Effect of Heat-Treatment Duration on Antioxidant Activities of Muscle, Liver and Other Parts of Grass Turtle (Chinemys reevesii)
Effect of Heat-Treatment Duration on Antioxidant Activities of Muscle, Liver and Other Parts of Grass Turtle (Chinemys reevesii)
Md. Serajul Islam1,2, Hongxin Wang1,2,*, Amer Ali Mahdi1, Mohamed Ismael Ahamed2, Zaixiang Lou1 and Fu An Wei3
1Food Nutrition and Functional Factors Research Center, School of Food Science and Technology, Jiangnan University, 1800 Lihu Avenue, Wuxi-214122, Jiangsu province, China
2State key Laboratory of Food Science and Technology, Jiangnan University, 1800 Lihu Avenue, Jiangsu Province, China
3 Guangxi Zhongtaikang Technology Industry Co., Ltd., Nanning-530029, Guangxi, P.R. China
ABSTRACT
The aim of the study was to determine the effect of duration of thermal treatment on the antioxidant activities (ABTS, DPPH, and FRAP) of muscle, liver, hard shell, bone, and skin from grass turtle (Chinemys reevesii) by direct QUENCHER procedure. The results showed that the highest ABTS capacity was found in cooked muscle at 10 min (110.133± 4.153 g trolox Eq./kg). On the other hand, DPPH and FRAP capacity were found in cooked muscle at 20 min which were 68.966±0.937 and 37.437±1.027 g trolox Eq./kg, respectively, and raw liver 31.508±1.091 and 58.237±0.919 g trolox Eq./kg, respectively. The total antioxidant capacities of the samples increased by thermal treatment at 180°C for 5 min in liver, 10 min in muscle and hard shell, 20 min in skin and bone by ABTS assay; 15 min in skin, 20 min in muscle, liver, hard shell, and bone by DPPH assay, but FRAP value decreased by heating at different time. These results suggested that grass turtle could be used as food additives to improve the functional food properties.
Article Information
Received 21 November 2019
Revised 22 July 2020
Accepted 05 March 2021
Available online 08 October 2021
(early access)
Published 23 May 2022
Authors’ Contribution
MSI presented the concept of the study, performed the experiments and
wrote the manuscript. HW supervised the study and edited the manuscript. AAM performed the data analyses. MIA helped in editing the manuscript. ZL helped in the analysis with constructive discussions. FAW did the formal data analysis and raw materials supply.
Key words
Antioxidant capacity, Grass tortoise, Heating treatments,QUENCHER method.
DOI: https://dx.doi.org/10.17582/journal.pjz/20191121081123
* Corresponding author: hxwang@jiangnan.edu.cn
0030-9923/2022/0005-2003 $ 9.00/0
Copyright 2022 by the authors. Licensee Zoological Society of Pakistan.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Introduction
A ntioxidants are components that inhibit oxidation reaction. Zhang et al. (2015) reported that meat and meat products quality deteriorates by oxidative chemical reaction. The intrinsic oxidative stability of meat depends on the dietary background of animals because the balance between pro-oxidant and antioxidant substances in muscle can be strongly affected by the diet (Luciano et al., 2013). Antioxidant activity evaluation is related to nutritional quality and antioxidant equivalents intake (Serpen et al., 2007). Cho et al. (2011) reported that have a positive correlation of natural antioxidants that contribute to be reduced coronary heart diseases, cancer mortality, and longer life expectancy. Meat profiles changes could significantly affect the total antioxidant capacity during cooking (Palka and Daun, 1999; Pérez-Jiménez and Saura-Calixto, 2005). The secondary and tertiary proteins structure and their physical properties are modified by thermal treatments (Sante-Lhoutellier et al., 2007; Tironi et al., 2002). Elias et al. (2007) found that unfolding proteins led to increase their antioxidant capacity to scavenge radicals. Therefore, a reliable estimation total antioxidant capacity (TAC) value of muscle, liver, hard shell, bone, and skin can be useful in the field of natural antioxidant and to determine modifications of TAC values during thermal processing.
Grass turtle (Chinemys reevesii) is an aquatic species native to China, Taiwan, Japan, and Hong Kong (Dai et al., 2012). Recently, many researches have focused on the practical utilization of various aquatic species and their by-products e.g. skins, bones, and shell (Nalinanon et al., 2008). Chinese softs shell turtle (Pelodiscus sinensis) is a commercially valuable because it high contains nutritional and medicinal values such as antioxidation, anticancer, and decreasing blood pressure (Zou et al., 2017). Many methods are used to extract and determine antioxidant capacity but QUENCHER procedure is a new and direct method used for this purpose. Since currently most of useful to measurement antioxidant activities by ABTS, DPPH, and FRAP assay based on QUENCHER procedure (Vural et al., 2009). Serpen et al. (2012) reported that the raw meat of chicken, pork, beef and fish contain antioxidant activities.
Several studies have focused on origin, trade, cultivation, traditional medicine, and pets of turtles (Chen et al., 2009) and investigated chemical composition of some tortoise species (Kienzle et al., 2006), but no information is available on antioxidant capacity of grass turtle. To improve the oxidative stability of meat products, synthetic antioxidants (BHA, BHT, and TBHQ) have been widely used but most of the consumers have shown a growing interest in natural antioxidants for preservative safety and toxicity (Dudonné et al., 2009; O’Grady et al., 2006; Sureshk et al., 2010; Vasta and Luciano, 2011). Therefore, the aim of this study was (i) to evaluate and compare the antioxidant activities of different parts of grass turtle, and (ii) to evaluate the effect of thermal treatment on the antioxidant activities.
Materials and methods
Chemical reagents
Potassium peroxdisulfate (K2S2O8), glacial acetic acid (CH3COOH), sodium acetate (C2H3NaO2), ferric chloride (FeCl3.6H2O), absolute methanol (CH3OH) and ethanol (CH3CH2OH) were purchased from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China) and α-Cellulose powder (C6H10O5)n; trolox [6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (C14H18O4)]; ABTS [2,2′-azinobis (3-ethylbenzothiazoline-6-sulfonic acid)]; DPPH [1,1 diphenyl-2-picryl-hydrazyl (C18H12N5O6)]; TPTZ [2,4,6-tris-2,4,6-tripyridyl-2-triazine (C18H12N6)] were purchased from Aladdin (Shanghai, China). All other chemical reagents and solvents used were pure and analytical grade.
Sample collection and thermal treatments
Grass turtle (Chinemys reevesii) were obtained from Guangxi Zhongtaikang Technology Industry Co., Ltd, Nanning, Guangxi, China. The grass turtle(s) immediately were slaughtered by using knife, clean and divided into five selected parts (muscle, liver, hard shell, bone, and skin). Samples were put in the fresh box with ice bag when transported to laboratory (Nutrition and Function Factors Food Research Center, Jiangnan University). After consultation with relevant Chinese authorities, it was not an experimental animal, and it was unnecessary to issue animal ethics certificate. These samples were cooked according to method of Serpen et al. (2012) with minor modification. Briefly, the samples were minced by using blender machine (600 Y, Yang kang, Boou, China) and then prepared to cylinder shaped (2.5 cm diameter and 0.5 cm thickness) and spreading by aluminum caps. The samples were cooked into air oven (101-2A, Shanghai, China) at 180 °C for different time including 5, 10, 15, and 20 min. Then samples were lyophilized and grinded by using a mortar to get powder.
Measurement of TE antioxidant capacity by Quencher method
Sample preparation to Quencher procedure
The Quencher procedure was used to prepare of samples for determination of antioxidant capacity according to Serpen et al. (2012) with slight modifications. Primarily the dilution was implemented by mixing the freeze dried powdered sample was mixed with cellulose powder at the ratio from 1:1 to 1:10 (w:w) and shaken rigorously by a vortex mixer (XW-80A) for proper mixing. Our preliminary tests showed that a 1:10 (w:w) solid state dilution was suitable for ABTS, DPPH, and FRAP assay.
Preparation of ABTS•+, DPPH• and FRAP radical solutions
ABTS [2, 2’-azino-bis (3-ethylbenzothiazoline-6-sulphonic acid)] solution was prepared according to a method of Serpen et al., (2012) with slight modification. The stock solution of ABTS•+ was incubated in dark for 12-16 h at room temperature before use (Descalzo et al., 2007). For working solution preparation, 10 mL of stock solution was diluted with about 800 mL of H2O:C2H5OH (50:50, v:v) to obtain final absorbance value 0.75–0.80 at 734 nm by using Spectrophotometer (UV-2100, Unico, Shanghai, China).
The DPPH• solution was prepared according to a method of Brandwilliams et al. (1995). For working solution preparation, 200 mL stock solution of DPPH was diluted with about 800 mL of [H2O:C2H5OH (50:50, v:v)] solution.
The FRAP solution was prepared according to a method by Benzie and Strain (1996) with slight modifications. Briefly, reagent was prepared by diluting of 10 mM TPTZ in 40 mM of HCl, and 20 mM ferric chloride, and 0.3 M sodium acetate buffer (pH 3.6) adjusted by 16 mM CH3COOH. Finally, FRAP working solution was prepared at ratio of 1:1:10 (v:v:v) (TPTZ: ferric chloride: sodium acetate buffer).
Standard curve of trolox
Trolox was used as standard of each sample for the trolox equivalent antioxidant capacity (TEAC). Standard trolox solution was diluted into an absolute methanol at a concentration of 0-700 μg/mL for ABTS•+, DPPH• and 0-600 μg/mL for FRAP. Then 0.1 mL each trolox solution was added into 9.9 mL of ABTS•+, DPPH•, and FRAP radical solution and kept in a dark place for incubation at room temperature for 30 min. Finally, the radical solution (3 mL) was transferred into a cuvette to absorbance analyzed at 734, 525, and 593 nm for ABTS, DPPH, and FRAP assay, respectively.
Standard calibration curves were built by plotting % inhibition [equation (1)] against the concentration of trolox at 734 and 525 nm for ABTS and DPPH, respectively (Fig. 1A, B).
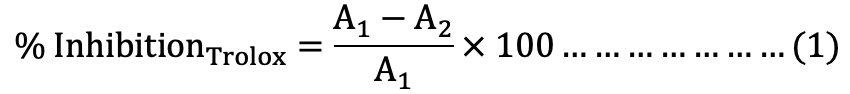
Where, A1 is absorbance of blank, and A2 is absorbance of trolox.
Procedure for measurement of antioxidant activities by direct Quencher method
Each powder sample (10 ±1.0 mg) was mixed with cellulose in a centrifuge tube at the ratio of 1:10 for ABTS, DPPH, and FRAP probe. The reaction was started by adding 10 mL working solution of ABTS•+, DPPH•, FRAP and tube was firstly shaken rigorously for 1 min, then shaken at 300-400 rpm for 30 min by orbital shaker at room temperature to facilitate the surface reaction between the solid particles and solution. After shaking, the solution was centrifuged at 4000 rpm for 2 min. Finally, 3 mL of clear supernatant was transferred into a cuvette to determine the absorbance of ABTS, DPPH, and FRAP assay at 734, 525, and 593 nm respectively by using Spectrophotometer (UV-2100, Unico, Shanghai, China). The percentage of inhibition of the ABTS•+ and DPPH• radicals were calculated by the using of formula [equation (2)]:
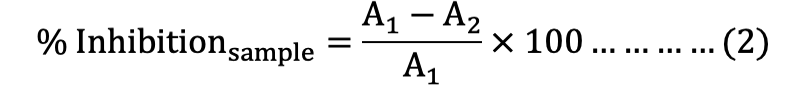
Where, A1 is absorbance of blank, and A2 is absorbance of samples.
Trolox calibration curve was expressed as the TEAC between the percentage of inhibition within the sample and slope, which was used to indicate the ABTS and DPPH free radical scavenging capability of the samples on a dry basis by the following equation:
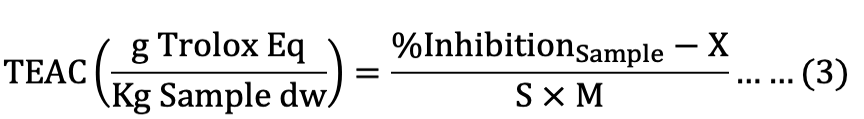
Where, X is the intercept of ABTS (XABTS= 0.0104) and DPPH (XDPPH= 0.0094), S is the slope of trolox calibration curve for ABTS (SABTS= 0.1436) and DPPH (SDPPH=0.1099), and M is the weight of sample (mg, on dry weight basis).
FRAP assay was measured according to absorbance value, a calibration curve was constructed the concentration against the absorbance at 593 nm (Fig. 1C). TEAC values of the samples were calculated by using following formula:
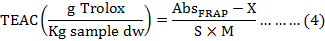
Where, AbsFRAP expresses the absorbance of sample, X and S represent the intercept (XFRAP= 0.1739) and the slope (SFRAP= 0.0013) of the trolox calibration curve, and M is the weight of sample (mg, on dry weight basis).
Statistical analysis
All experiments were performed in triplicate. The analysis data were statistically evaluated as the mean ± SD and measured by IBM SPSS Statistics for version 22.0 (SPSS, 2013). Significant differences between means were determined by one-way ANOVA and Duncan’s Multiple Range Test, p values less than 0.05.
Results and discussion
Antioxidants activity cannot be investigated acutely by a single assay (And and Sauracalixto, 2005). Two or more radical scavenging capacity assays are required to determine the mixed samples due to each assay involves with a different chemical mechanism that may reflect of their antioxidant properties. Antioxidants are reduced the oxidative changes in the meat and by-products. Oxidative changes have a negative effect on the quality of meats consequently their sensory and nutritional properties will be changes (Shah et al., 2014).
Antioxidant activity of muscle
The results of total antioxidant capacity (TAC) of raw and cooked muscle were evaluated by three assays ABTS, DPPH, and FRAP are presented in Figure 2A. As showed, ABTS and DPPH scavenging antioxidant capacity of raw muscle was lower than the cooked muscle, while FRAP result of raw muscle was higher. The TAC of raw muscle was ranged from 47.192±1.616 to 22.734±0.357 g trolox Eq./kg and cooked muscle was ranged from 35.952±1.006 to 97.277±2.286 g trolox Eq./kg at 5 min; 35.448±0.826 to 110.133±4.153 g trolox Eq./kg at 10 min; 32.556±0.565 to 98.371±1.435 g trolox Eq./kg at 15 min; 37.437±1.027 to 96.634±0.882 g trolox Eq./kg at 20 min in ABTS, DPPH, and FRAP assays, respectively. The TAC of 10 min cooked muscle (110.133±4.153 g trolox Eq./kg) was significantly (p <0.05) higher in ABTS assay than cooked muscle of 10 min by using DPPH and FRAP. On the other hand, cooked muscles of 5, 15, and 20 min had not significantly different (p <0.05) but raw muscle was significantly lower than the cooked muscle in ABTS assay. In Addition, DPPH result was no significant differences between 15 min and 20 min in cooked muscle but slightly differences was between 5 min and 10 min (Fig. 2A). Moreover, FRAP values of cooked muscle with no significant differences.
Figure 2A shows that the total antioxidant capacity has been influenced by thermal treatment of cooked muscle except FRAP assay. Whereas 10 min cooked muscle obtained positive effect on TAC by ABTS assay but more than 10 min TAC had negative effect. Similarly, 15 and 20 min cooked muscle contained a high antioxidant activity by DPPH test, while FRAP assay was the negative effect in cooked muscle. Serpen et al. (2012) reported ABTS scavenging capacity of the raw meat samples ranged from 25.9±1.0 to 51.7±1.2 mM trolox Eq./kg and DPPH
scavenging capacity was 19.1±1.8 to 31±0.9 mM trolox Eq./kg, whereas our raw muscle provided 140.400±1.586 and 90.829±1.427 mM trolox Eq./kg in ABTS and DPPH scavenging antioxidant capacity and 188.551±6.455 mM trolox Eq./kg in FRAP.
Antioxidant activity of liver
ABTS, DPPH, and FRAP assays in the raw and cooked liver evaluated are revealed in Figure 2B. The results showed that the highest TAC of raw liver was 58.237±0.919 g trolox Eq./kg in FRAP. On the other hand, cooked liver had the highest value 68.457±1.455 g trolox Eq./kg at 5 min followed by 40.666±1.696 g trolox Eq./kg at 15 min in ABTS assay. Similarly, DPPH showed the best result 39.048±1.086 g trolox Eq./kg at 20 min but 15 min later cooked liver was decrease (32.607±0.368 g trolox Eq./kg) with significantly. The highest activity FRAP was 53.446±1.512 g trolox Eq./kg after 5 min cooking. Comparing the raw and cooked liver values in ABTS, DPPH, and FRAP, overall the cooked liver (except 20 min) was significantly higher than raw sample, but in ABTS was no significant differences between 10 min and 15 min at 180oC. Similarly, cooked liver at 20 min was significantly higher (p<0.05) than 5, 10, and 15 min treatment liver when used DPPH assay. On the other hand, when applied FRAP assay, the raw sample was significantly higher than cooked sample.
The thermal treatment had affected TAC in cooked liver as shown by ABTS and DPPH probes but had no effect on FRAP. Martínez et al. (2015) reported that TAC levels decreased with the increasing the heating time of meat samples treatment. In this study, FRAP capacity of cooked liver was decreased by heating, which may be due to reduction of the Fe+3–TPTZ (2,4,6-tris-2,4,6-tripyridyl-2-triazine) complex for the ferrous form at a low pH value. Martínez et al. (2015) mentioned that TAC estimate of the standard diet around 29006 μmol trolox Eq. per intake whole diet per day, whereas our sample gave the value of about 273510.728±5814.962 μmol trolox Eq./Kg in ABTS, 156009.695±4337.742 μmol trolox Eq./Kg in DPPH and 232676.840±3670.451 μmol trolox Eq./Kg in FRAP on the dry basis.
Antioxidant activity of hard shell
The results of total scavenging antioxidant capacity of cooked hard shell followed the gradation ABTS>FRAP>DPPH whereas the raw sample followed FRAP>ABTS>DPPH gradation (Fig. 2C). There were statistically no significant differences (p <0.05) between DPPH and FRAP both alone in the raw and cooked hard shell. ABTS ranged from 25.707±0.336 to 54.913±1.615 g trolox Eq./kg, DPPH ranged from 4.858±0.051 and 6.767±0.131 g trolox Eq./kg, and FRAP assay showed the range of 14.406±0.223 to 17.192±0.781 g trolox Eq./kg in cooked hard shell. Turtle shell is widely used in medicine and cosmetics (Chen et al., 2009) so antioxidant capacity is an important to enhance the nutritional quality and shelf life of the products. TAC values were significantly increased until 10 min in ABTS, while DPPH result increased at every treatment time. Similarly, FRAP showed slightly increased values until 15 min and then significantly decreased.
Antioxidant activity of bone
The TAC values of raw and cooked bone are presented in Figure 2D. The FRAP value of raw bone was higher (16.423±0.324 g trolox Eq./kg) than the ABTS (11.511±0.150 g trolox Eq./kg) and DPPH (3.455±0.112 g trolox Eq./kg). The highest value of ABTS was 37.690±1.150 g trolox Eq./kg in cooked bone at 20 min, and DPPH value was 7.761±0.206 g trolox Eq./kg at 5 min, while FRAP value was 17.595±0.291 g trolox Eq./kg at treatment time of 5 min. It is clear from these results that the ABTS and DPPH of cooked bone were significantly (p< 0.05) higher than the raw sample but FRAP results did not show significant difference between the raw and cooked bones.
Antioxidant activity of skin
Figure 2E showed TAC of raw and cooked skin as evaluated by ABTS, DPPH, and FRAP assays. The highest result of DPPH in raw skin was 33.151±0.909 g trolox Eq./kg followed by ABTS (32.708±1.127 g trolox Eq./kg and the lowest value of FRAP (26.477±0.744 g trolox Eq./kg). On the other hand, TAC of cooked skin was found in the range of 27.793±0.259 to 40.245±1.045 g trolox Eq./kg for ABTS assay, 6.466±0.075 to 12.724±0.302 g trolox Eq./kg for DPPH assay, and 19.182±0.216 to 27.556±0.122 g trolox Eq./kg for FRAP assay.
Figure 2E showed that the ABTS was the highest (40.245±1.045 g trolox Eq./kg) at 20 min followed by 15 min (35.051±0.775 g trolox Eq./kg), 5 min (32.269±0.533 g trolox Eq./kg), and 10 min (27.793±0.259 g trolox Eq./kg), respectively. On the other hand, there were no significant difference (p < 0.05) between raw and cooked samples at 5, 10, and 15 min in FRAP values. But the FRAP value didn’t differ significantly (p < 0.05) between raw and cooked skin at 20 min. Previous report found that meat led to decrease the TAC value with increasing the heating time, this reason may be due to denaturation of proteins (Serpen et al., 2012). Chinese Softshell Turtle (Pelodiscus sinensis) skin has been widely used in medicine and cosmetics (Zou et al., 2017). Therefore, authors suggest that the thermal treatment lead to enhance the FRAP and ABTS capacity, which could be contributes to improve the functional and pharmaceutical properties of the products.
Conclusions
The antioxidant capacity of ABTS, DPPH, and FRAP of five parts of grass turtle were evaluated by using a direct QUENCHER procedure. Cooked muscle, hard shell, and bone positively influenced on total antioxidant capacity (ABTS and DPPH) but FRAP value not affected by treatment in muscle and bone except treatment of 5 min. Nonetheless, the FRAP value in the hard shell was positively affected by different treatment times. On the other hand, ABTS of cooked liver was significantly affected by cooking time except for 20 min, while DPPH was not affected after 5 and 10 min cooking. Similarly, antioxidant capacity of cooked skin showed negative effect in ABTS except for 15 and 20 min and in contrast DPPH showed negative affect after 10 and 20 min, whereas FRAP showed positive affected after 15 and 20 min of thermal treatment. Further studies are required to investigate the effect of antioxidant activities by an enzymatic hydrolysis process in raw and cooked samples to develop the functional food properties.
Acknowledgements
This work was financially supported by the National first-class discipline program of Food Science and Technology (JUFSTR20180204), China Scholarship Council (CSC No. 2017GXZ017968), Beijing, China.
Statement of conflict of interest
The authors have declared no conflict of interests.
References
And, J.P. and Sauracalixto, F., 2005. Literature data may underestimate the actual antioxidant capacity of cereals. J. Agric. Fd. Chem., 53: 5036-5040. https://doi.org/10.1021/jf050049u
Benzie, I.F. and Strain, J.J., 1996. The ferric reducing ability of plasma (FRAP) as a measure of antioxidant power: the FRAP assay. Anal. Biochem., 239: 70-76. https://doi.org/10.1006/abio.1996.0292
Brandwilliams, W., Cuvelier, M.E. and Berset, C., 1995. Use of a free radical method to evaluate antioxidant activity. LWT Fd. Sci. Technol., 28: 25-30. https://doi.org/10.1016/S0023-6438(95)80008-5
Chen, T.H., Chang, H.C. and Kuangyang, L., 2009. Unregulated trade in turtle shells for Chinese traditional medicine in East and Southeast Asia: The case of Taiwan. Chelonian Conserv. Biol., 8: 11-18. https://doi.org/10.2744/CCB-0747.1
Cho, M., Lee, H.S., Kang, I.J., Won, M.H. and You, S., 2011. Antioxidant properties of extract and fractions from Enteromorpha prolifera, a type of green seaweed. Fd. Chem., 127: 999-1006. https://doi.org/10.1016/j.foodchem.2011.01.072
Dai, S., Ota, H., Oh, H.S. and Hikida, T., 2012. Origin of Japanese populations of Reeves’ pond turtle, Mauremys reevesii (Reptilia: Geoemydidae), as inferred by a molecular approach. Chelonian Conserv. Biol., 10: 237-249. https://doi.org/10.2744/CCB-0885.1
Descalzo, A.M., Rossetti, L., Grigioni, G., Irurueta, M., Sancho, A.M., Carrete, J. and Pensel, N.A., 2007. Antioxidant status and odour profile in fresh beef from pasture or grain-fed cattle. Meat Sci., 75: 299-307. https://doi.org/10.1016/j.meatsci.2006.07.015
Dudonné, S., Vitrac, X., Coutière, P., Woillez, M. and Mérillon, J.M., 2009. Comparative study of antioxidant properties and total phenolic content of 30 plant extracts of industrial interest using DPPH, ABTS, FRAP, SOD, and ORAC assays. J. Agric. Fd. Chem., 57: 1768-1774. https://doi.org/10.1021/jf803011r
Elias, R.J., Mcclements, D.J. and Decker, E.A., 2007. Impact of thermal processing on the antioxidant mechanisms of continuous phase β-lactoglobulin in oil-in-water emulsions. Fd. Chem., 104: 1402-1409. https://doi.org/10.1016/j.foodchem.2007.01.072
Kienzle, E., Kopsch, G., Koelle, P. and Clauss, M., 2006. Chemical composition of turtles and tortoises. J. Nutr., 136: 2053S-2054S. https://doi.org/10.1093/jn/136.7.2053S
Luciano, G., Biondi, L., Scerra, M., Serra, A., Mele, M., Lanza, M. and Priolo, A., 2013. The effect of the change from a herbage- to a concentrate-based diet on the oxidative stability of raw and cooked lamb meat. Meat Sci., 95: 212-218. https://doi.org/10.1016/j.meatsci.2013.05.015
Martínez, J., Nieto, G. and Ros, G., 2015. Total antioxidant capacity of meat and meat products consumed in a reference Spanish standard diet. Int. J. Fd. Sci. Technol., 49: 2610-2618. https://doi.org/10.1111/ijfs.12577
Nalinanon, S., Benjakul, S., Visessanguan, W. and Kishimura, H., 2008. Improvement of gelatin extraction from bigeye snapper skin using pepsin-aided process in combination with protease inhibitor. Fd. Hydrocoll., 22: 615-622. https://doi.org/10.1016/j.foodhyd.2007.01.012
O’Grady, M.N., Maher, M., Troy, D.J., Moloney, A.P. and Kerry, J.P., 2006. An assessment of dietary supplementation with tea catechins and rosemary extract on the quality of fresh beef. Meat Sci., 73: 132-143. https://doi.org/10.1016/j.meatsci.2005.11.008
Palka, K. and Daun, H., 1999. Changes in texture, cooking losses, and myofibrillar structure of bovine M. semitendinosus during heating. Meat Sci., 51: 237-243. https://doi.org/10.1016/S0309-1740(98)00119-3
Pérez-Jiménez, J. and Saura-Calixto, F., 2005. Literature data may underestimate the actual antioxidant capacity of cereals. J. Agric. Fd. Chem., 53: 5036-5040. https://doi.org/10.1021/jf050049u
Sante-Lhoutellier, V., Aubry, L. and Gatellier, P., 2007. Effect of oxidation on in vitro digestibility of skeletal muscle myofibrillar proteins. J. Agric. Fd. Chem., 55: 5343. https://doi.org/10.1021/jf070252k
Serpen, A., Capuano, E., Fogliano, V. and Gökmen, V., 2007. A new procedure to measure the antioxidant activity of insoluble food components. J. Agric. Fd. Chem., 55: 7676. https://doi.org/10.1021/jf071291z
Serpen, A., Gökmen, V. and Fogliano, V., 2012. Total antioxidant capacities of raw and cooked meats. Meat Sci., 90: 60-65. https://doi.org/10.1016/j.meatsci.2011.05.027
Shah, M.A., Bosco, S.J.D. and Mir, S.A., 2014. Plant extracts as natural antioxidants in meat and meat products. Meat Sci., 98: 21-33. https://doi.org/10.1016/j.meatsci.2014.03.020
SPSS, 2013. IBM SPSS statistics for windows, version 22.0. IBM Corp., Armonk, NY.
Sureshk, D., Narsaiah, K. and Borah, A., 2010. Anti-oxidant effect of extracts of kinnow rind, pomegranate rind and seed powders in cooked goat meat patties. Meat Sci., 85: 155-159. https://doi.org/10.1016/j.meatsci.2009.12.019
Tironi, V.A., Tomás, M.C. and Añón, M.C., 2002. Structural and functional changes in myofibrillar proteins of sea salmon (Pseudopercis semifasciata) by interaction with malonaldehyde (RI). J. Fd. Sci., 67: 929-935. https://doi.org/10.1111/j.1365-2621.2002.tb09430.x
Vasta, V. and Luciano, G., 2011. The effects of dietary consumption of plants secondary compounds on small ruminants’ products quality. Small Rumin. Res., 101: 150-159. https://doi.org/10.1016/j.smallrumres.2011.09.035
Vural, G., Arda, S. and Vincenzo, F., 2009. Direct measurement of the total antioxidant capacity of foods: The ‘Quencher’ approach. Trends Fd. Sci. Technol., 20: 278-288. https://doi.org/10.1016/j.tifs.2009.03.010
Zhang, Y., Luo, H., Liu, K., Jia, H., Chen, Y. and Wang, Z., 2015. Antioxidant effects of liquorice (Glycyrrhiza uralensis) extract during aging of longissimus thoracis muscle in Tan sheep. Meat Sci., 105: 38-45. https://doi.org/10.1016/j.meatsci.2015.03.002
Zou, Y., Xu, P., Li, P., Cai, P., Zhang, M., Sun, Z. and Wang, D., 2017. Effect of ultrasound pre-treatment on the characterization and properties of collagen extracted from soft-shelled turtle (Pelodiscus sinensis). Fd. Sci. Technol., 82: 72-81. https://doi.org/10.1016/j.lwt.2017.04.024
To share on other social networks, click on any share button. What are these?










