Effect of Vitamins and Hormones during different stages of Lactation in Lohi Sheep and Beetal Goats
Research Article
Effect of Vitamins and Hormones during different stages of Lactation in Lohi Sheep and Beetal Goats
Sonia Kanwal1, Muhammad Naeem1*, Zaib Un Nisa1, Rabia Farhat1 and Zia Ur Rehman2
1Institute of Pure and Applied Biology (Zoology Division), Bahauddin Zakariya University, Multan, Pakistan; 2Department of Physiology and Pharmacology, University of Agriculture, Faisalabad, Pakistan.
Abstract | This study was conducted to evaluate the effect of vitamins and hormones during different stages of lactation in Lohi sheep and Beetal goats. The experiments were performed on 30 ewes and 30 goats between 1.5 to 5 years age group were selected from Bahadar Nagar Farm, Okara, Pakistan. Both the sheep and goats were grouped into three different lactating stages: [lac I (n=10), lac II (n=10) and lac III (n=10)]. Blood samples were collected during all production stages and serum has been preserved at -20 0C and used to evaluate the differences in vitamin E, vitamin C, Triiodothyronine (T3), Thyroxine (T4) and Cortisol. The statistical analysis was done by two way ANOVA and Duncan Multiple Range Test (DMRT). T3 and T4 showed significantly different results during various stages of lactation in Lohi sheep and Beetal goats. However, Vitamin E, Vitamin C and Cortisol did not reveal significantly different results during lactation in both ewes and does. It could be postulated that the evaluation of vitamins and hormones during various stages of lactation were good indicators for farm maintenance and production of these animals.
Received | January 15, 2022; Accepted | May 30, 2022; Published | October 18, 2022
*Correspondence | Muhammad Naeem, Institute of Pure and Applied Biology (Zoology Division), Bahauddin Zakariya University, Multan, Pakistan; Email: dr_naeembzu@yahoo.com
Citation | Kanwal, S., M. Naeem, Z.U, Nisa, R. Farhat and Z.U. Rehman. 2022. Effect of vitamins and hormones during different stages of Lactation in Lohi Sheep and Beetal Goats. Sarhad Journal of Agriculture, 38(4): 1462-1467.
DOI | https://dx.doi.org/10.17582/journal.sja/2022/38.4.1462.1467
Keywords | Lohi sheep, Beetal goat, Lactation, Vitamins, Hormones
Copyright: 2022 by the authors. Licensee ResearchersLinks Ltd, England, UK.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Introduction
Immediately before parturition as well as during the first stage of lactation, increased mammary gland activity results in energy deficiency. Changes in metabolites and hormones occur as a result of increased metabolic demands during lactation and animals become more susceptible to a number of metabolic diseases at this stage than during other life periods compromising productivity (Ashmawy, 2015). Homeostasis is the coordinated control in metabolism of body tissues necessary to support a physiological state (Abdulkareem, 2013). Lactation period represents a critical life phase, since have to adjust metabolically to the increase in energy and nutrient requirements needed to ensure milk production (Fiore et al., 2017).
Additionally increased demands for energy during early lactation would initiate oxidative reactions thus inducing reactive oxygen species (ROS) formation (Fassaha et al., 2015) and animals may face “oxidative stress”. Development of ROS are counterbalanced through antioxidant defense system. The system includes enzymatic (SOD, Catalase and GSHPx) and non-enzymatic includes, Vitamin E, C, zinc, glutathione, carotene, beta carotene (Pierce et al., 2004).
Vitamin E is a fat soluble antioxidant with most active homologues α-tocopherol that when react with ROS produce oxidative stress and defend the cell membranes from lipid peroxidation (Traber and Atkinson, 2007). Vitamin C or ascorbic acid is a water soluble antioxidant against ROS and defends DNA damage. It can be used in a chain breaking antioxidant which helps to inhibit the synthesis of the peroxidative process and also facilitates the recycling of glutathione and oxidized vitamin E (Chan, 1993).
During lactation, the mammary gland secretary cells utilize 80% of the circulating metabolites in the blood for milk synthesis (Karapehlivan et al., 2007). Thyroid hormones play an important role in lipid metabolism and concentration of these parameters in the blood gives a clearer picture of nutritional and health status of the animal. Serum T3 and T4 concentrations were significantly decreased during parturition and early lactation (Fiore et al., 2018) and then increased in T3 and T4 concentrations were observed during the late lactation. The cortisol is well-accepted indicator for stress (Schwinn et al.,2018) and its level varies during lactation. There is enhancement of cortisol during lactation period due to increased adrenocorticotrophic hormone that in turn causes the release of glucocorticoids from adrenal gland (ALkalby and Mohammad, 2013).
Materials and Methods
Clinically healthy Lohi sheep (n=30) and Beetal goats (n=30) aged between 1.5-5 years were selected from Livestock Production Research Institute Bahadur Nagar Farm, Okara, Pakistan . The lactating groups were divided into early, peak and low lactation stages as Lac I (30 days), Lac II (60 days) and Lac III (90 days-onwards) in both Lohi sheep and Beetal goats. Blood samples were collected and serum has been preserved at -20 0C. The analyses were done in the laboratory of Institute of Pharmacy, Physiology and Pharmacology at the University of Agriculture, Faisalabad, Pakistan.
Vitamin E (Vit. E; µmol/L) and Vitamin C (Vit. C; µmol/L)
Method of Rutkowski and Grzegorczyk (2007) was used for the evaluation of vitamin E and C. By using the following formula, the concentrations of vitamin E and vitamin C were calculated as followed:
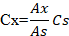
Where;
Cx: Concentration of vitamin E/C; Ax: Absorbance of the sample; As: Absorbance of the standard; Cs: Concentration of the standard solution.
Triiodothyronine (T3: ng/mL) and Thyroxine (T4; µg/dL)
T3 and T4 Elisa kit (Biocheck Inc., Foster City, CA-94404, USA, Lot No. RN - 41498) was used for evaluation of T3 and T4 concentrations. By means of using lin-log method for data reduction, concentrations of all samples were determined. Linear portions of curve were used to calibrate the readings.
Cortisol (ng/mL)
A microplate EIA kit provided by Accubind (Monobind Inc., Lake Forest, CA-92630, USA, Lot No. 3625-300) was used for quantitative evaluation of serum cortisol concentration. Linear portions of curve were used to calibrate the readings.
Statistical analysis
Data obtained was subjected to two way analysis of variance (ANOVA) technique (Steel et al., 1997). Duncan Multiple Range (DMR) test was used to explain significant difference (Duncan, 1955) among various studied groups.
Results and Discussion
Results for ANOVA in Lohi sheep and Beetal goats during various stages of Lactation
Data concerning the difference between Lohi sheep and Beetal goats for vitamin E, vitamin C, Triiodothyronine (T3) Thyroxine (T4) and cortisol activities during various stages of lactation were analyzed by two way ANOVA and the results have been shown in Table 1. Sheep and goats groups as well as the interaction between the groups and stages did not differ significantly in Vitamin E and vitamin C. The results for serum T3 and T4 concentrations revealed that both the groups pertaining to different lactation stages as well as groups × stages interaction presented statistically different results (P ≤ 0.01). However, the results concerning cortisol in Lohi sheep and Beetal goats pertaining to different lactation stages were different significantly. However, the results of groups G x S interaction did not show significant results (Table 1).
Table 1: Analysis of variance (ANOVA) of Vitamin E, C, T3, T4 and Cortisol of Lohi sheep and Beetal goats during various stages of Lactation.
|
Parameters (F-Value) |
Source of Varition |
||
|
Groups |
Stages |
G x S |
|
|
Vitamin E |
0.125 N.S. |
138.078** |
0.153 N.S. |
|
Vitamin C |
0.512 N.S. |
317.328** |
2.177 N.S. |
|
Triiodothyronin (T3) |
14.779** |
39.751** |
11.184** |
|
Thyroxine (T4) |
287.342** |
138.298** |
49.798** |
|
Cortisol |
136.707** |
192.347** |
0.685 N.S. |
**Significance = P ≤ 0.01; N.S. = Non-significant.
Results for Mean ± SE in Lohi sheep and Beetal goats during various stages of Lactation
Vitamin E (Vit. E; μmol/L) and vitamin C (Vit. C; μmol/L)
Vitamin E levels in ewes and does were significantly similar during lactation stage-I, II and III. In sheep and goats, vitamin E concentration decreased non-significantly from lactation stage-I to II and then an increase, though non-significant was observed during stage-III. Interaction between sheep and goats groups showed significantly same results among all the stages of lactation. Overall mean vitamin E concentrations in both Lohi sheep and Beetal goats groups were significantly the same (Table 2). It had been reported that vitamin E participate in fighting against oxidative stress during postpartum and decreased in lipid peroxidation (Bouwstra et al., 2008). Results of the present study are in consistent with those of Festila et al. (2012) had not discovered difference in vitamin E supplementation in milk production in dairy cows. Konvicná et al. (2015) reported a significant increase in vitamin E concentration with the advancement of lactation.
Vitamin C concentrations were significantly similar among all the stages of lactation. A non-significant increase was observed from stage-I to stage-II which decreased non-significantly during lactation stage-III in both the groups. Interaction between sheep and goats groups was significantly similar among all the stages of lactation. Overall mean Vitamin C concentrations for both sheep and goats groups were significantly the same (Table 2). The findings of present study agree with that of Ognik et al. (2015) who had reported that there was no change in Vitamin C concentration during various stages of lactation. Howida et al. (2015) concluded that vitamin C supplementation enhanced to the adverse effects of oxidative stress and improved milk quality.
Triiodothyronine (T3;ng/mL)and Thyroxine (T4; µg/dL)
Serum triiodothyronine concentration was observed to be significantly different (P ≤ 0.01) during stage-I in Lohi sheep. In Beetal does, significantly different results were observed in lactation stage-I and stage-II. The interaction between ewes and does manifested different results during lactation stage-I and stage-II of lactation. Overall mean T3 concentrations were also significantly different in both Lohi sheep and Beetal goats groups (Table 2).
Serum thyroxine level was significantly different in lactation stage-I in Lohi sheep and during lactation stage-III in Beetal goats. The interaction between Lohi sheep and Beetal goats groups showed significantly different (P ≤ 0.01) results for T4 concentration during stage-I and stage-II of lactation. Overall mean T4 concentrations were resulted into significantly different values in Lohi sheep and Beetal goats groups (Table 2). The results of present study are in consistent with Paulikova et al. (2017) and Fiore et al. (2018), concluded a decrease in both T3 and T4 concentrations during lactation. These results are in consistent with resulted into decreased T4 level during lactation.
The reason for low T3 and T4 could be breakdown of fat and protein in mammary tissue (Riis and Madsen, 1985). During early lactation due to limited supply of food (energy malnutrition), T3 and T4 levels decreased probably due to increased metabolism of T3 and T4 in peripheral tissues to inhibit secretary power of thyroid gland (Huszenicza, et al., 2002). However, ALkalby and Mohammad (2013) observed an increase in thyroid hormones during lactation.
Cortisol (ng/mL)
Serum cortisol level during various lactation stages exhibited significantly the same results. A non-significant decrease in cortisol concentration was observed
Table 2: Mean ± SE of Vitamin E, Vitamin C, T3, T4 and Cortisol of Lohi sheep and Beetal goats during various stages of Lactation.
|
PARAMETERS |
Lactation Period |
|||
|
Lactation (Stage I) |
Lactation (Stage II) |
Lactation (Stage III) |
Overall Mean |
|
|
Vitamin E |
||||
|
Lohi Sheep Beetal Goats |
1.40±0.01 1.40±0.01 |
1.29±0.02 1.30±0.02 |
1.51±0.01 1.51±0.01 |
1.40±0.02 1.40±0.02 |
|
Vitamin C |
||||
|
Lohi Sheep Beetal Goats |
4.03±0.04 3.97±0.04 |
4.91±0.08 4.74±0.06 |
3.07±0.08 3.18±0.09 |
4.00±0.14 3.96±0.12 |
|
T3 |
||||
|
Lohi Sheep Beetal Goats |
1.68±0.05 a 1.45±0.05 b |
1.37±0.01 b 1.25±0.01 c |
1.33±0.01 bc 1.39±0.02 bc |
1.46±0.03 A 1.37±0.02 B |
|
T4 |
||||
|
Lohi Sheep Beetal Goats |
32.75±0.55 b 39.30±0.52 a |
26.55±0.44 d 37.60±0.54 a |
27.40±0.28 cd 29.15±0.41 c |
28.90±0.57 B 35.35±0.87 A |
|
Cortisol |
||||
|
Lohi Sheep Beetal Goats |
3.53±0.09 2.75±0.06 |
2.58±0.08 1.86±0.10 |
2.02±0.06 1.41±0.03 |
2.71±0.12 A 2.01±0.11 B |
A-B, a-d; Values in a row (a-d) or column (A-B) with different letters were significantly different (P ≤ 0.01; ANOVA, DMRT).
from stage-I of lactation to stage-III in both ewes and does. An interaction between Lohi sheep and Beetal goats groups for cortisol concentration demonstrated significantly the same results throughout all the stages of lactation. Although, statistically different results were observed for overall mean cortisol concentrations in both Lohi sheep and Beetal goats groups (Table 2).
Ghanem et al. (2012) and Howida et al. (2015) observed the same non-significant results for cortisol concentration during different stages of lactation in cows. Similarly, ALkalby and Mohammad (2013) had observed a significant decrease in cortisol concentration with the advancement of lactation.
Conclusions and Recommendations
Lactation is the physiological period of increased metabolic demand that could provoke a threat to homeostasis. Variations in vitamins and hormones revealed negative energy balance during lactation and should be consider to improve health, welfare and production of these animals.
Novelty Statement
The study was carried out in Lohi sheep and Beetal goats and the results suggested that helps the farmers for better maintenance and production of their animals and to improve their income.
Author’s Contribution
Sonia Kanwal: Carried out the research work and has been written the first draft of the manuscript.
Muhammad Naeem: Supervised the research work and improved the manuscript.
Zaib Un Nisa: Provided aid during data analyses.
Rabia Farhat: Helped out during sampling process.
Zia Ur Rahman: Provided all the means to make this research possible. All authors read and proved the final manuscript.
Conflict of interest
The authors have declared no conflict of interest.
Abdulkareem, T.A. 2013. Some hematological and blood biochemical attributes of Iraqi riverine buffaloes (Bubalus bubalis) around calving and postpartum periods. Al-Anbar J. Vet. Sci., 6: 143–150. https://doi.org/10.6000/1927-520X.2013.02.02.4
ALkalby, J.M.A. and Mohammad, A.Q. 2013. Effect of Late Pregnancy, Parturition and Early Lactation on T3, T4 and Cortisol level of Heifers and cows. J. Thi-Qar Sci., ISSN 1991-8690.
Ashmawy, N.A. 2015. Blood metabolic profile and hormones conc. in Egyptian buffalo during physiological states. Asian J. Anim. Vet. Adv., 10(6): 271–280. https://doi.org/10.3923/ajava.2015.271.280
Bouwstra, R.J., Goselink, R.M.A., Dobbelaar, P., Nielen, M. Newbold, J.R. and Vanwerven, T. 2008. The relation between oxidative damage and Vit. E in blood, milk, and liver from Vit. E supplemented and non-suppl. Peri-parturient heifers. J. Dairy Sci.,91: 977-987. https://doi.org/10.3168/jds.2007-0596
Chan, A.C. 1993. Partners in defense, vitamin E and C. Can. J. Physiol. Pharmacol., 71: 725-731. https://doi.org/10.1139/y93-109
Duncan, D.B. 1955. Multiple range and multiple F-tests. Biometrics, 11: 1-42. https://doi.org/10.2307/3001478
Fassaha, D.M., Khotijaha, L., Atabanyb, A., Mahyardiania, R.R., Puspadinia, R. and Putraa, A.Y. 2015. Blood Malondialdehyde, Reproductive, & Lactation performances of ewes fed high PUFA rations supplemented with different antioxidants. Med. Peternakan., 38(1):48-56. https://doi.org/10.5398/medpet.2015.38.1.48
Festila, I., Miresan, V., Raducu, C., Cocan, D. and Constantinescu, R. 2012. Evaluation of oxidative stress in dairy cows through antioxidant enzymes GPX and SOD. Bullet. UASVM Anim. Sci. Biotech., 69(1–2): 107-110.
Fiore, E., Arfuso, F., Gianesella, M., Vecchio, D., Morganle, M. and Mazzotta, E. 2018. Metabolic and hormonal adaptation in Bubalus bubalis around calving and early lactation. PLOS ONE 13(4): e0193803. https://doi.org/10.1371/journal.pone.0193803
Fiore, E., Giambelluca, S., Morgante, M., Contiero, B., Mazzotta, E. and Vecchio, D. 2017. Changes in some blood parameters, milk composition and yield of buffaloes (Bubalus bubalis) during the transition period. Anim. Sci. J., 88: 2025–2032. https://doi.org/10.1111/asj.12872
Ghanem, M.M., Mahmoud, M.E., Abd El-Raof, Y.M. and El-Attar, H.M. 2012. Metabolic profile test for monitoring the Clinical, Haematological and Biochemical alterations in cattle during peri-parturient period. Benha Vet. Med. J., 23(2): 13-23.
Howida, M.A., Abd-El-Rahman., Ibrahim, M.A., Dohreig, R.M.A. and Asfour, H.A.E. 2015. The relation between oxidative status, milk quality and conception rate in Dairy goats supplemented with vitamin C. Assiut Vet. Med. J., 61: 145. https://doi.org/10.21608/avmj.2015.170208
Huszenicza, G., Kulcsar, M. and P. Rudas. 2002. Clinical Endocrinology of thyroid gland functions in ruminants. Vet. Med. Czech., 47(7): 199-210. https://doi.org/10.17221/5824-VETMED
Karapehlivan, M., Atakisi, A., Atakisi, O., Yugayurtr, R. and Pancarci, S.M. 2007. Blood biochemical parameters during the lactation and dry period in Tuj ewes. Small Rum. Res., 73: 267-271. https://doi.org/10.1016/j.smallrumres.2006.12.006
Konvicná, J., Vargová, M.V., Paulíková, I., Kováč, G. and Kostecká, Z. 2015. Oxidative stress and antioxidant status in dairy cows during prepartal and postpartal periods. Acta Vet. Brno., 84: 133-140. https://doi.org/10.2754/avb201584020133
Ognik, K., Patkowski, K., Gruszecki, T. and Kostro, K. 2015. Redox status in blood of ewes in the perinatal period and during lactation.Bullet. Vet. Inst. Pulawy., 59: 557-561. https://doi.org/10.1515/bvip-2015-0083
Paulikova, I., Seidel, H., Nagy, O., Tothova, C.S., Konvicna, J., Kadasi, M. and Kovac, G. 2017. Thyroid Hormones, Insulin, body Fat, and Blood biochemistry indices in dairy cows during the Reproduction/Production cycle. Folia Vet., 61(1): 43-53. https://doi.org/10.1515/fv-2017-0007
Pierce, J.D., Cackler, A.B. and Arnett, M.G. 2004. Why should you care about free radicals? Reg. Nurse J., 67: 38-42.
Riis, P.M. and Madsen, A. 1985. Thyroxine concentration and secretion rate in relation to pregnancy, lactation, energy balance in goats. J. Endocrinol., 107: 421-427. https://doi.org/10.1677/joe.0.1070421
Rutkowski, M and Grzegorczyk, K. 2007Modifications of spectrophotometric methods for antioxidative vitamins determination convenient in analytic practiceActa Scientiarum Polonorum Technologia Alimentaria., 6(3): 17-28
Schwinn, A.C., Sauer, F., Gerber, V., Bruckmaier, R.M and Gross, J.J. 2018. Technical note: Free and bound cortisol in plasma and saliva during ACTH challenge in dairy cows and horses. J. Anim. Sci., 96: 76–84. https://doi.org/10.1093/jas/skx008
Steel, R.G.D., Torrie, J.H. and Dieky, D.A. 1997. Principles and procedures of statistics. 3rd Ed. McGraw Hill Book Co. Inc. New York.
Traber, M.G. and Atkinson. 2007. Vitamin E, antioxidant and nothing more. Free Rad. Biol. Med., 43: 4-15. https://doi.org/10.1016/j.freeradbiomed.2007.03.024
To share on other social networks, click on any share button. What are these?








