Effect of Heat Stress on the Physiological and Hematological Profiles of Horned and Polled Bali Cattle
Research Article
Effect of Heat Stress on the Physiological and Hematological Profiles of Horned and Polled Bali Cattle
Sukandi Sukandi1,4, Djoni Prawira Rahardja2*, Herry Sonjaya2, Hasbi Hasbi2, Sudirman Baco2, Sri Gustina3, Kirana Dara Dinanti Adiputra1
1Department of Animal Science and Technology, Faculty of Animal Science, Hasanuddin University, Jl. Perintis Kemerdekaan KM. 10 Tamalanrea, Makassar, 90245, Indonesia; 2Department of Animal Production, Faculty of Animal Science, Hasanuddin University, Jl. Perintis Kemerdekaan KM. 10 Tamalanrea, Makassar, 90245, Indonesia; 3Department of Animal Science, Faculty of Animal Science and Fisheries, Universitas Sulawesi Barat, Jl. Prof. Dr. Baharuddin Lopa, Tande Timur, Majene, 91412, Indonesia; 4Livestock and Animal Health Service of South Sulawesi Province, Jl. Veteran Selatan No. 234, Makassar, 90131, Indonesia.
Abstract | Bali cattle are local cattle owned by Indonesia that play a role in meeting the lack of meat demand in the country. In its development, both horned and hornless Bali cattle were found. This study aims to determine the influence of heat stress on the physiological and hematological profiles of horned and polled Bali cattle. Eight male Bali cattle (four horned and four polled) with an age range of 2.5–4.5 years were used. Parameters observed were the body’s physiological response (rectal temperature, skin surface temperature, respiration rate, and pulse rate) and hematological profile (erythrocytes, hemoglobin, hematocrit, and leukocytes). Body physiological and hematological data were analyzed using factorial analysis to compare Bali cattle (horned and polled) and the time factor of measurement (morning and afternoon), followed by Duncan’s test. The results showed that horned and polled Bali cattle were significantly (P<0.05) different in rectal temperature, erythrocytic counts, hemoglobin levels, and hematocrit values but not in skin surface temperature, respiration rate, pulse rate, and leukocyte counts. The time factor of measurement (morning and afternoon) showed significant (P<0.05) differences in rectal temperature, skin surface temperature, respiration rate, pulse rate, erythrocyte counts, and hemoglobin levels, but not in hematocrit values and leukocytic counts. Compared to horned Bali cattle, polled Bali cattle showed higher increases in rectal temperature, skin surface temperature, and respiration rate caused by the absence of horns. A higher hematological profile supports higher metabolic processes.
Keywords | Bali Cattle, Heat Stress, Hematological, Physiological, Polled
Received | March 16, 2023; Accepted | April 17, 2023; Published | May 03, 2023
*Correspondence | Djoni Prawira Rahardja, Department of Animal Production, Faculty of Animal Science, Hasanuddin University, Jl. Perintis Kemerdekaan KM. 10 Tamalanrea, Makassar, 90245, Indonesia; Email: djonipra@gmail.com
Citation | Sukandi S, Rahardja DP, Sonjaya H, Hasbi H, Baco S, Gustina S, Adiputra KDD (2023). Effect of heat stress on the physiological and hematological profiles of horned and polled Bali cattle. Adv. Anim. Vet. Sci. 11(6):893-902.
DOI | https://dx.doi.org/10.17582/journal.aavs/2023/11.6.893.902
ISSN (Online) | 2307-8316
Copyright: 2023 by the authors. Licensee ResearchersLinks Ltd, England, UK.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
INTRODUCTION
Bali cattle (Bos sondaicus, Bos javanicus, or Bos/Bibos banteng) are one of Indonesia’s indigenous livestock genetic resources. According to Minister of Agriculture Decree No. 325/Kpts/OT.140/1/2010, Bali cattle are characterized by a brick red coat on females and a blackish brown on adult males, with white on the lower legs, back of the pelvis (rump), and upper and lower lips. On the back, there is a black eel line (elongated) and also a black color at the tip of the tail (MOA, 2010). Bali cattle are beef cattle that contribute significantly to the development of the livestock industry in Indonesia by meeting the country’s meat demand. Bali cattle have a higher carcass yield compared to the other native Indonesian cattle (Purwantara et al., 2012; Nuraini et al., 2019) because they are non-selective, can eat low-quality feed, adapt well to their environment, and can even live and produce well on critical land (Leo et al., 2012; Purwantara et al., 2012; Baco et al., 2020a).
Bali cattle are a breed that has horns, both on males and females. However, in its development, it was found that there are Bali cattle that are naturally hornless, also known as polled (Zulkharnaim et al., 2017; 2020a; Baco et al., 2020b; Hasbi et al., 2021). Polled cattle are inherited through an autosomal dominant pattern (Medugorac et al., 2012; Glatzer et al., 2013; Gehrke et al., 2020), where PP (polled), pp (horned), with heterozygous (Pp) cattle usually polled but commonly scurred (Wiedemar et al., 2014; Grobler et al., 2018; Randhawa et al., 2020; Aldersey et al., 2020). Polled cattle are usually easier to handle, safer for workers, and less aggressive towards each other (Glatzer et al., 2013), so polled cattle have their advantages in the maintenance process, and the risk of injuring their bodies and the other cattle can be minimized. However, Parés-Casanova and Caballero (2014) assumed that horns in animals influence thermoregulation.
Increased ambient temperature causes stress and affects the adaptive mechanisms of livestock (Nussa et al., 2018). Bali cattle have good physiological performances and can adapt to climatic conditions in Indonesia (Aritonang et al., 2017a, b), but polled Bali cattle that appear in this development are not yet known for their physiological responses to climatic changes (IPCC, 2014), which is one of the threats to the livestock industry (Nardone et al., 2010; Ganaie et al., 2013; Herbut et al., 2019), including Bali cattle farming. Several physiological indicators of heat stress include body temperature, pulse rate, respiration rate, and hematological profiles (Induk and Pareek, 2015; Berian et al., 2019). Therefore, this study was conducted to determine the physiological and hematological profiles of horned and polled Bali cattle during heat stress exposure.
MATERIALS AND METHODS
Period and place of research
The study was carried out between July and November of 2022. Evaluation of physiological parameters (rectal temperature, skin surface temperature, respiration rate, and pulse rate) and blood sampling were conducted at one of the farms in Tanete Riaja District, Barru Regency, South Sulawesi, Indonesia (4o29’17” south latitude and 119o38’50” east longitude; 10 msal). The dry season lasts 6 months, from May to November, and the month with the fewest wet days is August. Hematological parameters were examined using a Prokan Hematology Analyzer Model PE-6800 Vet (Shenzhen Prokan Electronics Inc., China) at the Laboratory of Pathology and Toxicology, Maros Veterinary Center, Maros, Indonesia.
Research animals
This study used eight male Bali cattle aged 2.5–4.5 years, 4 horned and 4 polled, respectively. The cattle were kept in a barn with 1x1.5 m individual stalls equipped with feed and drink container. Cattle were fed concentrate (20%) and elephant grass (80%) twice a day, in the morning and evening, while drinking water was given ad libitum. All cattle have been vaccinated against foot and mouth disease (FMD).
Implementation procedure
Each animal was measured twice: in the morning, from 6:30 to 7:30 am (before eating and engaging in other activities), and in the afternoon, from 12:30 to 2:00 pm (given an exercise to walk in the hot sun for 5 min before measurement). Physiological measurements, including rectal temperature, skin surface temperature, respiration rate, and pulse rate were conducted on the study animals.
Blood was drawn from the jugular vein and placed in a 3 mL Vaculab® EDTA-K3 vial (One Med, Indonesia). The collected samples were transferred in a cooler box to the laboratory and kept 4oC.
Measured parameters
Environmental parameters
The environmental parameters measured in this study were temperature, humidity, and temperature-humidity index (THI).
Temperature and humidity
The ambient temperature and humidity were measured using a thermohygrometer (Thermohygrometer Clock HTC-2), which was placed on the location to be measured and then left for 3–5min before taking the readings.
Temperature-humidity index (THI)
The relationship between air temperature and humidity was calculated using the THI (Temperature Humidity Index) to measure the comfort level of the livestock environment after Thompson and Dahl (2012), namely:
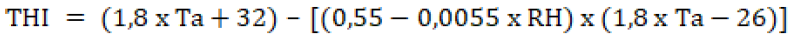
Where; Ta = Ambient Temperature (oC) and RH = Relative Humidity (%).
Body physiological parameters
Parameters measured for the physiological body of horned and polled Bali cattle in this study include rectal temperature, skin temperature, respiration rate, and pulse rate.
Rectal temperature
Measured using an Omron digital thermometer model MC-343F (Omron Healthcare Co. Ltd., Japan) by inserting the thermometer 6-7 cm into the rectum at an angle toward the rectal wall (Rashamol et al., 2018).
Skin surface temperature
Measured with an Omron infrared thermometer model MC 720 (Omron Healthcare Co. Ltd., Japan) 1–5 cm from the skin surface at four measurement points: the back (A), chest (B), upper legs (C), and lower legs (D). The average skin surface temperature was calculated after McLean et al. (1983) in Pamungkas et al. (2020) as follows:
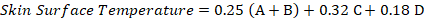
Respiration rate
Measured visually after Shilja et al. (2016) by counting flank movements with a stopwatch.
Pulse rate
measured by attaching a Littmann Classic IIITM stethoscope (3M Healthcare, USA) to the left chest of the cattle. When this was not possible, it was palpated on the coccygeal artery under the middle of the tail, about 10 cm from the anus (Nugraha et al., 2020).
Hematological parameters
Samples were tested using a Prokan hematology analyzer model PE-6100 Vet for the erythrocytic count, hemoglobin level, hematocrit value, and leukocytic count. The examination was conducted following the procedures of the Prokan model PE-6100 vet hematology analyzer.
Data analysis
Data on environmental parameters were analyzed using independent sample T-test to compare difference between morning and afternoon. Physiological and hematological data obtained were analyzed using factorial analysis to determine the effect of Bali cattle type (horned and polled) and the time factor of measurement (morning and afternoon), followed by Duncan’s test. The p-value had to be less than 0.05 for the parameter to be considered significant. All analysis was performed using SPSS version 25.
RESULTS
Microclimatic conditions of the research site
The climate is one of the external factors that has a significant impact on livestock productivity and physiology. The average microclimatic conditions during the study are presented in Table 1.
Table 1 showed that morning and afternoon were significantly (P<0.05) different in averages ambient temperature, humidity, and THI.
Body’s physiological response
Table 2 displayed physiological parameters including rectal temperature, skin temperature, respiration rate, and pulse rate.
Horned and polled Bali cattle were significantly (P<0.05) different in rectal temperature but not in skin surface temperature, respiration rate, and pulse rate.
The time factor of measurement (morning and afternoon) had significant (P<0.05) differences in rectal temperature, skin surface temperature, respiration rate, and pulse rate.
Hematological profile
Blood profile (hematological) parameters in the form of the erythrocytic count, hemoglobin level, hematocrit value, and leukocytic count are presented in Table 3.
The erythrocytic count, hemoglobin level, and hematocrit value of horned and polled Bali cattle were significantly (P<0.05) different rather than the leukocytic count.
The time factor of measurement (morning and afternoon) had significant (P<0.05) differences in erythrocytic count and hemoglobin level but not in hematocrit values and leukocytic count.
Table 1: Average temperature, humidity, and THI at the research site.
|
Time factor of measurement |
Ta (oC) |
RH (%) |
THI |
Environmental comfort categories* |
|
Morning |
24.20±0.52a |
93.25±1.76a |
74.86±0.74a |
Normal |
|
Afternoon |
32.90±1.08b |
67.50±8.50b |
85.19±0.71b |
Severe heat stress |
Description: Ta: ambient temperature; RH: relative humidity; THI: temperature humidity index. a, b Significant differences (P<0.05) are indicated by different superscripts in the same column. *Bulitta et al., 2015.
Table 2: Average values of physiological parameters of horned and polled Bali cattle.
|
Parameters |
Bali cattle |
Time factor of measurement |
Average |
|
|
Morning |
Afternoon |
|||
|
Rectal temperature (oC) |
Horned |
37.35±0.24a |
38.30±0.22b |
|
|
Polled |
37.66±0.26a |
38.73±0.24c |
||
|
Average |
||||
|
Skin surface temperature (oC) |
Horned |
35.63±0.42a |
36.80±0.08b |
36.21±0.69 |
|
Polled |
35.68±0.34a |
37.30±0.20c |
36.49±0.91 |
|
|
Average |
||||
|
Respiration rate (breath/min) |
Horned |
21.25±1.71a |
35.25±0.96b |
28.25±7.59 |
|
Polled |
21.50±1.73a |
37.50±1.30c |
29.50±8.67 |
|
|
Average |
||||
|
Pulse rate (beats/min) |
Horned |
54.75±8.66a |
93.50±1.91b |
74.13±21.51 |
|
Polled |
55.00±5.01a |
97.50±5.07b |
76.25±23.20 |
|
|
Average |
||||
Notes: a, b, c Different superscripts in rows and columns within the same parameter indicate significant differences (P<0.05). x, y Different superscripts in the same row indicate significant differences (P<0.05). p, q Different superscripts in the same column indicate significant differences (P<0.05).
Table 3: Average values of hematological parameters of horned and polled Bali cattle.
|
Parameter |
Bali cattle |
Time factor of measurement |
Average |
|
|
Morning |
Afternoon |
|||
|
Erythrocytic count (x106/mm3) |
Horned |
5.47±0.30a |
5.29±0.40a |
5.38±0.34p |
|
Polled |
7.11±0.39c |
6.06±0.34b |
6.59±0.65q |
|
|
Average |
||||
|
Hemoglobin level (g/dl) |
Horned |
11.63±0.54a |
11.25±1.06a |
11.44±0.81p |
|
Polled |
17.23±0.40c |
14.33±1.09b |
15.78±1.72q |
|
|
Average |
||||
|
Hematocrit (%) value |
Horned |
33.93±1.30a |
32.83±2.42a |
33.38±1.89p |
|
Polled |
43.83±3.21b |
40.95±3.86b |
42.39±3.63q |
|
|
Average |
38.88±5.76 |
36.89±5.27 |
||
|
Leukocytic Count (103/mm3) |
Horned |
12.65±3.81 |
11.73±3.35 |
12.19±3.36 |
|
Polled |
13.55±2.05 |
13.25±2.93 |
13.40±2.35 |
|
|
Average |
13.10±2.87 |
12.49±3.03 |
||
Notes: a, b, c Different superscripts in rows and columns within the same parameter indicate significant differences (P<0.05). x, y Different superscripts in the same row indicate significant differences (P<0.05). p, q Different superscripts in the same column indicate significant differences (P<0.05).
DISCUSSION
The temperature rises throughout the day until it reaches a peak when the sun shines perpendicular to the earth’s surface. The humidity decreases during the day, indicating that when the ambient temperature is high, the humidity will decrease. According to Yani et al. (2007), the ambient temperature in Indonesia tends to be high, ranging from 24-34oC and the humidity is also high, at 60–90% annually. The temperature and humidity are considered normal for the tropics (Putra et al., 2019), but may affect livestock and therefore require improved management.
The temperature-humidity index (THI) is a simple combination of temperature and humidity that is often used to estimate livestock comfort based on environmental conditions. The higher the THI value, the more severe the heat stress that can be inflicted on cattle. A THI value of <74 is ideal for beef cattle, 75–78 is mild heat stress, 79–83 is moderate heat stress, and >84 is severe heat stress (Bulitta et al., 2015). The results showed that THI values during the day can put cattle under severe stress, making it difficult to maintain thermoregulatory mechanisms and normal temperatures (Habeeb et al., 2018), which can have an impact on livestock productivity. Although the threshold value of THI concerning stress is unknown due to limited information, it may affect behavioral changes, the ability to maintain body homeostasis by increasing respiration rate and heart rate, and changes in hematological profile.
The results showed that both horned and polled Bali cattle experienced an increase in physiological parameters after being exercised under direct sunlight, but polled Bali cattle tended to experience a higher increase (rectal temperature, skin temperature, and respiration rate) than horned Bali cattle. Although horns are not one of the factors that influence the process of thermoregulation, the presence of horns is thought to have a contribution. Parés-Casanova and Caballero (2014) explained that heat exchange works by pumping blood around the horn core (the part containing blood vessels). When this blood passes through the outside of the horn, heat is lost to the environment, and cooled blood is returned to the cattle’s body.
Rectal temperature is considered an optimal parameter for representing body temperature (Rashamol et al., 2018; Reuter et al., 2010) because it reflects the heat produced and heat released (Hermawansyah et al., 2020). In hot weather, an increase in body temperature is a function of heat accumulation and heat dissipation with the environment (Lees et al., 2019). Heat absorption on the animal’s body surface during hot weather contributes to raising the animal’s body temperature. This condition forces animals to maintain a constant body temperature (homeostasis) with thermoregulation mechanisms (Terrien et al., 2011; Mota-Rojas et al., 2021; Renaudeau et al., 2012) to remove excess heat from the body.
The rectal temperature of Bali cattle in this study is still in the normal category. Beef cattle have a normal rectal temperature range of 36.7-39.1oC (average 38.3oC) (Reece, 2015) or 37.8-39.2oC (Abdisa, 2017). The rectal temperature of polled Bali cattle increased more than that of horned Bali cattle during the day. Rectal temperatures in both Bali cattle are not very different from those reported by Aritonang et al. (2017a), who researched in the same district (Barru Regency) and reported that Bali cattle have rectal temperatures in the range of 37.6-38.6oC. Putra et al. (2021) also reported that the rectal temperature of Bali cattle increased from 37.5±0.3oC in the morning to 38.7±0.2oC in the afternoon. Contrary to Prahesti et al. (2021), who reported that exercised Bali cattle had higher rectal temperatures between 39.16-40.06oC. Prasanna et al. (2022) reported Sahiwal and Sahiwal cross cattle had higher average rectal temperatures during summer than other seasons, which could be due to the excessive heat production due to the increased metabolic rate.
Animals try to maintain their body temperature by releasing heat through various efforts. The respiration rate is the first thermoregulatory mechanism used by the animal to maintain body temperature (Seixas et al., 2017). Polled Bali cattle during the daytime showed a higher increase in respiration rate. Putra et al. (2021) reported an average respiration rate of 22.6±5.3 breaths/min in the morning, which increased to 37.4±4.4 breaths/min during the day. When exposed to high environmental heat, peripheral receptor activation sends nerve impulses to the hypothalamic thermal center, which then stimulates the cardio-respiratory center to send impulses to the diaphragm and intercostal muscles to accelerate breathing (Ganaie et al., 2013; Atkins et al., 2018), so that body heat can be lost through evaporative breathing. Animals that have increased respiration rates do not necessarily indicate that they have been able to maintain a stable body temperature, but rather that the animal has overheated and is trying to restore its body heat balance (Ganaie et al., 2013). Low respiration rates in hot environments indicate a greater tolerance (Prasanna et al., 2022). The normal respiration rate for beef cattle, according to Abdisa (2017) is 25–30 breaths/min.
The skin acts as a heat radiator, allowing heat to radiate from the body surface to the environment (Collier et al., 2019). Animals release heat through vasodilation and sweating. Vasodilation begins when the heart increases blood flow in the capillaries to the upper periphery of the skin to facilitate the transfer of body heat to the environment (Habeeb et al., 2018; Shilja et al., 2016; Wang et al., 2020). When this mechanism is unable to reduce body temperature or the external environment is higher, the brain responds by releasing sweat on the skin to carry additional heat from the body from the liquid to vapor phase (Habeeb et al., 2018).
The increase in skin surface temperature in this study is similar to the report of Putra et al. (2021), where the skin surface temperature in Bali cattle increased from 32.6±1.0oC in the morning to 37.0±1.6 oC during the day. Berian et al. (2019) also reported that crossbred dairy cows that experienced heat stress had a higher skin surface temperature (38.69±0.09oC) than those kept at a comfortable ambient temperature (37.31±0.04oC). The skin surface temperature of horned and polled Bali cattle in this study did not differ much from Nuriyasa et al. (2015), who reported that Bali cattle have a skin temperature range of 36.66-37.89oC. The skin surface is the part of the body that is directly exposed to sunlight. The skin color of these cattle is thought to have a correlation with skin surface temperature. Brown-Brandl et al. (2006) explained that cattle breeds with dark skin (Angus and MARC III) have higher surface temperatures than cattle breeds with light-colored skin (Charolais and Gelbvieh). Male polled Bali cattle have a color that tends to be slightly darker than horned Bali cattle (Zulkharnaim et al., 2020b) so during the day they have a higher skin surface temperature.
Bali cattle had a higher pulse rate in the afternoon after exercise under sun exposure compared to that in the morning. Exposure of animals to heat stress induces changes in circadian rhythms in the heart of the animal (Rashamol et al., 2018). Putra et al. (2021) showed an increase in the pulse rate of Bali cattle from 55.5±10.6 beats/min to 64.5±11.3 beats/min when the ambient temperature increased. Prahesti et al. (2021) also reported an increase in pulse rate from 70.00±5.00 beats/min to 96.66±6.00 beats/min after Bali cattle were given exercise. Under normal conditions, the pulse rate of cattle generally ranges from 40 to 70 beats/min (Kubkomawa et al., 2015) or 60 to 90 beats/min (Abdisa, 2017).
The increase in pulse rate in hot environmental conditions is due to sympathetic activity. Heart rate is controlled by the activity of the autonomic nervous system, namely the sympathetic nervous system, which increases heart rate, and the parasympathetic nervous system, which decreases heart rate (Kasahara et al., 2021). When the environment is hot, animals try to control their body temperatures by releasing heat from their bodies into the environment. Sarangi (2018) explained that the increase in heart rate is caused by two things, namely: (1) increased muscle activity involved in regulating respiration rate, and (2) decreased vascular and arterial peripheral resistance. Heat release can be achieved through water diffusion from the skin by increasing blood flow from the core to the surface (Gupta et al., 2013), where the heart will be more active in pumping blood.
The results revealed that polled Bali cattle have higher erythrocytic counts, hemoglobin levels, and hematocrit values than horned Bali cattle. During the day after exercise, polled cattle had decreased erythrocytic counts and hemoglobin levels, while horned Bali cattle showed no significant disturbances in their hematological profile. High hematological values indicated that polled Bali cattle have a high metabolic rate.
Red blood cells, also known as erythrocytes, are blood cells that function as oxygen transportation from the lungs to body tissues and provide the oxygen needed by body cells (Mohanty et al., 2014). The average erythrocytic counts in horned Bali cattle were not much different from the research of Adam et al. (2015), which was 5.49±0.88 x106/mm3 (male); 4.89±0.53 x106/mm3 (female), Siswanto (2011), which was 5.2 x106/mm3 and Suharti et al. (2017), which was 5.18±0.41 x106/mm3. Meanwhile, polled Bali cattle have a higher erythrocyte count but are still within the normal range when referring to the erythrocytic count of Roland et al. (2014). The erythrocytic count of horned Bali cattle did not change, while that of polled Bali cattle decreased after morning exercise.
The decrease in the erythrocytic count in polled Bali cattle indicates that sun exposure and exercise during the day can interfere with erythrocytes, as explained by Tamzil et al. (2014) that heat stress can affect the erythropoietin hormone in the spinal cord so that the production and development of erythrocytes are inhibited. Abduch et al. (2022) reported that daytime sun exposure and exercise had a negative impact on the number of erythrocytes in Caracu beef cattle. Ondruska et al. (2011) also reported that rabbits exposed to 36°C daily, for 12 h, within 4 weeks were able to reduce the erythrocytic count. Another report by Perumal et al. (2022) reported lower erythrocytic counts in local Andamanese cattle during the dry season than during the wet season. Piemontese cattle reared in a hotter environment showed lower erythrocyte counts (Mazzullo et al., 2014).
Hemoglobin is the main component of erythrocytes and functions as the carrier of oxygen and carbon dioxide. The average hemoglobin levels of horned and polled Bali cattle were higher than the hemoglobin levels reported by Siswanto (2011) and Diparayoga et al. (2014) but still within the range of reports by Septiarini et al. (2020) and Suharti et al. (2017) which had hemoglobin levels of 12.53-14.53 g/dl and 10.62±1.21 g/dl, respectively, and in general similar to the result reported by Roland et al. (2014).
According to Srikandakumar and Johnson (2004), heat stress can result in erythrocyte lysis because of an increase in free radicals in the erythrocyte membrane, which is rich in fat. This can lower hemoglobin levels or lead to a lack of nutrients for the synthesis of hemoglobin because the animal consumes less feed. Lawrence et al. (2017) explain that hemoglobin correlates with the number of erythrocytes; when the number of erythrocytes decreases, hemoglobin levels also decrease. Hemoglobin synthesis occurs at the beginning of erythrocyte formation. When erythrocyte formation is disrupted, hemoglobin synthesis is also disrupted. Perumal et al. (2022) reported that local Andamanese cattle during the dry season were significantly had lower erythrocytic count and hemoglobin than cattle reared during the wet season.
Hematocrit, also known as packed cell volume (PCV), is a term used to describe the proportion of erythrocytes in 100 mL of blood (Reece, 2015). The hematocrit value of horned Bali cattle is not significantly different from those reported by Diparayoga et al. (2014) and Suharti et al. (2017), but the hematocrit values of horned and polled Bali cattle was still higher than the reports of Siswanto (2011) and Adam et al. (2015), but still in the normal category when referring to result reported by Roland et al. (2014).
Providing exercise under sunlight did not impair hematocrit values. In contrast, Iranian cattle reported by Mirzadeh et al. (2010) and local Andamanese cattle reported by Perumal et al. (2022) showed lower hematocrit values when reared in summer compared to colder seasons. An increase or decrease in hematocrit value is related to erythrocytic counts (Merdana et al., 2020), which affects the viscosity of the blood, with high or low values having an impact on increasing or slowing blood flow and heart (Merdana et al., 2020).
White blood cells, also known as leukocytes, are blood cells that play a role in protecting the body from disease-causing infections. Horned and polled Bali cattle have similar leukocytic counts. The leukocytic count in this study is not much different from the results of Novanty et al. (2022), who reported the leukocytic count of Bali cattle at 12.26±2.85 x103/mm3 although the contrast was higher than Suharti et al. (2017), who reported 7.13±0.80 x103/mm3.
Exercise under sunlight did not affect the leucocytic count of horned and polled Bali cattle. This indicates that the immunity of both Bali cattle was not impaired during heat stress. Pandey et al. (2017) reported leukocyte counts of Tharparkar and Karan Fries cattle that showed no difference in cattle exposed to heat stress and oxygen levels compared to controls. In contrast, Perumal et al. (2022) reported that local Andamanese cattle have lower leukocytic counts during the dry season than during the rainy season.
The absence of horns in polled Bali cattle is expressed by a higher rectal temperature, skin surface temperature, and respiration rate. This condition was supported by the hematological profile of polled Bali cattle, which has higher erythrocytic counts, hemoglobin levels, and hematocrit values to compensate for higher metabolic processes. During the day after exercise under the hot sun, horned Bali cattle did not experience significant changes, while polled Bali cattle experienced significant decreases in several hematological parameters. This circumstance does not imply that polled Bali cattle are less resilient to heat stress than horned Bali cattle. Polled Bali cattle may be able to adapt and survive at high altitudes with low oxygen levels (Pathak et al., 2022). Low oxygen availability at high altitudes requires physiological adjustments that allow adequate tissue oxygenation (Gassmann et al., 2019). High erythrocytic counts and hemoglobin levels are the most important hematological indicators for high-altitude acclimatization.
CONCLUSIONS and Recommendations
Horned and polled Bali cattle both experience an increase in physiological parameters during the day after being given exercise in the sun, but polled Bali cattle tend to experience a higher increase (rectal temperature, skin surface temperature, and respiration rate) compared to horned Bali cattle. Higher rectal temperature, skin surface temperature, and respiration rate compensate for the absence of horns in polled Bali cattle. These conditions are balanced by the higher hematological profile of polled cattle to compensate for the faster metabolic processes.
ACKNOWLEDGMENT
The authors would like to thank The Directorate General of Higher Education, Research, and Technology (DGHERT) of the Ministry of Education, Culture, Research, and Technology (MOECRT) of the Republic of Indonesia for funding this research.
NOVELTY STATEMENT
The physiological and hematological profiles of Bali cattle are only found in horned Bali cattle, not in polled Bali cattle. This study is preliminary research that discusses the differences in physiological and hematological profiles of horned and polled Bali cattle.
AUTHOR’S CONTRIBUTION
SS and DPR conception and design of the study, data collection, data analysis, initial writing drafts, writing-review and editing. HS conception and design of the study; data analysis; correcting and editing a draft. HH data collection; data analysis. SB data analysis. SG data collection. KDDA; data collection, data analysis.
Ethical approval
The Animal Ethics Committee of Hasanuddin University, Makassar, Indonesia, approved all procedures in the present study.
Conflict of interest
The authors have declared no conflict of interest.
REFERENCES
Abdisa T (2017). Review on practical guidance of veterinary clinical diagnostic approach. Int. J. Vet. Sci. Res., 3(2): 06-25. https://doi.org/10.17352/ijvsr.000020
Abduch NG, Pires BV, Souza LL, Vicentini RR, Zadra LEF, Fragomeni BO, Silva RMO, Baldi F, Paz CCP, and Stafuzza NB (2022). Effect of thermal stress on thermoregulation, hematological and hormonal characteristics of Caracu beef cattle. Animals, 12(24): 3473. https://doi.org/10.3390/ani12243473
Adam M, Lubis TM, Abdyad B, Asmilia N, Muttaqien, Fakhrurazy (2015). Total erythrocytes count and haematocrit value of Aceh and Bali cattle in Leumbah Seulawah, Aceh Besar. J. Med. Vet., 9(2): 115-118. https://doi.org/10.21157/j.med.vet..v9i2.3810
Aldersey JE, Sonstegard TS, Williams JL, Bottema CDK (2020). Understanding the effects of the bovine POLLED variants. Anim. Genet., 51(2): 166-176. https://doi.org/10.1111/age.12915
Aritonang SB, Yuniati R, Abinawanto, Imron R, Bowolaksono A (2017a). Studies of adaptive traits of Bali cattle in Buleleng District, Bali and Barru District, South Sulawesi. AIP Conf. Proc., 1844: 060003. https://doi.org/10.1063/1.4983443
Aritonang SB, Yuniati R, Abinawanto, Imron M, Bowolaksono A (2017b). Effect of thermal stress on HSP90 expression of Bali cattle in Barru District, South Sulawesi. AIP Conf. Proc., 1862: 030104. https://doi.org/10.1063/1.4991208
Atkins I, Cook N, Mondaca M, Choi C (2018). Continuous respiration rate measurement of heat-stressed dairy cows and relation to environment, body temperature and lying time. Trans. ASABE, 61: 1475-1485. https://doi.org/10.13031/trans.12451
Baco S, Malaka R, Zulkharnaim, Hatta M (2020a). The body condition and reproduction performances of Bali cattle cows through the improved feeding in the intensive management system. IOP Conf. Ser. Earth Environ. Sci., 492: 012101. https://doi.org/10.1088/1755-1315/492/1/012101
Baco S, Zulkharnain, Malaka R, Moekti GR (2020b). Polled Bali cattle and potentials for the development of breeding industry in Indonesia. Hasanuddin J. Anim. Sci., 2(1): 23–33.
Berian S, Gupta SK, Sharma S, Ganai I, Dua S, Sharma N (2019). Effect of heat stress on physiological and hemato-biochemical profile of cross bred dairy cattle. J. Anim. Res., 9(1): 95–101. https://doi.org/10.30954/2277-940X.01.2019.13
Brown-Brandl, T.M., Nienaber JA, Eigenberg RA, Mader TL, Morrow JL, Dailey JW (2006). Comparison of heat tolerance of feedlot heifers of different breeds. Livest. Sci., 105(1-3): 19-26. https://doi.org/10.1016/j.livsci.2006.04.012
Bulitta FS, Aradom S, Gebresenbet G (2015). Effect of transport time of up to 12 hours on welfare of cows and bulls. J. Sci. Manag., 8: 161-182. https://doi.org/10.4236/jssm.2015.82019
Collier RJ, Baumgard LH, Zimbelman RS, Xiao Y (2019). Heat stress: physiology of acclimation and adaptation. Anim. Front., 9(1): 12-19. https://doi.org/10.1093/af/vfy031
Diparayoga IMG, Dwinata IM, Dharmawan NS (2014). Erythrocytes total, hemoglobin, pack cell volume, and erythrocytes index of Bali cattle infected with Cysticercus bovis. Indonesia Med. Vet., 3(3): 206-212.
Ganaie AH, Shanker G, Bumla NA, Ghasura RS, Mir NA, Wani SA, Dudhatra GB (2013). Biochemical and physiological changes during thermal stress in bovines. J. Vet. Sci. Technol., 4(1): 1-6.
Gassmann M, Mairbäurl H, Livshits L, Seide S, Hackbusch M, Malczyk M, Kraut S, Gassmann NN, Weissmann N, Muckenthaler MU (2019). The increase in hemoglobin concentration with altitude varies among human populations. Ann. N. Y. Acad. Sci., 1450: 204-220. https://doi.org/10.1111/nyas.14136
Gehrke LJ, Capitan A, Scheper C, König S, Upadhyay M, Heidrich K, Russ I, Seichter D, Tetens T, Medugorac I, Thaller G (2020). Are scurs in heterozygous polled (Pp) cattle a complex quantitative trait? Genet. Sel. Evol., 52(1): e6. https://doi.org/10.1186/s12711-020-0525-z
Glatzer S, Merten NJ, Dierks C, Wöhlke A, Philipp U, Distl O (2013). A single nucleotide polymorphism within the interferon gamma receptor 2 gene perfectly coincides with polledness in Holstein cattle. PLoS One, 8(6): 1-7. https://doi.org/10.1371/journal.pone.0067992
, C (2018). Validation of the POLLED Celtic variant in South African Bonsmara and Drakensberger beef cattle breeds. Livest. Sci., 217: 136–139. https://doi.org/10.1016/j.livsci.2018.10.003
Gupta M, Kumar S, Dangi SS, Jangir BL (2013). Physiological, biochemical and molecular responses to thermal stress in goats. Int. J. Livest. Res., 3: 27-38. https://doi.org/10.5455/ijlr.20130502081121
Habeeb AA, Gad AE, Atta MA (2018). Temperature-humidity indices as indicators to heat stress of climatic conditions with relation to production and reproduction of farm animals. Int. J. Biotechnol. Recent Adv., 1(1): 35-50. https://doi.org/10.18689/ijbr-1000107
Hasbi H, Prahesti KI, Sonjaya H, Baco S, Wildayanti W, Gustina S (2021). Characteristics of libido and testosterone concentrations of Bali polled and horned bulls. IOP Conf. Ser. Earth Environ. Sci., 788: 012141. https://doi.org/10.1088/1755-1315/788/1/012141
Herbut P, Angrecka S, Godyń D, Hoffmann G (2019). The physiological and productivity effects of heat stress in cattle. A review. Ann. Anim. Sci., 19(3): 579–594. https://doi.org/10.2478/aoas-2019-0011
Hermawansyah, Salundik, Priyanto R (2020). Physiological response of reared Bali cattle based on different peat land characterstic. Chalaza J. Anim. Husband., 5(1): 12-21. https://doi.org/10.31327/chalaza.v5i1.1254
Indu S, Pareek A (2015). A review: Growth and physiological adaptability of sheep to heat stress under semi arid environment. Int. J. Emerg. Trends Sci. Technol., 2(9): 3188-3198. https://doi.org/10.18535/ijetst/v2i9.09
Intergovermental Panel on Climate Change (IPCC) (2014). Climate change 2014: Impacts, adaptation, and vulnerability. part A: global and sectoral aspects. In: (eds. Field, C.B., V.R. Barros, D.J. Dokken, K.J. Mach, M.D. Mastrandrea, T.E. Bilir, M. Chatterjee, K.L. Ebi, Y.O. Estrada, R.C. Genova, B. Girma, E.S. Kissel, A.N. Levy, S. MacCracken, P.R. Mastrandrea, and L.L. White). Contribution of working group II to the fifth assessment report of the intergovernmental panel on climate change. Cambridge (UK)/New York (NY): Cambridge University Press; pp. 1132.
Kasahara Y, Yoshida C, Saito M, Kimura Y (2021). Assessments of heart rate and sympathetic and parasympathetic nervous activities of normal mouse fetuses at different stages of fetal development using fetal electrocardiography. Front. Physiol., 12: 652828. https://doi.org/10.3389/fphys.2021.652828
Kubkomawa IH, Emenalom OO, Okoli IC (2015). Body condition score, rectal temperature, respiratory, pulse and heart rates of tropical indigenous zebu cattle. Int. J. Agric. Innov. Res., 4(3): 448-454. https://ijair.org/index.php/component/jresearch/?view=publicationandtask=showandid=633
Lawrence KE, Forsyth SF, Vaatstra BL, McFadden AMJ, Pulford DJ, Govindaraju K, Pomroy WE (2017). Clinical haematology and biochemistry profiles of cattle naturally infected with Theileria orientalis Ikeda type in New Zealand. N. Z. Vet. J., 66(1): 21-29. https://doi.org/10.1080/00480169.2017.1391142
Lees AM, Lees JC, Sejian V, Sullivan ML, Gaughan JB (2020). Influence of shade on panting score and behavioural responses of Bos taurus and Bos indicus feedlot cattle to heat load. Anim. Prod. Sci., 60(2): 305-315. https://doi.org/10.1071/AN19013
Lees AM, Sejian V, Wallage AL, Steel CC, Mader TL, Lees JC, Gaughan JB (2019). The impact of heat load on cattle. Animals, 9(6): 322. https://doi.org/10.3390/ani9060322
Leo TK, Leslie DE, Loo SS, Ebrahimi M, Aghwan Z, Panandam JM, Alimon AR, Karsani SA, Sazili AQ (2012). An evaluation on growth performance and carcass characteristics of integration (oil palm plantation) and feedlot finished Bali cattle. J. Anim. Vet. Adv., 11(18): 3427-3430. https://doi.org/10.3923/javaa.2012.3427.3430
Mazzullo G, Rifici C, Cammarata F, Caccamo G, Rizzo M, Piccione G (2014). Effect of different environmental conditions on some haematological parameters in cow. Ann. Anim. Sci., 14(4): 947–954. https://doi.org/10.2478/aoas-2014-0049
McLean J, Downie A, Jones C, Stombaugh D, Glasbey C (1983). Thermal adjustments of steers (Bos taurus) to abrupt changes in environmental temperature. J. Agric. Sci., 100(2): 305-314. https://doi.org/10.1017/S0021859600033451
Medugorac I, Seichter D, Graf A, Russ I, Blum H, Göpel KH, Rothammer S, Förster M, Krebs S (2012). Bovine polledness an autosomal dominant trait with allelic heterogeneity. PLoS One, 7(6): e39477. https://doi.org/10.1371/journal.pone.0039477
Merdana IM, Sulabda IN, Tiasnitha NMWA, Gunawan IWNF, Sudira IW (2020). Erythrocyte, hemoglobin and hematocrit profile of Bali cattle during the various periods of parturition. J. Anim. Health Prod., 8(2): 75-79. https://doi.org/10.17582/journal.jahp/2020/8.2.75.79
Ministry of Agriculture of the Republic of Indonesia (2010). Determination of Bali cattle breeds decree of the minister of agriculture, Number: 325/Kpts/OT.140/1/2010. Directorate General of Animal Husbandry and Animal Health, Jakarta.
Mirzadeh K, Tabatabaei S, Bojarpour M, Mamoei M (2010). Sex, physiological status and season in Iranian cattle. J. Anim. Vet. Adv., 9(16): 2123-2127. https://doi.org/10.3923/javaa.2010.2123.2127
Mohanty JG, Nagababu E, Rifkind JM (2014). Red blood cell oxidative stress impairs oxygen delivery and induces red blood cell aging. Front. Physiol., 5: 84. https://doi.org/10.3389/fphys.2014.00084
Mota-Rojas D, Titto CG, de Mira Geraldo A, Martínez-Burnes J, Gómez J, Hernández-Ávalos I, Casas A, Domínguez A, José N, Aldo Bertoni, Reyes B, Pereira AMF (2021). Efficacy and function of feathers, hair, and glabrous skin in the thermoregulation strategies of domestic animals. Animals, 11: 3472. https://doi.org/10.3390/ani11123472
Nardone A, Ronchi B, Lacetera N, Ranier MS, Bernabucci U (2010). Effects of climate change on animal production and sustainability of livestock systems. Livest. Sci., 130: 57–69. https://doi.org/10.1016/j.livsci.2010.02.011
Novanty NPG, Sulabda IN, Dharmawan NS (2022). Total leukocytes and differential leukocytes of male Bali cattle after transportation to the pesanggaran slaughterhouse Denpasar. Indones. Med. Vet., 11(1): 21-29. https://doi.org/10.19087/imv.2022.11.1.21
Nugraha AK, Afnan R, Taufik E, Satyaningtijas AS (2020). Study of Bali cattle physiological parameters during sea transport on camara Nusantara ships. J. Ilmu-ilmu Peternakan, 30(3): 190-197. https://doi.org/10.21776/ub.jiip.2020.030.03.03
Nuraini H, Aditi EL, Brahmantiyo B (2019). Meat quality of Indonesian local cattle and buffalo. In: Bovine science. A key to sustainable development. Intech Open, 5: 65-74. https://doi.org/10.5772/intechopen.79904
Nuriyasa IM, Dewi GAMK, Budiari NLG (2015). Indeks kelembaban suhu dan respon fisiologi sapi Bali yang dipelihara secara feed lot pada ketinggian berbeda. Majalah Ilmiah Peternakan, 18(1): 5-10.
Nussa HT, Aritonang SB, Puteri SA, Rahmani AF, Bowolaksono A, Lestar R (2018). Thermal stress effect on ATP1A1 gene expression in Bali cattle (Bos sondaicus) in Barru District, South Sulawesi. AIP Conf. Proc., 2023: 020152. https://doi.org/10.1063/1.5064149
Ondruska L, Rafay J, Okab AB, Ayoub MA, Al-Haidary AA, Samara EM, Parkanyi V, Chrastinova L, Jurcik R, Massanyi P, Lukac N, Supuka P (2011). Influence of elevated ambient temperature upon some physiological measurements of New Zealand white rabbits. Vet. Med., 56(4): 180–186. https://doi.org/10.17221/3150-VETMED
Pamungkas FA, Purwanto BP, Manalu W, Yani A, Sianturi RG (2020). Use of infrared thermography for identifying physiological and hematological conditions of young Sapera dairy goats. J. Ilmu Ternak Vet. 25(3): 120-130. https://doi.org/10.14334/jitv.v25i3.2522
Pandey P, Hooda OK, Kumar S (2017). Impact of heat stress and hypercapnia on physiological, hematological, and behavioral profile of Tharparkar and Karan fries heifers. Vet. World, 10(9): 1146-1155. https://doi.org/10.14202/vetworld.2017.1149-1155
Parés-Casanova, PM, Caballero M (2014). Possible tendency of polled cattle towards larger ears. Rev. Colomb. Pediatr. Pueric., 27: 221-225.
Pathak AS, Singh P, Shahi BN, Verma MK, Rahman JU (2022). Comparative analysis of haematobiochemical profile of Badri cattle at different altitudes of Uttarakhand. J. Vet. Pharmacol. Toxicol., 21(1): 35-37. https://www.indianjournals.com/ijor.aspx?target=ijor:jvpatandvolume=21andissue=1andarticle=009
Perumal P, De AK, Bhattacharya D, Sunder J, Kundu A (2022). Hematology and biochemical profiles of endangered local cattle of Andaman and Nicobar Islands. Indian J. Anim. Sci., 92(1): 82-88. https://doi.org/10.56093/ijans.v92i1.120930
Prahesti KI, Malaka R, Baco S (2021). Stamina prediction of cows and goats to exercise changes by measuring body temperature, heart rate, and respiratory rate. Hasanuddin J. Anim. Sci., 3(1): 1-7. https://doi.org/10.20956/hajas.v3i1.14130
Prasanna JS, Rao STV, Prakash MG, Rathod S, Kalyani P, Reddy BR (2022). Effect of seasons on pysiological responses in Sahiwal and Crossbred cows. Indian J. Anim. Res., 56(10): 1202-1205.
Purwantara B, Noor RR, Andersson G, Martinez HR (2012). Banteng and Bali cattle in Indonesia: Status and forecasts. Reprod. Domest. Anim., 47(1): 2-6. https://doi.org/10.1111/j.1439-0531.2011.01956.x
Putra TD, Panjono P, Bintara S, Widayati DT, Baliarti E, Putra B (2021). Characteristics of skin coat as well as the physiological status of F1 crossing Bali (Bos sondaicus) x Angus (Bos taurus) for early identification of adaptability in tropical environment. MOJ Ecol. Environ. Sci., 6(3): 82-86. https://doi.org/10.15406/mojes.2021.06.00219
Putra TD, Bintara S, Widayati DT, Panjono, Baliarti E (2019). Physiological conditions of Bali cattle based on oil palm plantation. IOP Conf. Ser. Earth Environ. Sci., pp. 012125. https://doi.org/10.1088/1755-1315/387/1/012125
Randhawa IAS, Burns BM, McGowan MR, Porto-Neto LR, Hayes BJ, Ferretti R, Schutt KM, Lyons RE (2020). Optimized genetic testing for polledness in multiple breeds of cattle. G3 Genes, Genome, Genet., 10(2): 539-544. https://doi.org/10.1534/g3.119.400866
Rashamol VP, Sejian V, Bagath M, Krishnan G, Archana PR, Bhatta R (2018). Physiological adaptability of livestock to heat stress: An updated review. J. Anim. Behav. Biometeorol., 6(3): 62–71. https://doi.org/10.31893/2318-1265jabb.v6n3p62-71
Reece WO (2015). The composition and functions of blood. In: Dukes Physiology of Domestic Animals, 13th ed. (Editor: W.O. Reece; Assosiate editor: (H. Erickson, J. P. Goff, E. E. Uemura). Iowa, USA: John Wiley and Sons, Inc.
Renaudeau D, Collin A, Yahav S, de Basilio V, Gourdine JL, Collier RJ (2012). Adaptation to hot climate and strategies to alleviate heat stress in livestock production. Animal, 6(5): 707–728. https://doi.org/10.1017/S1751731111002448
Reuter RR, Carroll JA, Hulbert LE, Dailey JW, Galyean ML (2010). Technical note: Development of a self-contained, in dwelling rectal temperature probe for cattle research. J. Anim. Sci., 88(10): 3291-3295. https://doi.org/10.2527/jas.2010-3093
Roland L, Drillich M, Iwersen M (2014). Hematology as a diagnostic tool in bovine medicine. J. Vet. Diagn. Invest., 26(5): 592-598. https://doi.org/10.1177/1040638714546490
Sarangi S (2018). Heat adaptability in livestock in the tropics: A scenario. Pharma Innov. J., 7(3): 345-350.
Seixas L, de Melo CB, Tanure CB, Peripolli V, Manus CM (2017). Heat tolerance in Brazilian hair sheep. Asian-Australas. J. Anim. Sci., 30(4): 593-601. https://doi.org/10.5713/ajas.16.0191
Septiarini AAIA, Suwiti NK, Suartini IGAA (2020). Hematological value of total erythrocytes and hemoglobin level of Bali cattle with organic forage feed. Bull. Vet. Udayana, 12(2): 144-149. https://doi.org/10.24843/bulvet.2020.v12.i02.p07
Shilja S, Sejian V, Bagath M, Mech A, David CG, Kurien EK, Varma G, Bhatta R (2016). Adaptive capability as indicated by behavioral and physiological responses, plasma HSP70 level, and PBMC HSP70 mRNA expression in Osmanabadi goats subjected to combined (heat and nutritional) stressors. Int. J. Biometerol., 60(9): 1311-1323. https://doi.org/10.1007/s00484-015-1124-5
Siswanto (2011). The erythrocyte profile of the female Bali cattle (sloughter house study). Bull. Vet. Udayana, 3(2): 99-105.
Srikandakumar A, Johnson EH (2004). Effect of heat stress on milk production, rectal temperature, respiratory rate and blood chemistry in Holstein, Jersey and Australian Milking Zebu Cows. Trop. Anim. Health Prod., 36: 685–692. https://doi.org/10.1023/B:TROP.0000042868.76914.a9
Suharti S, Khotijah L, Nasution AR, Warmadewi DW, Cakra IGLO, Arman C, Wiryawan KG (2017). Productive and reproductive performances and blood profile of Bali cows supplemented with calcium soap-soybean oil. Pak. J. Nutr., 16: 882-887. https://doi.org/10.3923/pjn.2017.882.887
Tamzil MH, Noor RR, Hardjosworo PS, Manalu W, Sumantri C (2014). Hematological response of chickens with different heat shock protein 70 genotypes to acute heat stress. Int. J. Poult. Sci., 13(1): 14-20. https://doi.org/10.3923/ijps.2014.14.20
Terrien J, Perret M, Aujard F (2011). Behavioral thermoregulation in mammals: A review. Front. Biosci., 16: 1428-1444. https://doi.org/10.2741/3797
Thompson IM, Dahl GE (2012). Dry-period seasonal effects on the subsequent lactation. Prof. Anim. Sci., 28(6): 628-631. https://doi.org/10.15232/S1080-7446(15)30421-6
Wang J, Li J, Wang F, Xiao J, Wang Y, Yang H, Li S, Cao Z (2020). Heat stress on calves and heifers: A review. J. Anim. Sci. Biotechnol., 11(79): 1-8. https://doi.org/10.1186/s40104-020-00485-8
Wiedemar N, Tetens J, Jagannathan V, Menoud A, Neuenschwander S, Bruggmann R, Thaller G, Drögemüller C (2014). Independent polled mutations leading to complex gene expression differences in cattle. PLoS One, 9(3): e93435. https://doi.org/10.1371/journal.pone.0093435
Yani A, Suhardiyanto H, Hasbullah R, Purwanto BP (2007). Analisis dan simulasi distribusi suhu udara pada kandang sapi perah menggunakan computational fluid dynamics (CFD). Media Peternakan, 30(3): 218−228.
Zulkharnaim, Baco S, Yusuf M, Rahim L (2017). Comparison of body dimension of Bali polled and horned cattle in South Sulawesi. Int. J. Sci. Basic Appl. Res., 36(5): 133–139.
Zulkharnaim, Baco S, Yusuf M, Rahim L (2020a). Morphological and mating behavioral characteristics polled Bali cattle. IOP Conf. Ser. Earth Environ. Sci., 492: 012105. https://doi.org/10.1088/1755-1315/492/1/012105
Zulkharnaim, Baco S, Rahim L, Yusuf M (2020b). Identification of qualitative characteristic Bali polled cattle. Hasanuddin J. Anim. Sci., 2(2): 70-75.
To share on other social networks, click on any share button. What are these?






