Effect of Supplemented Algal Carotenoid Diets on Skin Color of Tomato Clownfish, Amphiprion frenatus
Effect of Supplemented Algal Carotenoid Diets on Skin Color of Tomato Clownfish, Amphiprion frenatus
Müge Aliye Hekimoğlu*, Kürşat Fırat, Şahin Saka, Cüney Süzer, Aysun Kop and Yaşar Durmaz
Aquaculture Department, Faculty of Fisheries, Ege University, 35100 Bornova, Izmir, Turkey
ABSTRACT
This study analyzes the effect of Nannochloropsis oculata and Porphyridium cruentum as natural pigment sources on skin color of tomato clownfish, Amphiprion frenatus. Two groups, each of 6 fish were fed on feed containing (69.8±9.158 mg.g-1) N. oculata (Group A), and 67.21±7.068 mg.g-1 of P. cruentum (Group B). The third group (Group C) was fed on control basal diet (34.93±29.07 mg.g-1). Total carotenoid content of fish skin was determined at 30-day intervals. At the end of the experiment the highest weight gain was found to be 1.73±0.37g in Group B, whereas the lowest performance (1.29±0.38 g) was received in Group C. The best feed conversion ratio was found in at Group B. The total carotenoid content of skin of fish were found to be 0.77±0.61 µg.g-1 on the initial day in the experimental group. As a result of the measurements performed on the 120th day, the pigment values were determined as 30.39±0.39 µg.g-1 in Group A, 39.07±1.12 µg.g-1 in Group B and 35.68±10.69 µg.g-1 in the Group C. Group B pigment source is more effective on the color of tomato clownfish A. frenatus.
Article Information
Received 09 January 2016
Revised 08 April 2016
Accepted 29 August 2016
Available online 2 April 2017
Authors’ Contributions
MAH, KF and YD conceived and designed the study. MAH executed the experimental work. MAH and KF statistically analyzed the ata, looked after fish, analyzed the fish colour and wrote the article. AK helped in fish feed preparation. CS anayzed the fish feed. SS helped in statistically analysis.
Key words
Ornamental fish, Carotenoid, Amphiprion frenatus, Porphyridium cruentum, Nannochloropsis oculata.
DOI: http://dx.doi.org/10.17582/journal.pjz/2017.49.2.663.668
* Corresponding author: mugehekimoglu@gmail.com
0030-9923/2017/0002-0663 $ 9.00/0
Copyright 2017 Zoological Society of Pakistan
INTRODUCTION
The tomato clownfish, Amphiprion frenatus, is an important ornamental fish for the aquarium industry because of its color. The tomato clown fish is characterized by a white band just behind the eyes, being dominated by colors orange/red in the natural environment. The tomato clown fish also has distinctive differences from the fish being collected from the nature, in terms of color tone, brightness and saturation. In this context, it is required to impose carotenoids, which give color to the fish, from the outside in order to increase the visual effect (Yasir and Qin, 2010).
The coloration of the fish skin is dependent on absorption and deposition of carotenoid pigments from the diet, since fish, like other vertebrates, are unable to synthesize carotenoids de novo (Goodwin, 1984). Carotenoids are biosynthesized by plants, algae, and certain yeast and bacteria (Ong and Tee, 1992). Carotenoids are responsible for the red, orange, and yellow colors of fish and crustaceans (Packer, 1992). Dietary carotenoids play an important role in regulating fish color because fish, like other animals, are unable to synthesize carotenoids and their skin color is highly dependent on carotenoids from the diet (Torrissen et al., 1989). Upon intake, fish can modify alimentary carotenoids and store them in the integument and other tissues (Ha et al., 1993).
In this study, the unicellular red alga Porphyridium cruentum, which is a member of the Rodophyta, and Nannochloropsis oculata, a marine unicellular alga belonging to the Eustigmatophyceae family, were used as naturel pigment sources. P. cruentum’s carotene has Phycoerythrin. Pigment composition of algae from the genus Nannochloropsis is characterized by chlorophyll a (not chlorophyll b or chlorophyll c), carotene, violaxanthin and vaucheriaxanthin as major pigments. These algae also contain some minor carotenoids among which canthaxanthin and astaxanthin, both with ketonic groups; stand out (Whittle and Casselton, 1975; Karlson et al., 1996).
Although there are studies on the effects of pigment materials, being mixed in feed for a number of saltwater fish and other clown fish species, upon color changes on skin (Yasir and Qin, 2009), studies on A. frontalis only concentrate on the spawning management (Nakamura et al., 1994) micro habitat (Hattori, 2005) and diseases (Silphaduang et al., 2000). There is not any study about the effect of coloration on the tomato clown fish; A. frenatus. Therefore, this study was undertaken for the first time to determine the effect of microalgae’s pigment on skin of coloration of A. frenatus.
MATERIALS AND METHODS
Fish and experimental design
The tomato clown fish, which have been produced in a commercial aquaculture facility in Akvatek Company (Turkey), was used in the research. 45 days old 180 clown fish were used for experiment. Their average wet-weight were 0.26± 0.02 g and average total length was 1.29±0.01 cm. This study was conducted in rectangular glass aquaria in 50 L working volume (47 x 37 x 29 cm) with 20 fish in each. The aquaria were supplied with seawater though a recirculating system treated with biofilter and mechanical filter. Oxygen was maintained at 6.8±0.2 mgL-1 with air stone. The environmental variables were maintained at 25.5±0.4°C, ‰35±0.2 salinity and light was supplied by florescent lamps: with a power of 1200 lx at water surface and photoperiod applied 16 h light: 8 h dark. During the experiment the major physical and chemical parameters were maintained at stable conditions.
Algal culture
N. oculata and P. cruentum were cultured in laboratory in 10-liter balloon as a batch culture at 18°C at the Plankton Laboratory at Ege University (Izmir, Turkey). The alga was cultured in enriched seawater with F/2 medium. The culture was kept illuminated with florescent lamps at photon flux density of 116 μEm−2 s−1. Culture was continuously aerated by the air. Harvesting of the culture was done by centrifugation. The pellet obtained was dried at 65˚C in the drying oven for used pellet feeds.
Preparation of feed and application
The experimental diets were formulated to meet the nutritional requirements of the tomato clownfish and prepared with using laboratory type pellet machine in the fish nutrition and fish feed technology laboratory at Ege University (Turkey). The experimental feeds were prepared with basic ingredients such as fish meal, soybean meal, wheat meal, corn gluten, and fish oil. The two natural carotenoid sources such as powdered Nannochloropsis oculata (Group A) and powdered Porphyridium cruentum (Group B) used in the experimental diet and the control diet (Group C), were not included any algae powder as a carotenoid source. So, only the pigment sources show differences in the feed, which were prepared as 3 groups.
Three replicates were used for each of the three experimental diets. Fish in each treatment only received one diet type twice a day (morning and evening) ad libitum. The feeds being used throughout the study were stored at +4°C.
Calculations and analyses
At the beginning and the end of the experiment, fish were measured and weighed. During the study the specific growth rate (SGR), whose formulation is given below, was used to determine growth rate of fish (Jensen, 1985):
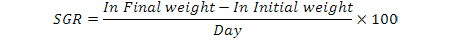
Daily growth rate (DGR) was calculated according to Guillaume et al. (1999):

Feed Conversion Ratio (FCR) was calculated as below formula:
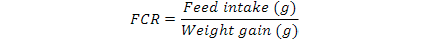
Assuming a complete of diets, the carotenoid retention rate were calculated by the following equation (Ingle De La Mora et al., 2006):
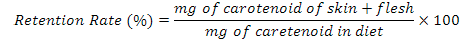
The crude protein, crude fat and ash in diets were determined with method of Padmore (1990).
Total β-carotene content of the fish was determined spectrophotometrically after extraction with acetone (Choubert and Storebakken, 1989). Three fish were randomly sampled from each aquarium. Fish were then anesthetized with MS-222 (70 ppm). The anaesthetized fish were put down with a needle right in the brain and the skin was dissected immediately from body area for carotenoid content determination. Skin samples were collected from both sides between the abdominal and dorsal region of the fish. 10 mg skin sample passed through the homogenization procedure with the addition of 5 ml acetone, they passed through the centrifuge procedure for 10 min at 3500 rpm. After that these samples were read in 475 nm wavelength on the spectrophotometer (Jenway 6305 model). A calibration curve was made using the absorbance values in 5 ml acetone solution which has 0.16, 1.63, 2.04, 3.27 and 4.09 mg g-1 β-carotene to determine the quantity of β-carotene.
Statistical analysis consisted nonparametric test of Kruskal Wallis, using the probability level of 0.05 for rejection of the null hypothesis. After ANOVA, significant differences among means were determined by Mann-Whitney U test. All statistical analysis were performed using SPSS 15.0 for Windows.
RESULTS
The crude protein, lipid content of all experimental diets did not vary in different experimental diets from each other (Table I). The protein content from Group A, Group B and Group C were 42.94%, 42.70% and 42.79%, respectively. The lipid value and crude cellulose from experimental diets groups were 7.90%, 7.05% 6.79 and%, 3.07%, 2.80%, 3.02%, respectively. Total β-carotenoid amount of experimental diets were determined as 69.8±9.158, 67.21±7.068 and 34.93±29.07 mg g-1, respectively.
Table I.- Feed composition and proximate chemical composition of diets.
|
Ingredient |
Group A |
Group Bb |
Group C |
| Fish meal | 35 | 35 | 35 |
| Soybean meal | 25 | 25 | 25 |
| Wheat meal | 19 | 16.8 | 20 |
|
Corn gluten |
11 | 10.2 | 15 |
| Fish oil | 2 | 2 | 2 |
| Nannochloropsis oculata | 5 | ||
| Porphyridium cruentum | - | 8 | - |
|
Mineral premix d |
3 | 3 | 3 |
| Total | 100 | 100 | 100 |
| Chemical Composition | |||
| % Crude protein | 42.94 | 42.70 | 42.79 |
| % Crude lipids | 7.90 | 7.05 | 6.79 |
|
% Crude cellulose |
3.07 | 2.80 | 3.02 |
| % Crude ash | 8.49 | 9.5 | 8.15 |
|
Gross energy (MJ. Kg-1) |
2540.3 | 2450.5 |
2604.7 |
aGroup A, Diet Nannochloropsis. feed included Nannochloropsis oculata. bGroup B, Diet Porphyridium, feed included Porphyridium cruentum. cGroup C, Diet Control, feed not included Algae. dMineral premix, g Per g mixture (vitamin A 342 IU, vitamin D3 329 IU, vitamin E 0.0274 IU, vitamin K3 48 mg, vitamin B1 2.05 mg, vitamin B2 3.42 mg, vitamin B3 20.5 mg, vitamin B5 5.48 mg, vitamin B6 2.05 mg, vitamin B12 2.74 mgm vitamin C 24.0 mg, biotin 0.411 mg, folic acid 0.685 mg, Zn 12.3 mg, Mn 4.80 mg, Cu 1.64 mg. I 0.274 mg, Se 0.0274 mg. Ca: 125 mg, K 189 mg). Agromey Feed Mill Company, Izmir, Turkey.
All experiment diets were equally accepted by fish. In this study, no mortality was observed. Fish showed a homogeneous distribution within the aquarium in all groups throughout the experimentation. At the end of the 8th day, individuals were observed chasing each other, attacking their tails and fins or swimming after each other. The fish generally displayed the behavior of grabbing feed on the water surface and rarely from the bottom, throughout the experimentation. At the end of the 20th day, the fish retreated in groups to certain areas of the aquarium.
At the end of the experimentation, there were no significant differences between the groups in terms of total length (p>0.05). The highest weight of experimental groups was measured as 1.73±0.37 g (Group B, Table II). Lower performance was measured as 1.29±0.38 g in Group C. The weight of both groups (Group A, Group B) were significantly different from the Group C (p<0.05). The best feed conversion ratio was measured at Group B. Similarly, the best SGR and growth values were measured at Group B (Table II).
Table II.- Effect of feeding carotenoids supplements on total length, survival rate, growth and feed utilization parameters after 120 days of experiment.
|
Group A |
Group B |
Group C |
||
| Total length (cm) | Initial | 1.29±0.02 | 1.29±0.02 | 1.29±0.02 |
| Final | 4.15±0.24 | 4.38±0.30 | 4.00±0.38 | |
| Total weight (gm) | Initial | 0.26±0.03 | 0.26±0.03 | 0.26±0.03 |
| Final | 1.59±0.35 | 1.73±0.37 | 1.29±0.36 | |
| Growth (DGR) |
4.26 |
4.71 |
3.30 |
|
|
SGRc |
1,59 |
1,68 |
1,39 |
|
|
FCRd |
2,02 |
1.83 |
2,61 |
|
| Survival rate (%) |
100 |
100 |
100 |
|
Values are mean ± S.D. of three groups per treatment. a, no significantly differences (P>0.05) were observed among treatments means. For diet composition of group A, B and C, see Table I.
The total carotenoid values of skin of the fish in experimental groups (Group A, Group B and Group C) were determined as 0.77±0.61 µg g-1 on the initial day. After other analyses conducted on the 120th day, a rapid decrease was observed in the carotenoid amount and it was determined that these values were lower than the initial value (Table III). The data were not found statistically significant (p>0.05).
Table III.- Total carotonoid amounts ( mean ± S.D.).
| Initial days | Group A | Group B | Group C |
| 30 | 130.91±118 | 140.05±67.71 | 140.33±136.49 |
| 60 | 78.77±44.131 | 85.84±46.45 | 57.68±21.81 |
| 90 | 29.88±14.52 | 50.88±18.57 | 50.67±6.85 |
| 120 | 30.39±0.39 | 39.07±1.12 | 35.69±10.69 |
As a result of the analyses performed on the 60th day, it was determined that the pigment accumulation of individuals in Group B did not differ from Group A and Group C; the acquired data were different from the initial value (p<0.05). As a result of the measurements performed on the 120th day, the pigment value was determined as 30.39± 0.39 µg.g-1 in Group A, 39.07±1.12 µg.g-1 in Group B and 35.68±10.69 µg.g-1 in the Group C. In addition to this, Group B showed a greater pigment accumulation compared to other groups, on the 120th day and no difference was determined between Group B and others (p>0.05).
DISCUSSION
The variation of colors and patterns of the fish enables the implementation of different functions such as warning, hiding and recognizing the species and sex. Coloration is controlled by the endocrine and nervous system. But dietary sources of pigment also play a role in determining color in fishes. Carotenoids are known to have a positive role in the intermediary metabolism of fish (Tacon, 1981; Segner et al., 1989; Chatzifotis et al., 2005) that could enhance nutrient utilization and may ultimately result in improved growth (Amar et al., 2001). As long as carotenoids are added to feeds as an extra material, they may prevent skin darkening caused by stress and production in the culture conditions. Accordingly, studies on the effect of carotenoids upon the color of fish showed that it could change according to the species, pigment of the fish, as well as the duration of application (Chatzifotis et al., 2005; Yasir and Qin, 2010).
Microalgae are significant organisms since they form the primary link in the food chain in seas. It is known that the success of any hatchery operation depends mainly on the availability of basic food such as microalgae and zooplanktons. N. oculata have been extensively utilized for mass production of zooplanktons such as rotifer, artemia, copepods etc. and are also used for generating “green water” in many hatcheries. Rodophyta tends to have a simple pigment composition with b-carotene, zeaxanthin and chlorophyll as the predominant thylakoid pigments (Grabowski et al., 2000). In addition to Beta carotene, the major xanthophylls pigment found in all Nannochloropsis species are vilaxanthin and a vaucherxanthin-like pigment (Karlson et al., 1996; Sukenik, 1999). Besides this, it was stated that pigments being added to the feeds of some saltwater fish and other fish had no effect upon growth (Chebbaki et al., 2002; Gomes et al., 2002; Nickell and Bomage, 1998).
Supplementing algal biomass to the basal diet has not had measurable effects on alteration of growth or voluntary feed intake by fresh water fish like rainbow trout (Gouveia et al., 2003). The study determined that the group which had N. oculata and P. cruentum in their feeds had a higher weight development, which could be explained through the fact that the algae being added to the feed positively affect the feed evaluation. This condition was also reported in other fish species.
A. frenatus, which is originally from Pacific Ocean, can breed all year long in the tropics but only in the warmer months of temperate locations. It is stated that while the tomato clownfish being collected from the nature have a few white bands on the neck during youth, these bands decrease as they age (Hoff, 2009). It is also stated that the tomato clownfish juveniles that are produced under controlled conditions take the color and band features of adults on the 30th day (Madhu et al., 2011). In the study, band changes were completed within a process of 4 months, which is thought to be caused by the broodstock management, environmental conditions and nurture regime differences.
The species being produced in the aquarium and marine aquarium sector, where the visual effect is in the forefront, have an utterly important pigmentation feature. In this context, it is inevitable to use materials involving synthetic and natural carotenoid as a feed additive. Although adding various synthetic materials into the feed increases the color intensity of the fish in a short time, a great majority of them are not suggested for use since they contain carcinogens. In addition to this, the most frequently used non-carcinogenic pigment materials are too expensive. Microalgae, on the other hand, are rich nutritional sources that are economically accessible, could be produced under convenient conditions and involve a rich carotenoid and nutritional value (Kop et al., 2010). Carotenoids are lipid soluble and follow the same absorptive pathways as other dietary lipids. Carotenoids are used by fisheries mainly due to the existence and place of the double bond and hydroxyl group in their structures. Absorptions, metabolic cycles and accumulations of carotenoid pigments differ according to the fish species. Trouts and shrimps use carotenoid of 4-4’ oxo that bear a double bond in their structures. Goldfish, on the other hand, use carotenoid of 3-3’ hydroxy (Torrissen et al., 1989). Trouts and some species such as shrimp only absorb linings and similar carotenoids and accumulate them in their tissues also as linings. Koi and goldfish, on the other hand, absorb carotenoids; however, they also metabolize and accumulate them in their tissues as linings.
Spirulina meal has also been used successfully to increase the skin coloration of red tilapia (Matsuno et al., 1980), red sword tail (James et al., 2006), blue gourami Trichogaster trichopterus (Alagappan et al, 2004), goldfish Carassius auratus (Gouveia et al., 2003; James et al., 2006) and yellow tail cichlid Pseudotropheus acei (Güroy et al., 2012).
CONCLUSIONS
In conclusion it was observed in the study that the two algae species created no statistically significant difference in the pigmentation of the fish, but this condition could be due to the low level of carotenoids in the algae being added in the feed. In addition, this study has consequently shown that adding algae species involving natural pigment materials to the feeds increases the total amount of carotenoids in tomato clown fish skins. Besides that the study is also thought to be important in terms of the first application of Porphyridium cruentum and Nannochloropsis oculatus, which are among natural pigment resources, on this species. Although the results do not show a statistical difference, appearances of the fish are observed to differ according to the colors of the fish in the Group C. This condition is especially observed in the group that had P. cruentum in their feeds. In addition to this, it will definitely have a greater meaning together with the increase of the algae amount that will be added to the feed in next studies and consequently the total pigment amount that will be added to the feed.
ACKNOWLEDGEMENTS
The authors kindly wish to thank the staff of the Akuatek Company, Izmir/TURKEY, where the experiments were conducted, for their most efficient technical assistance. This study was funded by Ege University, Scientific Research Project, 13-SUF-027.
Statement of conflict of interest
Authors have declared no conflict of interest.
REFERENCES
Alagappan, M., Vijula, K. and Sinha, A., 2004. Utilization of spirulina algae as a source of carotenoid pigment for blue gouramis (Trichogaster trichopterus, Pallas). J. Aquaricult. Aquat. Sci., 10: 1–11.
Amar, E.C., Kiron, V., Satoh, S. and Watanabe, T., 2001. Influence of various dietary synthetic carotenoids on bio-defence mechanisms in rainbow trout, Oncorhynchus mykiss (Walbaum). Aquacul. Res., 32: 162–163. https://doi.org/10.1046/j.1355-557x.2001.00051.x
Chatzifotis, S., Pavlidis, M., Jimeno, C.D., Vardanis, G., Sterioti, A. and Divanach, P., 2005. The effect of different carotenoid sources on skin coloration of cultured red porgy (Pagrus pagrus). Aquacul. Res., 36: 1517-1525. https://doi.org/10.1111/j.1365-2109.2005.01374.x
Chebbaki, K., Robaina, L., Vergara, J.M., Izquierdo, M.S., Fernandez-Palacios, H. and Chatzifotis, S., 2002. Effect of carotenoid sources on the red porgy (Pagrus pargus): effect on muscle and skin colour and skin carotenoid content. 10th International Symposium on Nutrition and Feeding of Fish, Rhodos, Greece1-7 June.
Choubert, G. and Storebakken, T., 1989. Dose response to astaxanthin and canthaxanthin pigmentation of rainbow trout fed various dietary carotenoids concentrations. Aquaculture, 81: 69-77. https://doi.org/10.1016/0044-8486(89)90231-7
Goodwin, T.V., 1984. The biochemistry of the carotenoids, Animals, II Chapman and Hall, London, New York. pp. 224. https://doi.org/10.1007/978-94-009-5542-4
Gomes, E., Dias, J., Silva, P., Valente, L., Empis, J., Gouveia, J.B. and Young, A., 2002. Utilization of natural and synthetic sources of carotenoids in the skin pigmentation of gilthead sebream (Sparus aurata). Eur. Fd. Res. Technol., 214: 287–293. https://doi.org/10.1007/s00217-001-0475-9
Gouveia, L., Rema, P., Pereira, O. and Empis, J., 2003. Colouring ornamental fish (Cyprinus carpio and Carassius auratus) with microalgal biomass. Aquacul. Nutr., 9: 123–129. https://doi.org/10.1046/j.1365-2095.2003.00233.x
Grabowski, B., Tan, S., Cunningham Jr. F.X.C. and Gantt, E., 2000. Characterization of the Porphyridium cruentum Chl a-binding LHC by in vitro reconstitution: LHCaR1 binds 8 Chl a molecules and proportionately more carotenoids than CAB proteins. Photosynth. Res., 63: 85-96. https://doi.org/10.1023/A:1006357107247
Guillaume, J., Kaushik, S., Bergot, P. and Metailler, R., 1999. Nutrition and feeding of fish and Crustaceans. Springer-Praxis Books in Aquaculture and Fisheries. 408: 15.
Güroy, D., Sahin, I., Güroy, B., Altin, A. and Merrifield, D.L., 2012. Effect of dietary protein levels on growth performance and nitrogen excretion of yellow tail cichlid Pseudotropheus acei., Israeli J. Aquacul. Bamidgeh, 684: 1-6.
Ha, B.S., Kang, D.S., Kim, J.H., Choi, O.S. and Ryu, H.Y., 1993. Metabolism of dietary carotenoids and e¡ects to improve the body color of cultured Flounder and red sea bream. Bull. Korean Fish. Soc., 26: 91-101.
Hattori, A., 2005. High mobility of the protandrous anemonefish Amphiprion frenatus: nonrandom pair formation in limited shelter space. Ichthyol. Res., 52: 57-63. https://doi.org/10.1007/s10228-004-0253-3
Hoff, F., 2009. Conditioning, spawning and rearing of fish with emphasis on marine clownfish. Florida Aqua Farms Inc. 3.
Ingle de la Mora, G., Arredondo-Figueroa, J.L., Ponce-Palafox, J.T., Barriga-Soca, I.D.A. and Vernon-Carter, J.E., 2006. Comparison of red chilli (Capsicum annuum) oleoresin and astaxanthin on rainbow trout (Oncorhyncus mykiss) fillet pigmentation. Aquaculture, 258: 487-495. https://doi.org/10.1016/j.aquaculture.2006.04.005
James, R., Sampath, K., Thangarathinam, R. and Vasudevan, I., 2006. Effect of dietary spirulina level on growth, fertility, coloration and leucocyte count in red swordtail, Xiphophorus helleri. Israel J. Aquacul. Bamidgeh, 58: 97–104.
Jensen, J.W., 1985. The potential growth of salmonids. Aquaculture, 48: 223-234. https://doi.org/10.1016/0044-8486(85)90126-7
Karlson, B., Potter, D., Kuylenstierna, M. and Andersen, R.A., 1996. Ultrastructure, pigment compostiona, and 18s rRNA gene sequence for Nannochloropsis granulata sp. nov. (Monodopsidaceae, Eustigmatophyceae), a marine ultraplankter isolated from Skagerrak. Northeast Atlan. Ocean. Phycol., 35: 253-260.
Kop, A., Durmaz, Y. and Hekimoglu, M., 2010. Effect of natural pigment sources on colouration of cichlid (Cichlasoma severum sp., Heckel, 1840). J. Anim. Vet. Adv., 9: 566-569. https://doi.org/10.3923/javaa.2010.566.569
Madhu, K., Madhu, R., Mathew, G. and Retheesh, T., 2011. Captive breeding of tomato clownfish, Amphiprion frenatus. Trop. Fish Hobby., 3: 80-84.
Matsuno, T., Matsutaka, H., Datsuyama, M. and Nagata, S., 1980. Occurrence of 3’-Epimer of Lutein (Calthaxanthin, 3’-Epilutein) from fishes. Bull. Jap. Soc. scient. Fish., 46: 337-340.
Nakamura, M., Mariko, T. and Nagahama, Y., 1994. Ultrastructural and in vitro steroidogenesis of the gonads in the protandrous clownfish. Amphiprion frenatus. Jpn. J. Ichthyol., 41: 47–56.
Nickell, D.C. and Bomage, N.R., 1998. The effect of timing and duration of feeding astaxanthin on the development and variation of fillet colour and efficiency of pigmentation in rainbow trout (Oncorhynchus mykiss). Aquaculture, 169: 233-246. https://doi.org/10.1016/S0044-8486(98)00385-8
Ong, A.S.H. and Tee, E.S., 1992. Natural sources of carotenoids from plants and oils. Meth. Enzymol., 213: 142-167. https://doi.org/10.1016/0076-6879(92)13118-H
Packer, L., 1992. Carotenoids, chemistry, separation, quantitation, and antioxidation. Meth. Enzymol., Part A, 213: 1-538.
Padmore, J.M., 1990. Animal feed. In: Official methods of analysis of the association of official analytical chemists 15th Edition, (ed. Kenneth Helrich), vol. 1, Association of Official Analytical Chemists, INC, USA, pp. 69-79.
Segner, H., Arend, P., Von Poeppinghaussen, K. and Schmidt, H., 1989. The effect of feeding astaxanthin to Oreochromis niloticus and Colisa labiosa on the histology of the liver. Aquaculture, 79: 381–390. https://doi.org/10.1016/0044-8486(89)90480-8
Silphaduang, U., Hatai, K., Wada, S. and Noga, E., 2000. Cladosporiosis in a tomato clownfish (Amphiprion frenatus). J. Zoo Wildl. Med., 31: 259-261. https://doi.org/10.1638/1042-7260(2000)031[0259:CIATCA]2.0.CO;2
Sukenik, A., 1999. Production of EPA by marine eustigmatophyte Nannochloropsis. In: Chemicals from microalgae (ed. E. Cohen). Taylor and Francis, London.
Tacon, A.G.J., 1981. Speculative review of possible carotenoid function in fish. The Progr. Fish-Cultur., 43: 205-208. https://doi.org/10.1577/1548-8659(1981)43[205:SROPCF]2.0.CO;2
Torrissen, O.J., Hardy, R.W. and Shearer, K.D., 1989. Pigmentation of salmonids-carotenoid deposition and metabolism. Rev. aquat. Sci., 1: 209-225.
Whittle, S. and Casselton, P., 1975. The chloroplast pigments of the algal class eustigmatophyceae and xantophyceae. i. eustigmatophyceae. Br. Phycol. J., 10: 179-191. https://doi.org/10.1080/00071617500650171
Yasir, I. and Qin, J., 2010. Effect of dietary carotenoids on skin color and pigments of false clownfish, Amphiprion ocellaris, Cuvier. J. Aquacul. Soc., 41: 308-318. https://doi.org/10.1111/j.1749-7345.2010.00373.x
Yasir, I. and Qin, J., 2009. Effect of light intensity on color performance of false clownfish. Amphiprion ocellaris Cuvier. J. Aquacul. Soc., 40: 337-350. https://doi.org/10.1111/j.1749-7345.2009.00254.x
To share on other social networks, click on any share button. What are these?










