Effect of Hibiscus Leaf on Protein Balance, Blood Profile, and Body Composition of Etawah Crossbreed Goats
Research Article
Effect of Hibiscus Leaf on Protein Balance, Blood Profile, and Body Composition of Etawah Crossbreed Goats
I Gusti Lanang Oka Cakra1*, Anak Agung Ngurah Badung Sarmuda Dinata2, I Gede Mahardika1, I Gusti Nyoman Gde Bidura1
1Faculty of Animal Husbandry, Udayana University, Denpasar, Bali, Indonesia; 2Bali Agricultural Technology Research Center, Denpasar, Bali, Indonesia.
Abstract | Hibiscus leaf was a native tropical plant that contains saponin that may be used as de-faunating agent in ruminants. In some areas, these plants commonly being used as an alternative feed for ruminant, particularly during the dry season, but the study of its effect was limited. This study aims to determine the effect of additional Hibiscus leaf flour (HLF) in the concentrate on protein balance, blood metabolic profile, and body composition of Etawah crossbreed goats. A total of 20 goats with an average initial body weight of 18.22 ± 3.09 kg were used in the study with a randomized block design consisting of 4 treatments and 5 replications. The four treatments tested were as follows: A: elephant grass + concentrate A (without HLF); B: treatment A + concentrate B (with 5% HLF); C: treatment A + concentrate C (with 10% HLF); and treatment D: Treatment A+ concentrate D (with 15% HLF). The protein balance, blood metabolic profile, body composition, and nutrient deposition were measured. The results showed that the goats that were treated D had the highest protein retention up to 9.89 g/head/day, but it was not significantly different from other treatments. Blood metabolic profile, body composition, and nutrient deposition were also not significantly different between all treatments. It can be concluded that the addition of HLF in concentrate does not affect the protein balance, blood metabolic profile, body composition, and nutrient deposition.
Keywords | Goat, Hibiscus, Protein, Blood and body composition
Received | January 20, 2022; Accepted | April 25, 2022; Published | June 15, 2022
*Correspondence | I.G.L.O. Cakra, Faculty of Animal Husbandry, Udayana University, Denpasar, Bali, Indonesia; Email: oka_cakra@unud.ac.id
Citation | Cakra IGLO, Dinata AANBS, Mahardika IG, Bidura IGNG (2022). Effect of Hibiscus leaf on protein balance, blood profile, and body composition of etawah crossbreed goats. Adv. Anim. Vet. Sci. 10(6):1325-1332.
DOI | https://dx.doi.org/10.17582/journal.aavs/2022/10.6.1325.1332
ISSN (Online) | 2307-8316
Copyright: 2022 by the authors. Licensee ResearchersLinks Ltd, England, UK.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
INTRODUCTION
The availability of adequate, high-quality feeds and forages has been a major challenge faced by the livestock sector, especially during the dry season when pasture and crop residues are scarce (Mtengeti et al., 2008; Maleko et al., 2018). Forage fodder (FP) is one of the feeds with a higher portion in goat rations. Elephant grass is the main forage plant that holds great importance, because forage contains almost all the substances needed by animals (Kastalani, 2017) Elephant grass has a lower nutritional content with a crude protein content of 6.8–10.8% (Mangiring et al., 2017), and is naturally less preferred than forage types of legumes. This can reduce consumption which has an impact on the reduced amount of nutrients that can be deposited in the body for growth. The utilization of elephant grass as goat feed can be done by increasing the palatability and digestibility of feed. One of the efforts to increase feed digestibility is through manipulation of rumen microbes with the addition of defaunation agents in concentrates. Rumen defaunation is carried out as an effort to reduce protozoan micro-fauna to create conducive ecological conditions in the rumen. Defaunation describes the removal of the protozoa population by means of chemical, anti-protozoa compounds or rumen washing (Li et al., 2018). Protozoa feed on rumen bacteria, resulting in an increase in rumen microbial N recycling and a 20-28% decrease in the supply of amino acids to the intestine (Yanuartono et al., 2019).
The consequence of elimination of rumen protozoa has largely impacted on reduced NH3 concentration in the rumen and increased microbial protein supply to the hosts (Nguyen et al., 2020). Viennasay et al. (2019) showed that the N-balance was improve when the digestibility of CP was high. In addition, rumen tannin-protein complex can support more protein available in lower-gut (Giang et al., 2017). Chemical compounds such as tannins, flavonoids, alkaloids and saponins contained in Hibiscus leaves have various biological activities. The four compounds are capable of being antibacterial against gram positive and gram negative bacteria, anticancer and antioxidants (Andriani et al., 2017; Samsudin et al., 2019). The content of saponins in Hibiscus leaves flour (HLF) has the potential as a defaunation agent (Armayanti et al., 2015). Rahayu et al. (2021) found that Hibiscus tiliaceus flower extract supplementation of 200 ppm/kg dry matter in the diet of bali cattle could increase the digestibility of dry matter (DMD), organic matter (OMD), neutral detergent fiber (NDFD), acid detergent fiber (ADFD). The addition of HLF at a dose of 0.32% in ammoniated rice straw-based feed was able to increase the digestibility of crude fiber (Nutigusti, 2011). The digestibility value of feed, especially protein, can be evaluated through a protein balance. A positive protein balance with a high protein retention value indicates that protein digestibility is more efficient. To determine the physiological status of the goat’s body, it is necessary to analyze the metabolic profile of the blood. Metabolic processes in the goat’s body play a role in converting food substances such as amino acids, fatty acids, and glucose into compounds needed for life processes.
The addition of HLF as a defaunation agent will affect the digestibility of feed which has an impact on the quality and quantity of nutrients that can be absorbed and metabolized in the body. Based on the above description, it is necessary to research to determine the effect of giving HLF in the concentrate on protein balance, blood metabolic profile, and body composition of Etawah crossbreed goats.
MATERIALS AND METHODS
Location and length of research
The in-vivo research was carried out at Sidemen Village, Sidemen Sub-district, Karangasem Regency, Bali Province. The study was conducted for 13 weeks, preceded by a feeding adjustment period of one week. Proximate analysis of rations, the protein content of feces, and urine was carried out at the Laboratory of Animal Nutrition, Faculty of Animal Husbandry, Udayana University. Blood metabolic profile analysis was carried out at the Mantra Medika clinical laboratory, Gianyar district.
Making Hibiscus leaf flour
Fresh Hibiscus leaves have been separated from the stems. cleaned of dirt, and air-dried without exposure to sunlight. The Hibiscus leaves were dried using an electric oven at 500C for 6 hours. The dried Hibiscus leaves were ground using a milling machine with a 60 mesh sieve.
Research design, livestock, and feed treatment
The experimental design used in this study was a Randomized Block Design (RBD) consisting of 4 treatments and 5 groups of animal body weight as replicates. This study used 20 male Etawah crossbreed goats aged 4-5 months with an average body weight of 18.22±3.09 kg. Goats were given worm drug at the start of this experiment. The cage building is made of wood and asbestos roof with a size of 6 × 9 m openly so that odors in the cage can be prevented by natural air flow. Inside the building, individual cages are made with a size 1.5 m long, 0.75 m wide and 0.75 m high from the floor. The stall has separate eating and drinking places. Cleaning of cages, feed and drinking equipment are carried out every morning before the animals are fed. At night, the cage is equipped with electric lighting. Each animal in the treatment was given elephant grass ad libitum and added concentrate according to treatment as much as 1% of body weight. Feed and water are given twice a day at 08.00 am and 04.00 pm. Drinking water comes from the drinking water company in Sidemen Village. The four treatments tested were as follows: treatment A: goats were fed elephant grass + concentrate A (without HLF); B: treatment A + concentrate B (with 5% HLF; C: treatment A + concentrate C (with 10% HLF); and D: Treatment A+ concentrate D (with 15% HLF). The composition and nutritional content of the treatment concentrate rations are presented in Table 1.
Protein balance
A sampling of feed, urine, and feces was carried out using the total collection method (balance trial). The goats were kept in individual metabolic cages, provided with feeder, water trough, and a separate system for collecting urine and faeces. Sampling was carried out every day for a total collection period of seven consecutive days. Feses and forage feed samples (elephant grass and concentrate) were taken as much as 200 g and naturally dried. The composite sample was then taken sub-samples based on the treatment in each group as much as 200 g. Stool samples were taken as much as 50 g while urine samples were taken 50 ml/day. Urine samples were added 2% HCL 75% (v/v) of the volume of urine samples, to prevent evaporation of nitrogen (Colbourne et al., 1968).
Table 1: Composition of ingredients and nutrient content of concentrate feed.
|
Feed ingredients (%) |
Consentrate |
Elephant Grass |
|||
|
A1) |
B |
C |
D |
||
|
Wheat bran |
82 |
77 |
72 |
67 |
- |
|
Rice bran |
10 |
10 |
10 |
10 |
- |
|
Hibiscus Leaf Flour (HLF) |
0 |
5 |
10 |
15 |
- |
|
Molasses |
5 |
5 |
5 |
5 |
- |
|
Lime (CaCO3) |
1 |
1 |
1 |
1 |
- |
|
Salt |
2 |
2 |
2 |
2 |
- |
|
Pignox |
0.5 |
0.5 |
0.5 |
0.5 |
- |
|
Nutrients (% DM) |
|||||
|
Dry Matter (%) |
88.7283 |
88.0807 |
88.0508 |
88.0971 |
13.7982 |
|
Organic Matter (%) |
85.3489 |
84.6310 |
84.4440 |
84.2319 |
75.4181 |
|
Crude Protein (%) |
13.3987 |
13.3018 |
13.1764 |
13.1234 |
10.3465 |
|
Crude Fat (%) |
3.2086 |
3.0896 |
2.9649 |
2.8571 |
5.7493 |
|
Crude Fiber (%) |
6.0817 |
6.3131 |
6.5282 |
6.7775 |
24.8023 |
|
Gross Energy (kcal/g) |
4.0986 |
4.0625 |
4.0177 |
3.9951 |
3.4175 |
|
Nitrogen Free Extract (%) |
70,6119 |
70,3066 |
70.1578 |
69.7797 |
42.9350 |
|
TDN (%) |
70.5859 |
70.3623 |
69.4945 |
68.9504 |
51.2964 |
1)A: Concentrate without HLF; B: Concentrate containing 5% HLF; C: Concentrate containing 10% HLF; D: Concentrate containing 15% HLF.
Samples of feed and feces were put into paper bags which the weight was known and then oven-dried at 70oC for 24 hours to determine the dry weight. The dried samples were ground with a 1 mm. sieve mill. Samples of feed, feces, and urine were analyzed for crude protein content using the Semi-Micro Kjeldahl (ICW) method (Ivan et al., 1974).
Blood metabolic profile
Blood samples were taken using a 3 ml vacutainer tube containing anticoagulant from the jugular vein before feeding and 4 hours after eating at the end of the collection. To separate the plasma, centrifugation was carried out at 500 rpm for 10 minutes. Blood plasma was stored at 20oC and then analyzed to determine the content of blood glucose levels, protein levels, blood urea nitrogen (BUN), triglycerides, low-density lipoprotein (LDL), high-density lipoprotein (HDL), and cholesterol.
Analysis of glucose levels was carried out using the GOD-PAP method (Dias, 1999), BUN was carried out using the Beckman Synchron LX20 enzymatic colorimetric method (Henrry, 1991). Blood triglyceride levels were carried out using the Glycerol-3-Phosphate oxidase-p-aminophenazone GPO-PAP method (Human Gesellschaft fur Biochemica and Diagnostica mbH, 2002). Blood cholesterol levels were carried out using the Cholesterol oxidase -p-aminophenazone CHOD-PAP method (Hans et al., 1980). Determination of HDL and LDL levels was carried out using the COHD-PAP method (Hans et al., 1980) and LDL using the formula compiled by Fridewald et al. (2001).
Body composition and nutrient deposition
The body composition of livestock was measured using the urea space technique according to Bartle et al. (1983). This measurement was carried out once, namely at the end of the experiment. Blood samples were taken through the external jugular vein as much as 5 ml, and then injected urea solution with a concentration of 30% as much as 0.44 cc/kg W0.75 into the blood circulation through the external jugular vein with venoject within 2 minutes. After 12 minutes of injection, 5 ml of blood was taken from the external jugular vein. Furthermore, the blood sample is centrifuged to obtain plasma fluid. The plasma fluid was analyzed to determine blood urea levels before and after the injection of urea solution. Blood urea was measured using a photometric system using a spectrophotometer model UV-VIS mc2 Safas SP2000 with a wavelength of 365 nm using the Berthelot-Reaction method. Calculation of body composition can be determined by calculating the urea space (US) with the formula:
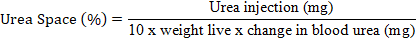
Estimation of empty body water content (empty body water = EBW), body fat and body protein was determined by the formula (Rule et al., 1986) namely: Body water (%) = 59.1 + 0.22 US-0.04 weight live (WL); Body fat (%) = 19.5-0.31 US+0.05 WL ; Body protein (%) = 16.5 + 0.07 US + 0.001 WL and body minerals (%) = 0.25 x % protein. Nutrient deposition can be determined by converting the daily body weight gain (DBWG) of livestock based on body composition. The calculated nutrient deposition is protein, fat and mineral deposition which can be calculated by the formula: Protein deposition (g/d) = % body protein × DBWG; Fat deposition (g/h) = % body fat × DBWG and mineral deposition (g/h) = % body minerals x DBWG.
Statistical analysis
The obtained data were analyzed with analysis of variance (ANOVA). If the treatment had a significant effect, it was followed by the Duncan’s Multiple Range Test based on the instructions of Gomez and Gomez (2010). Statistical Product and Service Solution (SPSS) Version 20 was utilized to facilitate data processing.
Ethical approval
The research procedure is in accordance with the principles of use and the principles of animal welfare and has received approval from the Animal Ethics committee of the Faculty of Veterinary Medicine, Udayana University No. B/29/UN14.2.9/PT.01.04/2022.
RESULTS AND DISCUSSION
Protein balance
Hibicus leaves are reported to contain 3% saponins, 48.2 ppm fumaric acid, and 78.6 ppm tannins. In addition, Hibiscus leaf extract using water as solvent is known contains anti-protozoal components, namely quinoline by 24.6% (Bata and Rahayu, 2017). The addition of HLF did not affect the protein balance. The addition of secondary metabolites has less effect on protein digestibility (Abarghuei et al., 2013) which causes no impact on protein balance. The results showed that the average retention of protein in goats it was not significantly different with all treatments (Table 2).
Protein excretion in feces in goats treated with D was significantly higher compared to treatments A and C, but not significantly different from treatment B. This was due to an increase in the tannin content in the ration in line with the increasing use of HLF in concentrate. Effects of tannin extract in a beneficial perspective on providing higher rumen by-pass protein with the appropriate level of tannin extract (Yanza et al., 2021). Tannins were associated with increasing levels of faecal N and decreasing levels of urinary N and the implication of the shift of N excretion from urine to feces (Kaitho et al., 1998). The tannin-protein complex bonds are stable at a pH of around 4-7 then will be broken in the abomasum because the pH is 2.5-3.5 which then enters the small intestine so that the protein can be digested and absorbed (Wiryawan et al., 1999). Increasing the amount of protein that escapes degradation in the rumen will increase protein retention.
Blood metabolic profile
Blood metabolism showed the supply of nutrients for livestock that comes from the quality of the feed given. In this study, the rations given to livestock had almost the same quality. This condition affects the supply of nutrients almost the same so that the metabolic levels of the rumen in the blood are not significantly different. The results revealed that only total blood cholesterol levels were significantly different. Goats treated with D had a blood cholesterol content of 70.2 mg/dl which was significantly higher (P<0.05) than goats treated with B, but not significantly different from goats treated with A and C (Table 3).
Blood cholesterol serves as a precursor for the biosynthesis of steroid hormones and bile acids. In addition, cholesterol in the blood is a response related to changes in the level of free fatty acids in the feed. Free fatty acids will be converted into co-acetyl-A which will turn into acetyl Co-A which is the main precursor for the formation of cholesterol (Maurya et al., 2004). Normal goat cholesterol values according to Astuti et al. (2011) of 65.86-70.26 mg/dl. Based on these data, the cholesterol levels of goats treated with A and D were in the normal range.
Table 2: Protein balance of goats fed HLF in concentrate.
|
Variable (g/head/d) |
Treatment1) |
|||
|
A |
B |
C |
D |
|
|
Protein consumption |
59.00±13.02 |
55.82±16.21 |
57.62±7.63 |
61.39±14.22 |
|
Protein in feces |
11.96 a2) ±1.10 |
12.99ab±3.83 |
11.41a±1.53 |
17.04b ±5.30 |
|
Amount of digestible protein |
47.05±12.37 |
42.83±12.45 |
46.21±7.00 |
44.35±9.71 |
|
Protein urine |
38.76±13.75 |
37.45±13.19 |
39.06±7.81 |
34.46±5.46 |
|
Protein retention |
8.23±3.59 |
5.42 ±3.63 |
7.12±2.19 |
9.89±4.90 |
|
Biological value |
18.74±11.49 |
13.23±9.41 |
15.77±5.94 |
21.39±7.04 |
|
Net Nitrogen utilization |
14.73±8.74 |
10.22±7.37 |
12.58±4.67 |
15.37±4.71 |
1)A: goats were fed elephant grass + concentrate A (without HLF); B: goats were fed elephant grass + concentrate B (with 5% HLF); C: goats were fed elephant grass + concentrate C (with 10% HLF); D: goats were fed elephant grass + concentrate D (with 15% HLF). 2) Values followed by different superscripts in the same row are significantly different (P<0.05).
Table 3: Metabolic profile of goat blood fed HLF in concentrate.
|
Variable (mg/dl) |
Teatments1) |
|||
|
A |
B |
C |
D |
|
|
Glucose |
47.12±6.90 |
45.96±8.50 |
38.94±8.36 |
38.34±5.15 |
|
Total protein (g/dl) |
5.10 ±0.50 |
5.41 ±0.53 |
5.05 ±0.95 |
5.70 ±0.57 |
|
BUN |
26.90±10.24 |
23.56±5.67 |
19.86±2.48 |
23.26±4.96 |
|
Triglycerides |
15.60±4.51 |
18.40±2.88 |
18.60±5.50 |
17.00±4.18 |
|
HDL |
44.22±11.14 |
42.74±7.26 |
43.36±4.68 |
41.32±5.31 |
|
LDL |
6.38±2.08 |
6.30±2.13 |
6.9±2.16 |
7.50±1.79 |
|
Total cholesterol |
66.8ab2)±7.82 |
56.2a±7.28 |
64.60ab±7.13 |
70.20b±5.02 |
Noted: 1) A: goats were fed elephant grass + concentrate A (without HLF); B: goats were fed elephant grass + concentrate B (with 5% HLF); C: goats were fed elephant grass + concentrate C (with 10% HLF); D: goats were fed elephant grass + concentrate D (with 15% HLF). 2) Values followed by different superscripts in the same row are significantly different (P<0.05).
Table 4: Body composition and nutrient deposition of goats fed Hibiscus Leaf Flour meal in concentrate.
|
Variable |
Treatment |
|||
|
A1) |
B |
C |
D |
|
|
Body composition |
||||
|
Water (%) |
58.29±0.15 |
58.3±0.12 |
58.35±0.09 |
58.26±0.21 |
|
Fat (%) |
20.52±0.19 |
20.48±0.15 |
20.43±0.11 |
20.55±0.26 |
|
Proteins (%) |
16.52±0.004 |
16.52±0.003 |
16.52±0.002 |
16.52±0.005 |
|
Minerals (%) |
4.13±0.001 |
4.13±0.001 |
4.13±0.001 |
4.13±0.001 |
|
Fat deposition (g/h/d) |
6.53±3.49 |
4.12±2.76 |
5.65±2.00 |
7.10±3.56 |
|
Protein deposition (g/h/d) |
5.25±2.81 |
3.33±2.23 |
4.57±1.60 |
5.69±2.77 |
|
Mineral deposition (g/h/d) |
1.31±0.70 |
0.83±0.56 |
1.13±0.40 |
1.42±0.69 |
1)A: goats were fed elephant grass + concentrate A (without HLF); B: goats were fed elephant grass + concentrate B (with 5% HLF); C: goats were fed elephant grass + concentrate C (with 10% HLF); D: goats were fed elephant grass + concentrate D (with 15% HLF).
Blood glucose level is a picture of energy supply in livestock. Glucose in the blood is released continuously to nourish various body tissues (Wahyuni et al., 2011). Blood glucose levels in this study were lower than Yupardi et al. (2014) found an average blood glucose level of Etawah crossbreed goats of 64.67 mg/dl. Glucose levels related to the level of consumption and digestibility of feed organic matter were not significantly different. This is because all components of the rations such as organic matter, carbohydrates, fats, and proteins in the ration, will be absorbed into the body into glucose. The results of carbohydrate digestion in ruminants are glucose, acetic acids, propionate, butyrate, CO2, and methane gas. Volatile fatty acids (VFA) derived from the digestion of feed in the rumen are absorbed into the blood circulation and then to the liver where they are converted into energy, fat, and glycogen. VFA is the largest energy source for ruminants (McDonald et al., 2010). Giving HLF containing saponins did not affect total blood protein. Blood protein levels associated with protein consumption were not significantly different (Table 2). This is in line with the research of Nasri and Salem (2012) who found that there was no effect of giving plant sources of saponins on total blood protein. The total protein in goat blood obtained in this study was lower than the results of the study. Rostini and Zakir (2017) found an average total protein level in the blood of Etawah goats of 7.47 mg/dl. Blood urea nitrogen (BUN) is the end product of protein metabolism. The average BUN in this study was in the range of 19.86-26.9 mg/dl. Kramer (2000) stated that the Normal BUN range is 10-26 mg/dL. The decrease in BUN may be due to decreased proteolysis in the rumen and decreased ammonia production. Saponins can reduce the production of ammonia in the rumen and thus affect the blood urea concentration (Wina et al., 2005). Lower rumen ammonia concentrations indicate increased protein digestion and utilization, thereby reducing the amount of ammonia released in the blood and converted by the liver to N-urea as a non-toxic end product, resulting in a decrease in BUN (Abeer et al., 2020). BUN concentrations were associated with rumen ammonia nitrogen concentrations Khattab et al. (2013). Crude protein will be degraded into ammonia in the rumen, and then ammonia is used by rumen bacteria for microbial protein synthesis. If there is an excess of ammonia production, it can cause the ammonia to be absorbed by the rumen wall and into the blood circulation which then enters the liver. In the liver, ammonia will be converted to urea with the help of the urea cycle enzyme (Peter et al., 2010). Then urea is secreted into the blood and can be measured through the BUN.
Blood triglyceride levels are a picture of the fat content consumed. Blood triglyceride levels with different concentrations were not significant, most likely because the supply of organic matter metabolized by livestock was almost the same in response to the presence of almost the same organic matter content in the rations and the level of consumption and digestibility of organic matter was not significantly different. This is because all components of the organic matter of the ration, both carbohydrates and protein in the ration, will be absorbed into the body and stored in the body in the form of triglycerides (fat) which will later be used as energy for livestock for maintenance, production, and reproduction.
Different triglyceride levels were not significantly different, causing the levels of high-density lipoprotein (HDL) and low-density lipoprotein (LDL) to be not significantly different. Blood HDL and LDL levels are part of the lipoprotein that contains cholesterol and triglycerides. In this study HDL levels were higher than LDL levels. High levels of HDL are important because HDL also functions as an antioxidant and anticoagulant that can prevent various diseases in the body of livestock Andersen et al. (2009).
Body composition and nutrient deposition
The main components of the body composition of livestock are water, protein, and fat. Water and protein are relatively constant, while the fat component varies. Measurement of body composition using the urea chamber technique showed that the water, fat, protein, and mineral levels were not significantly different between all treatments (Table 4). The values of fat, protein, and mineral deposition were also not significantly different between all treatments. This nutrient deposition is influenced by the nutritional content of the ration which is relatively the same. Fat deposition is related to glucose and triglyceride levels in the blood which causes the amount deposited in the body to be higher.
Protein deposition will determine livestock production and growth, the higher the protein deposition, the better the growth (Boorman, 1980). According to Maynard and Loosli (1969), protein deposition with a positive value causes an increase in body weight due to the addition of meat tissue, while a negative protein deposition will cause a decrease in body weight due to the disassembly of protein to meet the needs of life. The highest protein deposition in goats treated with D was associated with high protein consumption. High protein consumption will affect the increase in protein that escapes rumen degradation as a source of protein for the host.
HLF contains saponins and tannins which can bind proteins so that it is more difficult to be degraded by microbes in the rumen. Saponins that can suppress the number of protozoa and even bacteria in high doses will reduce the effectiveness of rumen microbial feed degradation. The presence of tannins that can proteins protect, further reduces the ability of microbial degradation in the rumen. This causes the protein content of Hibiscus leaves to pass directly to the abomasum as a source of by-pass protein for the landlady. Increased protein escaping from the rumen can be associated with increased body protein deposition, because increased protein escaping from the rumen is a source of protein for the livestock itself.
CONCLUSIONs and Recommendations
Hibiscus leaf flour supplementation in concentrate did not affect the protein balance, blood metabolic profile, body composition, and nutrient deposition of Etawah crossbreed goats basal fed elephant grass.
ACKNOWLEDGEMENTS
A greeting thanks also go to Head and Staff of Animal Nutrition and Feedstuff Laboratory, Faculty of Animal Husbandry, Udayana University on facilities for analyzing the data of this research. Finally, to all those who play a role, the authors would like to thank.
Novelty Statement
The presence of tannins and saponins in Hibiscus tiliaseus leaf flor has no effect on protein balance, blood metabolic profile, body composition, and nutrient deposition of Etawah crossbreed goats
AUTHOR’S CONTRIBUTION
IGLO Cakra research designed and drafted the manuscript. AANBS Dinata Statistical analysis and data collection, IG Mahardika and IGNG Bidura assisted in the research process. All authors make significant research contributions and approved the manuscript.
Conflict of interest
The authors have declared no conflict of interest.
REFERENCES
Abarghuei MJ, Rouzbehan Y, Salem AZM, Zamiri MJ (2013). Nutrient Digestion, Ruminal Fermentation and Performance of Dairy Cows Fed Pomegranate Peel Extract. Livest. Sci. 157: 452–461. Journal Homepage: www.elsevier.com/locate/livsci, https://doi.org/10.1016/j.livsci.2013.09.007
Abeer, El-Essawy, M., Anele, U. Y., Abdel-Wahad, A. M., Ahlam, R. Abdou, R and Khattab, I. M. (2020). Effects of Anise, Clove and Thyme Essential Oils Supplementation on Rumen Fermentation, Blood Metabolites, Milk Yield and Milk Composition in Lactating Goats. J. Pre-Proof : 1-39.
Andersen ML, Perry JC, Bignotto M, Tufik S (2009). Differential effect of sleep loss and chronic stressors on lipid metabolism. Sleep Sci., 2(3): 135-140.
Andriani Y, Mohamad H, Bhubalan K, Abdullah MI, Amir H (2017). Phytochemical analysis, anti-bacterial and anti-biofilm activities of mangrove associated Hibiscus tiliaceus extracts and fractions against pseudomonas aeruginosa. J. Sustain. Sci. Manag., 12(2): 45-51.
Armayanti AK, Nuswantasa IK, Achmadi J (2015). Combination of soybean meal and Hibiscus tiliaceus leaf flour in the goat diet: Effect of some parameters of carbohydrat metabolism. J. Indones. Trop. Anim. Agric., 40(3): 153-158. https://doi.org/10.14710/jitaa.40.3.153-158
Astuti DA, Baba AS, Wibawan IWT (2011). Rumen fermentation, blood metabolties and performance sheep. J. Anim. Sci. Tecnol., 34(3): 201-206. https://doi.org/10.5398/medpet.2011.34.3.201
Bata M, Rahayu S (2017). Evaluation of bioactive substances of Hibiscus tiliaceus and it’s potency to minimize methane emission and rumen efficiency. Curr. Bioact. Compd., 13(2): 157-164. https://doi.org/10.2174/1573407213666170109151904
Bartle SJ, Males JR, Preston RL (1983). Evaluation of urea dilution as an estimator of body composition in mature cows. J. Anim., 57: 410-417. https://doi.org/10.2527/jas1983.562410x
Boorman KN (1980). Dietary constrait on nitrogen retention dalam: (eds. P.J. Buttery and D.B. Lindsay). Protein Deposition in Animals. 1st Ed. London: Butterworth. https://doi.org/10.1016/B978-0-408-10676-4.50013-6
Coulborne, J.W., J.L. Even and C.F Ransminger. (1968). The Stage of Maturity and its Effect Upon the Chemical Composition of Four Nature rang Species. J.of Range Management, 26 (6) : 460-463.
Dias, T.S., (1999). Leaflet Glucose GOD PAP, Diacnostic System (Diasys) Internasional.
Fridewald NT, Levy RI, Frieddericson RI (2001). Estimation of the concentration of low density lipoprotein cholesterol plasma without use the prepagative ultracentri fugation. Clin. Chem., 18: 499-502. https://doi.org/10.1093/clinchem/18.6.499
Giang NTT, Wanapat M, Phesatcha K, Kang S (2017). Effect of inclusion of different levels of Leucaena silage on rumen microbial population and microbial protein synthesis in dairy steers fed on rice straw. Asian-Australas. J. Anim. Sci., 30: 181. https://doi.org/10.5713/ajas.15.0948
Gomez KA, Gomez AA (2010). Prosedur statistik untuk penelitian pertanian. Edisi Kedua. Jakarta (Indones): UI Press.
Hans F, Prafulla A, Heide K, Ingeborg K (1980). Use of a simple enzymatic assay for cholesterol analysis in human bile. J. Lipid Res., 21(1): 259. https://doi.org/10.1016/S0022-2275(20)39833-3
Henrry JB (1991). Clinical diagnosis and management by laboratory methods; 18th Ed. Philadelphia: W.B Saunders.
Human Gesellschaft fur Biochemica und Diagnostica mbH. (2002). Triglycerides Liquicolor. Germany.
Ivan, M., D. J. Clack, and White, G. J. (1974). Kjeldahl Nitrogen Determination. In Short Course on Poultry Production, Udayana University, Denpasar.
Kaitho RJ, Umunna NN, Nsahlai IV, Tamminga S, Van Bruchem J (1998). Utilization of browse supplements with varying tannin levels by Ethiopian Menz Sheep. 2. Nitrogen Metabolism. Agroforest. Syst. 39: 161–173. https://doi.org/10.1023/A:1005961018328
Kastalani K (2017). Pengaruh pemberian pupuk bokashi terhadap pertumbuhan vegetatif rumput gajah (Pennisetum purpureum). Ziraa’ah Majalah Ilmiah Pertanian, 42(2): 123-127.
Khattab IM, Salem AZM, Abdel-Wahed AM, Kewan KZ (2013). Effects of urea supplementation on nutrient digestibility, nitrogen utilisation and rumen fermentation in sheep fed diets containing dates. Livest. Sci., 155: 223-229. https://doi.org/10.1016/j.livsci.2013.05.024
Kramer JW (2000). Schlam’s veterinary hematology. Philadelphia (US): William and Wilkins.
Li Z, Deng Q, Liu Y, Yan T, Li F, Cao Y, (2018). Dynamics of methanogenesis, ruminal fermentation and fiber digestibility in ruminants following elimination of protozoa: a meta analysis [Internet]. Journal of Animal Science and Biotechnology. Available from: http://dx.doi.org/10.1186/s40104-018-0305-6
Mangiring W, Kurniawati N, Priyadi (2017). Production and quality pennisetum purpureum at shading condition and nitrogen fertilizer dosage. J. Penelitian Pertanian Terapan, 17(1): 58-65. ISSN 1410-5020 eISSN Online 2047-178. http://www.jptonline.or.id, https://doi.org/10.25181/jppt.v17i1.41
Maurya VP, Naqvi, SMK, Mitta JP (2004). Effect of dietry energi level on physiological responses and reproductive performance of malpura sheep in the hot semi-arid regions of India. J. Small Ruminant Res., 55: 117-122. https://doi.org/10.1016/j.smallrumres.2003.12.008
Maynard LA, Loosli JK (1969). Animal nutrition. 6th Edition. New Delhi: Mc. Graw Hill Book Company.
McDonald P, Edward RA, Greenhalgh JFD, Morgan A, Sinclair LA, Wilkinson RG (2010). Animal nutrition. Seventh Edition..Harlow-England: Pearson.
Maleko, D., Ng, W. T., Msalya, G., Mwilawa, A., Pasape, L., and Mtei, K. (2018). Seasonal variations in the availability of fodder resources and practices of dairy cattle feeding among the smallholder farmers in Western Usambara Highlands, Tanzania. Trop. Anim. Health Prod. 50, 1653–1664. doi: 10.1007/s11250-018-1609-4
Mtengeti, E. J., Phiri, E. C. J. H., Urio, N. A., Mhando, D. G., Mvena, Z., Ryoba, R., et al. (2008). Forage availability and its quality in the dry season on smallholder dairy farms in Tanzania. Acta Agric. Scand. A Anim. Sci. 58, 196–204. doi: 10.1080/09064700802492362
Nasri S, Salem BH (2012). Effect of oral administration of agave americana or quillaja saponaria extracts on digestion and growth of barbarine female lamb. Livest. Sci., 147: 59–65. https://doi.org/10.1016/j.livsci.2012.04.001
Nguyen SH, Nguyen HDT, Hegarty RS (2020). Defaunation and its impacts on ruminal fermentation, enteric methane production and animal productivity. Livest. Res. Rural Dev., 32, Article #60. Retrieved February 28, 2022.
Nutigusti PB (2013). Pengaruh penambahan tepung daun Hibiscus (Hibiscus tiliaceus) dalam Ransum Sapi Lokal Berbasis Jerami Padi Amoniasi terhadap Kecernaan Protein Kasar dan Serat Kasar. Jurnal Ilmiah Peternakan, 1(2): 669-676.
Peter R, Cheeke, Dierenfeld ES (2010). Comparative animal nutrition and metabolism. Cambridge (USA): Cambridge University Pr., https://doi.org/10.1079/9781845936310.0000
Rahayu S, Veven RB, Muhamad B (2021). Feed intake, blood parameters, digestibility and live weight gain of male bali cattle (Bos javanicus) fed ammoniation rice straw supplemented by waru (Hibiscus tiliaceus) flower extracts. Anim. Prod., 23(3): 171-179. https://doi.org/10.20884/1.jap.2021.23.3.12
Rostini T, Zakir I (2017). Performans produksi, jumlah nematoda usus, dan profil metabolik darah kambing yang diberi pakan hijauan rawa kalimantan. J. Vet., 18(3): 469-477. https://doi.org/10.19087/jveteriner.2017.18.3.469
Rule DC, Arnold RN, Hentges EJ, dan Beitz DC (1986). Evaluation of urea dilution as a technique for estimating body composition of beef steers in vivo: Validation of published equation and comparison with chemical composition. J. Anim. Sci., 63: 1935-1948. https://doi.org/10.2527/jas1986.6361935x
Samsudin MS, Andriani Y, Sarjono PR, Syamsumir DF (2019). Study on Hibiscus tiliaceus leaves as antibacterial and antioxidant agents. Alotrop Jurnal Pendidikan Dan Ilmu Kimia, 3(2): 123-131. https://doi.org/10.33369/atp.v3i2.9874
Viennasay B, Wanapat M, Phesatcha K, Phesatcha B, Ampapon T (2019). Replacement of rice straw with cassava-top silage on rumen ecology, fermentation and nutrient digestibilities in dairy steers. Anim. Prod. Sci., 59: 906–913. https://doi.org/10.1071/AN17477
Wahyuni RS, Retno B, Romziah S (2011). Profil total protein dan glukosa darah domba yang diberi starter bakteri asam laktat dan yeast pada rumput gajah dan jerami padi. J. Ilmiah Kedokteran Hewan, 4(1): 65-70.
Wina E, Mutetzel S, Becker K (2005). The impact of saponin-containing plant aterials on ruminant production. A review. J. Agric. Food Chem., 53: 8093-8105. https://doi.org/10.1021/jf048053d
Wiryawan, K. G., E. Wina & R. Ernawati. (1999). Utilization of calliandra tannins as a protective agent for some feed protein sources (in vitro). Proceedings of the Seminar on Research Results in the Life Sciences page: 278-289
Yanza YR, Fitri A, Suwignyo B, Elfahmi, Hidayatik N, Kumalasari NR, Irawan A, Jayanegara A (2021). The utilisation of tannin extract as a dietary additive in ruminant nutrition: A meta-analysis. Animals, 11: 3317. https://doi.org/10.3390/ani11113317
Yanuartono, Nururrozi A, Indarjulianto S, dan Purnamaningsih H (2019). Peran protozoa pada pencernaan ruminansia dan dampak terhadap lingkungan. J. Trop. Anim. Prod., 20(1): 16-28. https://doi.org/10.21776/ub.jtapro.2019.020.01.3
Yupardhi WS, Oka IGL, Mantra IB, dan Suyasa IN, Suranjaya IG (2014). Gambaran darah kambing gembrong, kambing peranakan eawah dan kambing kacang di bali. J. Vet., 15(4): 494-498.
To share on other social networks, click on any share button. What are these?






