for obtaining spermatozoa from the cauda epididymis of Iberian red deer. Theriogenology, 65: 471-485. https://doi.org/10.1016/j.theriogenology.2005.05.045
Meena D, Suradkar US, Chavhan DM, Singh H, Yadav S (2019). Effect of different level Aloe vera gel and mint extract incorporation in the development of chicken meat cutlets. Int. J. Chem. Stud.,7(1): 964-7.
Melo CC, Oliveira ÉC, Ramos RP, Lima CF, Rodrigues AE, Guerra MM. Renovação do diluidor Tris com gema de ovo ou Aloe vera sp (2014). Na viabilidade do sêmen canino refrigerado a 5°C–resultados preliminares. Acta. Vet. Bras., 8:142–143
Mona Mahmoud, Saber Abd-Allah, Abdel-Halim B.R., and Khalil A.A.Y., 2023. Assessment of Developmental Competence of Holstein Bulls Spermatozoa upon Addition of Aloe vera Raw Extract during In Vitro Capacitation. J. Appl. Vet. Sci., 8(4): 43-53.
Moussa M, Martinet V, Trimeche A, Tainturier D, Anton M (2002). Low density lipoproteins extracted from hen egg yolk by an easy method: cryoprotective affect on frozen-thawed bull semen. Theriogenology, 57: 1695-1706. https://doi.org/10.1016/S0093-691X(02)00682-9
Neamah HJ (2023). Evaluation of Adding Prickly Pear Extracts to the Diluted Ram’s Semen at Preservation. Journal of Animal Health and ProductionThis link is disabled., 11(1): 94–98. https://doi.org/10.17582/journal.jahp/2023/11.1.94.98
Neamah HJ, Houbi AA (2020). Effect of adding astaxanthin and taurine as antioxidants to improve characteristics of awassi ram semen after freezing storage. Plant Arch., 20: 604–608
Ni Y, Turner D, Yates KÁ, Tizard I (2004). Isolation and characterization of structural components of Aloe vera L. leaf pulp. Int. immunopharmacol., 4(14): 1745-1755. https://doi.org/10.1016/j.intimp.2004.07.006
Olugbenga OM, Olukole SG, Adeoye AT, Adejoke AD (2011). Semen characteristics and sperm morphological studies of the West African Dwarf Buck treated with Aloe vera gel extract. Iran. J. Reprod. Med., 9(2):83-8. PMID: 25587252; PMCID: PMC4216440.
Duncan DB (1955). Multiple range and multiple F tests.biometrics. 1; 11(1):1-42.
Olugbenga OM, Olukole SG, Adeoye AT, Adejoke AD (2011). Semen characteristics and sperm morphological studies of the West African Dwarf Buck treated with Aloe vera gel extract. Iran. J. Reprod. Med., 9(2): 83.
Rahman HU, Qureshi M, Khan R. (2014). Influence of dietary zinc on semen traits and seminal plasma antioxidant enzymes and trace minerals of beetal bucks. Rep. rod. Domest. Anim., 49(6): 1004-1007. https://doi.org/10.1111/rda.12422
Saleem A, Naureen I, Naeem M, Murad HS, Maqsood S, Tasleem G (2022). Aloe vera gel effect on skin and pharmacological properties. Scholars Int. J. Anat. Physiol., 5(1):1-8. https://doi.org/10.36348/sijap.2022.v05i01.001
Sánchez M, González-Burgos E, Iglesias I, Gómez-Serranillos MP (2020). Pharmacological update properties of Aloe vera and its major active constituents. Molecules, 13;25(6):1324. https://doi.org/10.3390/molecules25061324
Santaella JL, Pintus E (2021). Plant Extracts as Alternative Additives for Sperm Preservation. Antioxidants, 1: 772. https://doi.org/10.3390/antiox10050772
SAS (2012). Statistical Analysis System, User’s Guide . Statistical. Version 9.1th ed. SAS. Inst. Inc. Cary. N.C. USA.
Seshoeni K (2023). The effect of tris Aloe vera (barbadensis miller) gel on chilled and frozen-thawed bull spermatozoa quality (Doctoral dissertation). University of Venda, College of Science, Engineering and Agriculture.
Singh P, Agarwal S, Singh H, Verma PK, Pandey AK, Kumar S (2020). Antioxidant effects of Aloe vera as semen additive in cryopreservation of cattle bull semen. Int. J. Curr. Microbiol. App. Sci., 9(9): 1625-35. https://doi.org/10.20546/ijcmas.2020.909.202
Souza AP, Lima GL, Peixoto GC, Silva AM, Oliveira MF, Silva AR (2016). Use of Aloe vera–based extender for chilling and freezing collared peccary (Pecari tajacu) semen. Theriogenology, 85(8): 1432-1438. https://doi.org/10.1016/j.theriogenology.2016.01.007
Swanson EW, Beardon HJ (1951). An eosin nigrosin stain differentiating live and dead bovine spermatozoa. J. Anim. Sci., 10: 981-987. https://doi.org/10.2527/jas1951.104981x
Talukdar d, Talukdar p, Lwang ad, Sarma k, Deka d. Sharma d and Das b (2023). phytochemical and Nutrient Composition of Aloe vera (Aloe barbadensis Miller) in an Agro-climatic Condition of Mizoram, India. Asian J. Dairy Food Res., 42(1): 01-08. https://doi.org/10.18805/ajdfr.DR-2047
Watson PF, Martin IC (1975). Influence of some fractions of egg yolk on the survival of ram spermatozoa at 5 ºC. Aust. J. Biol. Sci., 28: 145-152. https://doi.org/10.1071/BI9750145
Yong SY, Nguang SI, Kari A (2017). Comparision between the effect of egg yolkbased extender and Aloe vera (Aloe Barbadensis)-based extender on red tilapia (Oreochromis Niloticus) sperm quality. J. Fund. Appl., 9: 813-820. https://doi.org/10.4314/jfas.v9i2s.50
Zanganeh Z, Zhandi M, Zare-Shahneh A, Najafi A, Nabi MM, Mohammadi-Sangcheshmeh A (2013). Does rosemary aqueous extract improve buck semen cryopreservation?. Small Rumin. Res., 114 (1): 120-5. https://doi.org/10.1016/j.smallrumres.2013.05.015
Zareie K, Farshad A, Rostamzadeh J, Azimi G, Ariyan F (2021). Freezability of Goat Epididymal Sperm using Aloe vera Extract and Trehalose in Diluents. Austin J. Vet. Sci. Anim. Husb., 8(2): 1078.
Zhang Y, Bao Z, Ye X, Xie Z, He K, Mergens B, Li W, Yatcilla M, Zheng Q (2018). Chemical investigation of major constituents in Aloe vera leaves and several commercial Aloe juice powders. J. AOAC Int., 101(6):1741-51. https://doi.org/10.5740/jaoacint.18-0122
Zhao HW, Li QW, Ning GZ, Han ZS, Jiang ZL, Duan YF (2009). Rhodiola sacra aqueous extract (RSAE) improves biochemical and sperm characteristics in cryopreserved boar semen. Theriogenology, 71: 849–857. https://doi.org/10.1016/j.theriogenology.2008.10.006
To share on other social networks, click on any share button. What are these?






