Effect of Harvesting Stages on Seed Yield and Quality of Okra (Abelmoschus esculentus L. Moench)
Research Article
Effect of Harvesting Stages on Seed Yield and Quality of Okra (Abelmoschus esculentus L. Moench)
Farzana Begum* and Gohar Ayub
Department of Horticulture, The University of Agriculture, Peshawar, Pakistan.
Abstract | Field experiments were conducted to study the effect of harvesting stages i.e. (mature pod of turning stage, brown undried stage and brown dried stage) on seed yield and quality attributes of okra sown at different dates (March 10, March 30, April 19 and May 9) at Agriculture Research Institute Tarnab Peshawar, during 2016 and 2017. A Randomized Complete Block Design (RCBD) with split plot arrangement having sowing dates as the main plot factor, while harvesting stages as sub plot factor was used. The seeds of okra, after harvesting and drying were packed in polythene bags and stored for 0, 3 and 6 months at room temperature. The seed quality attributes were evaluated at the specified storage duration. It is evident from the results that sowing dates, harvesting stages, storage duration and their interaction significantly affected seed yield and quality parameters of okra. The average of two years results showed that maximum pod length (15.23 cm), number of seeds pod-1(58.09), seed weight pod-1(3.57 g), 1000 seed weight (g), seed yield (1.72 t ha-1), germination (79.61 %) and seed viability (91.85 %) were observed in seeds harvested from plants sown on March 30 as compared to seeds harvested from late sown plants on May 9. Highest hard seed (23.69 %) was recorded in seeds collected from late sown plants on May 9 as compared to other sowing dates. As for as harvesting stages are concerned, maximum seed weight pod-1(3.57 g), 1000 seed weight (61.33 g), seed yield (1.67 t ha-1), germination (81.21 %) and seed viability (91.86 %) was recorded when pods were harvested at brown dried stage. Whereas maximum pod length (15.63cm), highest moisture content (12.57 %) and hard seed (26.85 %) were recorded at mature pod of turning stage. The data on seed quality attributes in relation to storage revealed that the highest seed moisture (13.21 %) and seed viability (91.86 %) was recorded in seeds stored for 0 months, but the mean maximum germination percentage (75.58%) and lowest hard seed (17.46 %) was recorded with seeds stored for 3 months. The seed moisture content, germination percentage and seed viability decreased, while hard seeds increased with increasing seed storage duration. The D x H interaction influenced most of the quality attributes such as seed weight pod-1, germination, hard seeds, but moisture content and seed viability of the seeds was not significantly affected. The H x S interaction also significantly affected the seed moisture content, germination percentage, hard seed and seed viability. So, it can be concluded that sowing on March 30 with pods harvested at brown dry stage resulted in maximum seed yield and quality and slowed down the deterioration of the seeds during storage.
Received | March 10, 2020; Accepted | December 31, 2021; Published | July 05, 2022
*Correspondence | Farzana Begum, Department of Horticulture, The University of Agriculture, Peshawar, Pakistan; Email: [email protected]
Citation | Begum, F. and G. Ayub. 2022. Effect of harvesting stage on yield and quality of Okra (Abelmoschus esculentus L. Moench) seed. Sarhad Journal of Agriculture, 38(3): 871-884.
DOI | https://dx.doi.org/10.17582/journal.sja/2022/38.3.871.884
Keywords | Harvesting stages, Okra, Quality, Sowing times, Seed yield
Copyright: 2022 by the authors. Licensee ResearchersLinks Ltd, England, UK.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Introduction
Seed is the basis of agriculture, and it is recognized as the first and most important step in every crop production programme (Elhag and Ahmad, 2014). The germination of okra seeds is a serious issue. A number of variables contribute to the low germination percentage of okra seeds, but one of the most important is the occurrence of hardseedness. Tegument impermeability is a feature of hardseed that acts as a primary obstruction against the absorption of moisture and emergence of radicle through seed during germination (Castro, 2005). Certain management practices are required for the successful production of okra seed (El-Waraky, 2014). Seedling vigour and viability determine the quality of the seed, which is influenced by a variety of factors such as the seed’s genetic potential, the inputs utilized, the harvesting stage, pods position on the plant and the environment in which the crop is cultivated (Verma et al., 2004). Locally produced seeds, on other hand, are of poor quality and purity, resulting in low yields (Vossen,1994).
Several growth and yield characteristics are significantly affected by sowing time, which were reduced by late planting dates. Okra is cultivated in tropical and the subtropical regions as early as possible in the spring when the soil temperature is favourable (Ghanti et al., 1991). Seed production is negatively affected by planting seeds late in the season owing to decrease in vegetative and reproductive attributes (Elhag and Ahmad, 2014). Temperature has an effect on seed quality during the seed-filling process. A variety of processes concerned in seed filling can differentially affected by high temperature. Seed composition can also be affected by heat stress. Functioning of the pollen and seed setting, pollen development, germination, and pollen tube expansion are all affected by environmental factors (Thuzar et al., 2010) and thus reduced the number of seeds and quality. Periodic evaluation of planting dates is critical due to changing agroclimatic conditions.
For optimal seed growth and maturity, the harvesting stage is crucial to increased seed yield, seed vigour and viability. For seed production pods are allowed to dry on the mother plant before being collected. To guarantee maximum germinability after harvest and during storage, the seed crop must be at proper stage of maturity, followed by thorough drying. This is due to the fact that seed longevity is known to be controlled by seed quality, which is influenced by the production process (Bortey and Dzomeku, 2016). Seed maturation is a positive process that occurs throughout seed growth and involves additive increases in seed parameters such as seed size, dry weight, viability, and vigour. When a seed reaches physiological maturity, it has reached its maximum dry weight, germination, and vigour (Ashok et al., 2005). Because seed quality peaks at physiological maturity, knowing when this stage of development occurs can help a seed farmer optimize following activities like harvesting and drying in order to maximize seed germination and vigor. According to Silva et al. (2017), seeds reach their peak quality toward the end of the seed filling stage, after which viability and vigour drop. Picking okra seed pods should be done when they are completely dry. Due to severe weather conditions, a delayed harvest may result in reduced germination and vigour in okra (Agrawal, 2011). The optimal harvesting stage has a significant effect on seed quality. Seed maturation, on the other hand, is linked to fruit maturity and full fruit drying (Ashok et al., 2005). Unfortunately, many farmers are unaware of how to manipulate harvest time for fresh fruit yield; also, the proper maturity period for harvesting okra for seed must also be considered. However, research on okra, a popular vegetable crop throughout Pakistan, is scarce. Keeping in view the consideration, an experiment was conducted to evaluate the effect of harvesting stage and sowing date on seed quality of okra stored for six months at room temperature.
Materials and Methods
The experiment entitled was carried out at Agriculture Research Institute Tarnab Farm Peshawar, Pakistan during the summer seasons of the years 2016 and 2017 in two phases. During the first phase, the field experiment was laid out in Randomized Complete Block Design (RCBD) with split plot arrangement having three replications. Date of sowing was kept in main plots, while harvesting stages were allotted to sub plots. Seeds of the okra cultivar Sabz Pari were pre-soaked in water for 24 hours before being planted with 60 cm between each row and 30 cm between plants within the rows. The plot size was 1.50× 2.40 m dimensions with total area 3.60 m2. During the second phase, the seeds after extraction and drying were packed in polythene bags and arranged in three factorial arrangements in Completely Randomized Design (CRD), and stored for 0, 3 and 6 months at room temperature. The seed quality attributes of each treatment were evaluated at the end of respective storage period. The Treatments details were as under.
|
Factor A:Sowing dates (D) |
Factor B: Harvesting stages (H) |
|
March 10 |
Mature pod of turning stage |
|
March 30 |
Brown undried stage |
|
April 19 |
Brown dried stage |
|
May 9 |
Data were recorded on the following parameters
Pod length (cm), number of seeds pod-1, seeds weight pod-1(g), seed yield (t ha-1), moisture content (%) germination (%), hard seed (%) and seed viability.
Seed yield (t ha-1)
The seed yield plot-1 was recorded and the seed yield (t ha-1) was computed using the following formula:
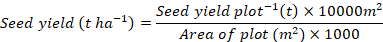
Moisture content (%)
Moisture content of seed was determined by constant temperature oven method (ISTA, 2017). Before drying the seeds were fine grinded. Then 4.5 g of grinded sample in a container with diameter < 8 cm was dried in hot air oven, maintained at 130-133oC for 2 hours. At the end the container was placed in desiccators to cool at ambient temperature and then the dry weight of the material, as well as the container, was recorded. The moisture content was calculated using the formula below;
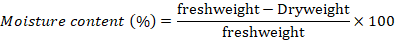
Germination (%)
100 seeds were put on two layers of blotter paper soaked with water equal to 2.5 times the substratum weight and germinated on Petri plates at 25°C in four replications. After 7 and 21 days, germination counts were made based on the number of normal seedlings. For each treatment, the mean germination percentage was computed (ISTA, 2017).
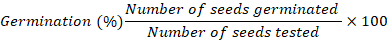
Hard seed (%)
100 seeds of each treatment were placed in seed germination trays with moist tissue paper to assess the hard seed percentage. The seeds were kept in germination trays at 25°C for ten days. The tissue in the trays was moistened on a regular basis to keep it wet. On a daily basis, the seed germination was monitored; seeds with protruding radicles through the coat were deemed germinated. The sprouted seeds were removed, and hard seed was defined as the percentage of seeds that did not germinate after 10 days (Auld et al., 1988).
Seed viability (%)
Two replications of 100 seeds were imbibed on moist media for 24 hours at 25°C to allow complete hydration of all tissues. The hydrated seeds were bisect longitudinally and were dipped in 0.1% tetrazolium chloride solution for 24 h at 30°C. Staining pattern reveals the live and dead areas of embryo and enables to evaluate the seed vigor. The number of viable seeds were then counted and their average was calculated (AOSA, Tetrazolium testing handbook, 2010).
Statistical analysis
The data recorded were analyzed statistically combined over years using analysis of variance techniques appropriate for RCB design with split plot arrangement and CRD. Means were compared using LSD test at 0.05 level of probability when the F values were significant (Steel and Torrie, 1980). The statistical software GenStat release 8.1 was used for the analysis of data.
Results and Discussion
Pod length (cm)
Data regarding pod length are shown in Table 1. Pod length was affected significantly by sowing dates and harvesting stages. The interaction effects for all the treatments remained non-significant. Sowing on
Table 1: Pod length (cm), seeds pod-1, seed weight pod-1 (g), 1000 seeds weight (g) and seed yield (t ha-1) of okra as affected by sowing dates and harvesting stages.
|
Sowing dates (SD) |
Pod length (cm) |
Seeds pod-1 |
Seed weight pod-1 (g) |
1000 seeds weight (g) |
Seed yield (t ha-1) |
|
March 10 |
15.05 a |
56.05 a |
3.19 b |
60.08 b |
1.61 b |
|
March 30 |
15.23 a |
58.09 a |
3.57 a |
62.57 a |
1.72 a |
|
April 19 |
14.97 a |
57.61 a |
3.51 a |
62.49 a |
1.64 b |
|
May 9 |
14.14 b |
51.61 b |
2.93 c |
55.17 c |
1.46 c |
|
LSD(0.05) |
0.54 |
2.33 |
0.18 |
1.27 |
0.05 |
|
Harvesting stages (H) |
|||||
|
Mature fruit of turning stage |
15.63 a |
55.09 |
3.01 c |
58.34 b |
1.55 c |
|
Brown undried stage |
14.96 b |
56.14 |
3.31 b |
60.57 a |
1.60 b |
|
Brown dried stage |
13.96 c |
56.29 |
3.57 a |
61.33 a |
1.67 a |
|
LSD(0.05) |
0.54 |
NS |
0.18 |
1.01 |
0.05 |
|
Years (Y) |
|||||
|
2016 |
14.75 |
54.56 b |
3.21 b |
59.39 b |
1.59 b |
|
2017 |
14.95 |
57.12 a |
3.39 a |
60.76 a |
1.63 a |
|
Interactions |
|||||
|
Y x D |
NS |
NS |
NS |
NS |
NS |
|
D x H |
NS |
NS |
* |
NS |
NS |
|
Y x H |
NS |
NS |
NS |
NS |
NS |
|
Y x D x H |
NS |
NS |
NS |
NS |
NS |
The means that are not preceded by the same letters are significantly different. * Indicates a 5% probability level. NS stands for non-significant.
March 30 resulted in maximum pod length (15.23cm) followed by (15.05cm) and (14.97cm) in plants sown on March 10 and April19 respectively, with difference being non-significant, while lowest pod length (14.14cm) were recorded in late sown plants on May 9. Pod length was also significantly influenced by stage of harvesting. Pod harvested at turning stage exhibited the maximum pod length (15.63cm) followed by (14.96cm) when pods were harvested at brown undried stage, while the minimum pod length (13.96 cm) were recorded at brown dried stage. This increment in pod length might be due to the effect of prevailing environmental conditions on flowering, pollination and subsequent pod development (Mohamed et al., 2016). Iremiren and Okiy (1986) observed that the growth response of okra to varied sowing dates is not consistent, and that okra seeded on April 1st had maximum pod length. Our findings are consistent with those of Shahid et al. (2015), who found that cultivars sown on March 30thhad the longest pod length, while cultivars sown on March 15thand May 30thhad the shortest pod length. Similarly, Incalcaterra et al. (2000) stated that okra seeded on April 1st had the longest pod length compared to okra seeded on April 15th.
Initially the fruit length was found to increase rapidly, however, towards the brown dried stage, fruit length decreased with maturity, which might be ascribed to drying and shrinking of the fruits. According to Gupta (2005), the longest pod length was reported when the pods were taken at their full green stage, which was identical to the length of pods harvested at mature yellow stage while the lowest was found when the pods were harvested at dry stage. Samnorata et al. (2002) stated that the length of okra pod was gradually increased, reaching at peaked at 18 and 24 days after anthesis and then began to decline.
Number of seeds pod-1
Data regarding number of seeds pod-1 are shown in Table 1. Number of seeds pod-1 was significantly influenced by sowing dates and year. The interaction effects for all the treatments remained non-significant. Sowing on March 30 resulted in maximum number of seeds pod-1 (58.09) followed by (57.61) and (56 05) in plants sown on April19 and March 10 respectively with difference being non-significant, while minimum number of seeds pod-1 (51.61) were recorded in late sown plants on May 9. Harvesting stage had no significant effect on number of seeds pod-1. However, the maximum number of seeds pod-1 was obtained, when pods were harvested at dry brown stage. The effect of year was also significant. More seeds pod-1 (57.12) was recorded during the year 2017 as compared to (54.56) seeds pod-1 in 2016.
Differences in number of seeds pod-1 with different sowing dates might be due to environmental condition prevailing during growth period (Thuzar et al., 2010). Lowest number of seeds pod-1 in late sowing might be due to temperature, as during the seed-filling period, seed quality is sensitive to temperature stress. Increasing temperature can accelerate to hasten seed growth rate, decrease the duration of seed filling which reduce seed number and seed weight (Singh et al., 2013). Mohamed et al. (2016) reported that significant highest number of seed pod-1 was recorded with sowing on 1stApril, while lowest number of seeds pod-1 were observed on 1stMay sowing in the first and second seasons, respectively.
When mature pods are presen tat the plant, has been shown to limit growth of plant and, in turn, fruit setting (Dhingra, 2009), and this effect may also apply to seeds inside the same fruit. Bortey and Dzomeku (2016) recorded the maximum seed pod-1 at 40 Days after anthesis. The seeds pod-1 reported at this stage of pod development were substantially higher than seeds harvested earlier or later. Similarly, Hedau et al. (2010) also recorded maximum number of seeds pod-1, when the pods were harvested at brown dry stage.
Seed weight pod-1 (g)
Seed weight pod-1 was significantly affected by sowing dates, harvesting stage and year according to the mean values in Table 1. Significant interaction was also found between D x H. Significantly maximum seed weight pod-1 (3.57 g) was obtained from plants sown on March 30followed by (3.51 g) in plants sown on April 19 with the difference being non-significant, while significantly less seed weight pod-1 (2.93 g) were noted in late sown plants on May 9. Seed weight pod-1 was also significantly influenced by stage of harvesting. Pod harvested at brown dry stage exhibited the maximum seed weight pod-1 (3.57 g) followed by (3.31 g) when pods were harvested at brown undried stage, while the minimum seed weight pod-1 (3.01 g) were recorded at mature pod of turning stage. The year effect was found significant. More seed weight pod-1 (3.39 g) was recorded during the year 2017 as compared to (3.21 g) seed weight pod-1 in 2016. The interaction between D x H revealed that sowing plants on March 30 harvested at brown dry stage exhibited the maximum seed weight pod-1 than other D x H interactions (Figure 1).
Seed weight is also influenced by environmental condition prevailing during seed filling duration (Singh et al., 2013). The seed-filling duration is sensitive to temperature. Increasing temperature during growth period can have an effect on the different processes involved in seed filling (Thuzar et al., 2010). Mohamed et al. (2016) observed that the heaviest seed weight pod-1 was recorded in case of sowing okra plants on 1st April. Significantly, the lowest values of seed weight pod-1 were observed on 1st May sowing in both seasons, respectively.
With the progression of physiological maturity, the weight of the seed may increase due to a reduction in moisture content and the accumulation of food material (Barnwal et al., 2017). Seed maturity is a polygenic variable that is regulated by genetic and environmental variables that affect the plant’s morphological, phenological and physiological characteristics (Gwathmey et al., 2016). According to Demir (1994) physiological seed maturity of okra, as measured by maximum seed dry matter weight and germination might occur 35 days following anthesis.
1000 seed weight (g)
Mean values of the data given in Table 1 indicated that 1000 seed weight was significantly affected by sowing dates harvesting stage and year. The interaction effect for all the treatments were non-significant. Sowing on March 30 had significantly maximum 1000 seed weight (62.57 g) followed by (62.49 g) in plants sown on April 19 with the difference being non-significant, while minimum 1000 seed weight (55.17 g) was produced in late sown plants on May 9. 1000 seed weight was also significantly influenced by stage of harvesting. Pod harvested at brown dry stage exhibited the maximum 1000 seed weight (61.33 g) and were statistically at par with (60.57 g) when pods were harvest at brown undried stage, while the minimum 1000 seed weight (58.34 g) were recorded at mature pod of turning stage. The year effect was found significant. More 1000 seed weight (60.76 g) was recorded during the year 2017 as compared to (59.39 g) 1000 seed weight in 2016.
Maximum seed weight with sowing in the month of March and April might be due to environmental condition as for higher seed yield, okra requires favorable climatic conditions. The optimum date of sowing determined the plant growth and seed yield (Ashok, 2002). Lowest seed weight in late sowing might be due to the direct effect of high temperatures on reproductive growth; mainly pollen development and function that can reduce the seed number and seed weight. Since warming speedup the reproductive process which resulted in small seed formation (Thuzar et al., 2010). The present results are in similarity with the results of (Mohamed et al., 2016) who stated that maximum 100 seeds weight was obtained from plants sown on 1stApril, while minimum 100 seed weight was obtained from plants sown on 1st May, in both seasons.
The weight of the seed increases as it reaches physiological maturity due to decrease in moisture content and accumulation of food material (Barnwal et al., 2017). The weight of seed is significantly influenced by environmental and genetic variables, and the amount is influenced by the maturity condition; these factors can cause a 20-30% reduction in 1000 seed weight. Same findings were also obtained by Hedau et al. (2010), who observed that 1000 seed weight was higher when the pods were harvested at brown dry stage. Ibrahim and Oladiran (2011) found that 100 seed weight increased from 14 to 35 days following anthesis, whereas Mistry and Hussain (2008) found that 100 seed weight was significantly maximum 30 days after flowering.
Seed yield (t ha-1)
The seed yield as affected by sowing dates, harvesting stage and year is presented in Table 1. Significant difference was found among the sowing dates, harvesting stage and year. None of the interactions were significant regarding seed yield. Sowing on March 30 produced the highest seed yield of (1.72 t ha-1) followed by (1.64 t ha-1) and (1.61t ha-1) in plants sown on April 19 and March 10 respectively, whereas late sown plants on May 9 produced least (1.46 t ha-1) seed yield. Pod harvested at brown dry stage exhibited the maximum seed yield (1.67 t ha-1) followed by (1.60 t ha-1) when pods were harvest at brown undried stage, while the minimum seed yield (1.55 t ha-1) were recorded at mature pod of turning stage. Data regarding the year revealed that seed yield was maximum (1.63 t ha-1) during 2017 as compared to (1.59 t ha-1) in 2016.
Seed yield was high due to vigorous vegetative development and high pod production. Sowing at the right time is a key part of getting the best yield. Crops’ quantitative and qualitative features are dependent on planting at the right time and in the right season (Farrag, 1995). Smililar results were obtained by Shahid et al. (2015), who stated that the higher seed yield was recorded from okra cultivars sown on 30th March 2014, while the least seed yield was obtained from cultivars sown on 14th April. The findings are similarly consistent with those of Incalcaterra et al. (2000), who found that April sowings had better vegetative growth and fruit set than March sowings.
Increased seed yield with harvesting at brown dry stage might be due to prolonged time of harvest, since the weight of seeds was increased until 40 days after anthesis and after that it was reduced (Mohamed et al., 2016). Fruit position on plant, seed maturity and growing season all influence seed quality in okra (Prabhakar et al., 1985). According to Nonnecke (1989), okra must be harvested at the most appropriate stage for maximum seed yield and quality. Hedau et al. (2010) observed the highest seed yield from fruits harvested at undried brown and dry brown stages of maturity. This assumption might support the findings of Bewly and Black (1994), who found that fruits picked between 25 and 30 DAA had larger seed yield, ranging from 70 to 80 seeds pod-1.
Seed moisture content (%)
The data regarding seed moisture content (%) as affected by harvesting stage and storage duration were significant, while there was no significant effect of sowing dates on seed moisture content (%) stated in Table 2. The interaction between harvesting stage and storage duration (H x S) also significantly affected the seed moisture content (%). The mean data in the Table 2 showed that the moisture content of the okra seed was the highest (12.57 %) in seed harvested at mature pod of turning stage that declined to 12.31% and 11.52% with delay in harvesting at brown undried and brown dried stage respectively. The moisture content of the seed decreased as storage time increased. During 0-month storage, the maximum moisture content (13.21%) dropped to 12.07 % and 11.12 %, respectively, with 3 and 6 months storage. The data regarding H x S interaction indicated that the moisture contents of the okra was highest in the seeds harvested at mature pod of turning stage, which declined to the minimum with seed harvested at brown dry stage. The least seed moisture content was recorded in seed harvested at brown dried stage and stored for 6 months (Figure 2).
Seed moisture content is high in the seed filling stage, followed by a dramatic loss of moisture throughout the maturity drying phase (Bewley and Black, 1994). When the fundamental features of the seed are fully grown, low seed moisture content may suggest maturity. A reduction in seed moisture content of sunflower was reported with an increase in germination percentage as harvesting stages progress (Adetunji, 1991). The decrease in seed moisture content as DAA increased might be attributed to maturation drying, as observed at various harvesting stages. Several studies have found that one of the most important aspects of seed development in fleshy-fruited vegetables is that seed moisture content maintains about 35-40% inside the fruit (Tekrony and Egli, 1991). After then, the seed moisture content will gradually decrease and the dry matter will continue to accumulate (El Balla, 2011). Increasing days after anthesis following declines in seed moisture content due to maturation drying.
Table 2: Moisture content (%), germination (%), hard seed (%) and viability (%) of okra seeds as affected by sowing dates, harvesting stages and storage duration.
|
Sowing dates (D) |
Moisture content (%) |
Germination |
Hard seed (%) |
Seed viability (%) |
|
March 10 |
12.20 |
74.56 c |
18.41b |
90.61 b |
|
March 30 |
12.12 |
79.61 a |
15.85 d |
91.85 a |
|
April 19 |
12.15 |
76.45 b |
17.33 c |
91.39 a |
|
May 9 |
12.07 |
64.89 d |
23.69 a |
87.61 c |
|
LSD (0.05) |
NS |
0.78 |
0.47 |
0.62 |
|
Harvesting stages (H) |
||||
|
Mature fruit of turning stage |
12.57 a |
62.24 c |
26.85 a |
88.35 c |
|
Brown undried stage |
12.31 a |
78.18 b |
15.82 b |
90.89 b |
|
Brown dried stage |
11.52 b |
81.21 a |
13.79 c |
91.86 a |
|
LSD (0.05) |
0.28 |
0.67 |
0.41 |
0.54 |
|
Storage duration (S) |
||||
|
0 Months |
13.21 a |
73.63 b |
19.00 b |
91.86 a |
|
3 Months |
12.07 b |
75.58 a |
17.46 c |
90.21 b |
|
6 Months |
11.12 c |
72.42 c |
20.00 a |
89.03 c |
|
LSD (0.05) |
0.28 |
0.67 |
0.41 |
0.54 |
|
Years (Y) |
||||
|
2016 |
12.10 |
73.95 |
18.86 |
90.31 |
|
2017 |
12.17 |
73.81 |
18.78 |
90.42 |
|
Interactions |
||||
|
D x H |
NS |
** |
** |
NS |
|
D x S |
NS |
NS |
NS |
NS |
|
H x S |
* |
* |
** |
* |
|
D x H x S |
NS |
NS |
NS |
NS |
|
Y x D |
NS |
* |
NS |
NS |
|
Y x H |
NS |
NS |
NS |
* |
|
Y x S |
NS |
NS |
NS |
NS |
|
Y x D x H |
NS |
NS |
NS |
NS |
|
Y x D x S |
NS |
NS |
NS |
NS |
|
Y x H x S |
NS |
NS |
NS |
NS |
|
Y x D x H x S |
NS |
NS |
NS |
NS |
The means that aren’t preceded by the same letters are significantly different. *, ** indicates a 5 and 1% probability level respectively. NS stands for nonsignificant.
During storage, seed conservation is strongly associated with moisture content which affects speed of metabolic activities and chemical composition of the seed (Marcos-Filho, 1999). Silva et al. (2017) reported that in the three phases of fruit maturity, there were minor fluctuations in seed moisture content throughout storage; a little decline was noted up to twelve months of storage. Khan (2019) also reported that the moisture content of the seed decreased dramatically as storage time increased. The maximum mean seed moisture content (12.89%) of fresh seeds (0 month storage) decreased to 12.18, 11.32 and 10.40% with 3, 6 and 9 months storage.
Germination (%)
Mean values of the data given in Table 2 indicated that germination (%) was significantly affected by sowing dates, harvesting stage and storage duration. The D x H, H x S, Y x D interactions remained significant, while rest of the interactions were not significant. Seeds harvested from plants sown on March 30 had significantly highest seed germination (79.61%) followed by in seeds harvested from plants sown on April 19 (76.45 %) and the least germination (64.89%) were recorded in seed harvested from late sown plants on May 9. The germination percentage of the seed was also significantly affected by stage of harvesting. Pod harvested at brown dry stage resulted in the highest seed germination (81.21%), followed by brown undried stage (78.18 %) and the least germination (62.24%) was recorded when pods were harvested at mature pod of turning stage. Germination percentages ranged from 73.63 % in fresh seeds (0 month storage) to 75.58 % in okra seeds held for three months. However, as the storage time was extended to 6 months, the germination rate dropped to 72.42 %. The D x H, indicated that sowing seed on March 30 and harvested at brown dry stage exhibited the highest seed germination than other D x H interactions (Figure 3). Interaction between H x S showed that the seeds harvested at brown dry stage and stored for 3 month had the maximum germination, while the lowest germination was recorded when seed harvested at mature fruit of turning stage stored for 6 months (Figure 4). The interactive effect of Y x D presented in (Figure 5) revealed that germination percentage was maximum in seeds harvested from plants sown on March 30 followed by April 19 sowing and that were decreased in May 9 sowing during both the year.
The lowest germination in seeds obtained from late sown plants might be due to temperature stress during seed filling duration, which can decrease germination by reducing the ability of the plant to provide the assimilates essential to synthesize the storage compounds necessary for the germination process (Hampton et al., 2013), and the seeds incur physiological injury to the point that they lose their capacity to germinate (Powell, 2006). Mohamed et al. (2016) stated that okra seed germination was affected greatly by planting dates in both seasons. Maximum germination (71.1% and 73.1 %) was recorded from seeds of okra plants sown on 1stApril, while, significantly minimum germination (53.1% and 57.3 %) was recorded in seeds collected from plants were sown on 1st May in the first and second seasons, respectively.
Premature harvesting or harvesting at the senescence stage can reduced seed germination (Setubal et al., 1994; Passam et al., 1998). The low germination of early harvested seeds may be due to the large proportion of immature seeds in these sets of seeds, as seeds become viable and vigorous when the embryo and endosperm grow properly and proportionately, and hence more germination occurred. Godon et al. (1979) also reported that immature seeds are known to germinate poorly. Early harvesting of soybeans, according to Mugnisjah and Nakamura (1984), might result in low germination and vigour. Verma et al. (2004) also observed that among the stages of harvesting, during both years, the brown undried stage and the brown dried stage showed the higher seed germination.
As the storage period extended, the germination rate decreased. Differential germination responses of okra seed to storage period were reported by Adam et al. (2017). This conclusion is in line with that of (Sultana et al., 2016), who found that, regardless of the containers used in the study, germination of stored okra seeds reduced significantly with increasing storage time. Similar reports were also available from certain researcher for other crop species. For example, (Verma and Tomer, 2003) found that as the storage duration of Brassica (Brassica campestris) seeds increased, seed germination, emergence rate, and seedling establishment decreased. Yilmaz and Aksoy (2007) also stated that, regardless of storage dutaion and conditioned, the germination of Rumex scutatus seeds decreased as storage duration increased.
Hard seed (%)
Mean values of the data given in Table 2 indicated that hard seed (%) was significantly affected by sowing dates, harvesting stage and storage duration. The D × H and H × S interactions remained significant, while rest of the interactions were not significant. Seeds harvested from plants sown on March 30 had significantly lowest hard seed (15.85%) followed by in seeds harvested from plants sown on April 19 (17.33 %) and the highest hard seed (23.69%) were recorded in seed harvested from late sown plants on May 9. The hard seed percentage was also significantly affected by stage of harvesting. Pod harvested at brown dry stage resulted in the lowest hard seed (13.79 %), followed by brown undried stage (15.82 %) and the maximum hard seed (26.85 %) was recorded when pods were harvested at mature pod of turning stage. The hard seed percentage was 19 % in fresh seeds (0 month storage), that decreased to the minimum of 17.46 % in okra seeds stored for 3 months. The hard seed percentage, however, increased to 20 % with further increase in storage duration to 6 months. The D × H and H × S interaction significantly affected the hard seed percentage of the okra. The D × H, indicated that sowing seed on March 30 and harvested at brown dry stage exhibited the lowest hard seed than other D × H interactions (Figure 6). Interaction between H x S showed that the seeds harvested at brown dry stage and stored for 3 month had the lowest hard seeds, while the highest hard seed was recorded when seed harvested at mature pod of turning stage stored for 6 months (Figure 7).
Differences in seedhardness with different sowing dates might be due to environmental condition prevailing during growth period (Thuzar et al., 2010). According to Passam et al. (1998), the proportion of hard seeds in okra is especially high during (July-August) periods of extreme heat, whereas Demir (1997) found that quick seed drying enhanced the hardness of the seed coat. According to Getzin (1983), late-maturing fruits contain more number of immature seeds, resulting in higher number of dead or hard seeds and less vigorous seedlings, lowering total germination.
When seeds are harvested before they have reached physiological maturity, may reduce vigour potentials and viability due to high moisture content and a large number of immature seeds with a low degree of embryo development (Dayal et al., 2017). Harvesting seeds at 40 DAA, according to sGhadir et al. (2013), may be a good method for minimising the hard seeds and enhancing percent germination of okra seeds.
With the increasing storage duration moisture content of the seed declined significantly (Khan, 2019). The proportion of hard seed raise as seed moisture level dropped, indicating that moisture level in seed is a key determinant in hardseedness formation. Helengeson (1932) stated that moisture content declined fast to approximately 25% during the ripening of seed of Trifolium specie, and at moisture content of 14%the seed coat became impermeable.
Seed viability (%)
Table 2 shows data on seed viability (%) in response to various sowing dates, harvesting stages and storage durations. As a source of variation, sowing dates, harvesting stages and storage time all had a significant effect on seed viability (%). While the year effect on seed viability was not significant. The interactions were not significant except H × S and Y × H interactions. The seed viability (91.85 %) was highest in seeds harvested from plants sown on March 30, followed by (91.39 %) in seeds obtained from plants sown on April 19 with the difference being non significant. Seeds collected from plants sown on May 9 resulted in lowest viability (87.61%). Regarding harvesting stages maximum seed viability (91.86%) were recorded in seeds harvested at brown dried stage followed by (90.89%) and (88.35 %) when the seeds were harvested at brown undried and mature pod of turning stage respectively. The viability percentage was 91.86% in fresh seeds (0 Month storage), that decreased to 90.21% in seeds stored for 3 months. The viability (%), however, declined to 89.03 % with further increase in storage duration to 6 months. The H × S interaction as presented in (Figure 8) indicated that the seeds harvested at brown dry stage and stored for 0 month had the maximum viability while the lowest viability was recorded when seed harvested at mature fruit of turning stage and stored for 6 months. The Y × H interaction as presented in (Figure 9) showed that maximum seed viability is recorded in seeds harvested at brown dry stages during 2017.
Differences in seed viability with different sowing dates might be due to environmental condition prevailing during growth period (Thuzar et al., 2010). Lowest seed viability in seeds collected from late sown plants might be due to temperature, as the physiological deterioration of seeds is induce or increases by high temperature during seed maturation period (Powell, 2006), and limited evidence suggests that at critical stages of seed development, a short periods of high temperature stress are required to decrease seed vigor and viability (Hampton et al., 2013). Caldwell (1972) observed that deterioration of seed during the post maturation, pre harvest environment is a serious seed production problem. Even one week exposure to rainy condition causes 20- 30% loss in cotton seed viability.
Due to a large number of immature seeds with a low degree of embryo development and high moisture content, early seed harvesting before physiological maturity may reduce vigour potentials and viability (Dayal et al., 2017). According to Getzin (1983), late-maturing fruits contain a greater number of immature seeds, resulting in a greater number of dead or hard seeds and less vigorous seedlings, lowering total germination. Ibrahim and Oladiran (2011) recorded highest seed viability and 97% germination in okra, when seeds were harvested at 42 days after anthesis. Seeds become more vigorous and viable due to proper development of embryo and endosperm when the seeds were harvested about 26 days after flowering i.e. 75 % or more germination occurred (Devadas et al., 1998).
During storage all seeds losses its viability with a loss of vigor followed by loss in germination. It is always indispensable to store seed, despite inevitable losses in vigor and viability. Storage times (from harvest to the next sowing) can range from six months to many years (Agrawal, 1986). Seeds undergo physiological and physico-chemical changes known as ageing during storage, which include breakdown of the chemical components of the seeds. Seed viability, on the other hand, declines with time as lipid per oxidation changes. The capacity of the seed to resist degradation and its protective systems determine how quickly it matures (Carvalho et al., 2017). Early indicators of membrane disorder have been linked to reduced germination rates, whereas the emergence of seedling abnormalities (in the final stages of degradation) have been linked to considerable tissue death in many seed parts, notably in meristematic tissues. Oladiran and Agunbiade (2000) reported a general decline in seed viability with increasing storage duration at all harvesting stages irrespective of the seed position on mother plant, could be attributed to seed deterioration.
Conclusions and Recommendations
On the basis of these findings, it could be inferred that:
- Sowing on March 30 proved superior to other sowing dates in the seed yield and quality as evident from the number of seeds pod-1, seed weight pod-1, 1000 seed weight, seed yield, germination and seed viability while, highest hard seed percentage was recorded in seeds collected from late sown plants on May 9.
- For seed production, pods harvested at undried brown and dry brown stages resulted in maximum seed yield and superior quality attributes by having the highest germination percentage, seed viability as well as the least hard seed percentage.
- The seed quality attributes were significantly affected by storage duration. The maximum seed moisture content, seed viability as well as the minimum hard seed percentage was in seed stored for 0 months, but germination percentage, was recorded with seed stored for 3 months, which decreased, while hard seed percentage increased with increasing the seed storage duration.
- March 30 is recommended for the local growers of Peshawar to produce higher seed yield and quality of okra crop.
- For seed production, mature pods should be picked at dry brown stage to obtain good quality seeds of okra.
- Storage duration resulted in a significant decline in seed quality attributes and caused seed deterioration however, sowing on March 30 and harvested at brown dried stage may retain the quality and slow down the deterioration of the seeds.
Novelty Statement
These experiments enhanced the seed yield and quality of Okra in terms of germination, viability and vigor.
Author’s Contribution
Farzana Begum: Principal author, who designed and conducted research, data analysis and wrote draft of the manuscript.
Gohar Ayub: Major supervisor, provided technical guidelines and designed the experiments.
Conflict of interest
The authors have declared no conflict of interest.
References
Adam O., A. Sunday, O. Mayowa, A. Gloria and O. Olabisi. 2017. Effects of storage conditions and duration on seed germination of okra (Abelmoscus esculentus L.M.). Inter. J. Plant Soil Sci., 20(6): 1-6. https://doi.org/10.9734/IJPSS/2017/38518
Adetunji, I.A. 1991. Effect of harvest date on seed quality and viability of sunflower in semi-arid tropics. Seed Sci. Tech. 19: 57 1-580.
Agrawal, P.K. 1980. Genotypic variation on seed dormancy of paddy and simple method to break it. Seed Res., 9(1): 20-27.
Agrawal, P.K. 2011. Climate change and its impact on agriculture. In: Souvenir-Indian Seed Congress, Feb. 22nd–23rd, Hyderabad. pp. 67–76.
AOSA/SCST. 2010. Tetrazolium Testing Handbook. Revised Edition , 2013.
Ashok S. Sajjan, M. Shekargouda, B.D. Biradar, K.N. Pawar and S.B. Devaranvadgi. 2005. Fruit development and seed maturation studies in Okra (Ablemoschus esculentus (L.) Moench). Indian J. Agric. Res., 39:310-312.
Ashok, S., Sajjan, M. Shekhargouda and V. P. Badanur. 2002. Influence of date of sowing, spacing and levels of nitrogen on yield attributes seed yield in okra. Karnataka J. Agric. Sci., 15 (2):267-274.
Auld, D.L., B.L. Bettis, J.E. Crock and K.D. Kephart. 1988. Planting data and temperature effects on germination and seed yield of chickpea. Agron. J., 80(6):909–914.
Barnwal, A.K., A.K. Pal, A. Tiwari, S. Pal and A.K. Singh. 2017. Effect of picking stages on fruit and seed development in Okra [Abelmoschus esculentus (L.) Moench] Cultivars Kashi Pragati and Kashi Kranti. Int. J. Agric. Environ. Biotechnol., 10(6): 695-701. https://doi.org/10.5958/2230-732X.2017.00086.9
Bewley, J.D. and M. Black. 1994. Seed: Physiology of Development and Germination. 2nd ed. Plenum Press, New York, NY, USA. pp. 445.
Bortey, H.M. and B.M. Dzomeku. 2016. Fruit and seed quality of okra [Abelmoschus esculentus (L.) Moench] as influenced by harvesting stage and drying method. Indian J. Agric. Res., 50 (4): 330-334. https://doi.org/10.18805/ijare.v50i4.11253
Bortey, H.M., A.O. Sadia and J.Y. Asibuo. 2016. Influence of Seed Storage Techniques on Germinability and Storability of Cowpea (Vigna unguiculata (L) Walp). J. Agric. Sci., 8(10): 241-248. https://doi.org/10.5539/jas.v8n10p241
Caldwell, W.P. 1972. Relationship of pre harvest environment factors to seed deterioration in cotton. Ph.D. Dissertation. Mississippi State Uni. United States.
Carvalho, C.F., V.G. Uarrota, C.A. Souza and C.M.M. Coelho. 2017. Physiological quality of soybean seed cultivars (Glycine max (L.) Merr) with different maturity groups. Res. J. Seed Sci., 10 (2): 59-72. https://doi.org/10.3923/rjss.2017.59.72
Castro, A., C. Bernis , S, Vigneron , J.C. Labbé and T. Lorca. 2005. The anaphase-promoting complex: a key factor in the regulation of cell cycle. Oncogene, 24(3): 314-25.
Dayal, A., V.S. Mor, O.S. Dahiya, R.C. Punia and H. Ovais. 2017. Effect of pickings on seed quality parameters of Gossypium hirsutum L. varieties. J. Pharmacogn. Phytochem., 6(4): 858-862.
Demir, I. 1994. Development of seed quality during seed development in okra. Acta Hort., 362:125-132. https://doi.org/10.17660/ActaHortic.1994.362.15
Devadas, V.S., T.G. Rani, K.J. Kuriakose, and S.R. Nair. 1998. A note on fruit and seed development in okra [Abelmoschus esculentus (L.) Moench]. Veg. Sci., 25(2):187-189.
Dhingra, H.R. 2009. Morphological reaturea and reproductive biology. Inc: Okra Handbook (Ed. Dhankhar, B.S. and Singh, R.). HNB Publication, New York. pp. 25-36.
El Balla, M.M.A., A.I. Saidahmed and M. Makkawi. 2011. Effect of moisture content and maturity on hardseededness and germination in okra (Abelmoschus esculentus L. Moench). Int. J. Plant Physiol. Biochem., 3(6): 102-107. https://academicjournals.org/journal/IJPPB/article-full-text-pdf/18ACE2A10338
Elhag, A.Z. and A.A. Ahmed. 2014. Effect of cultivar and sowing date on okra (Abelmoschus esculentus L. Moench.) seed yield. Universal J. Appl. Sci., 2(3): 64-67.
El-Waraky, Y.B. 2014. Effect of sowing date, plant density and phosphorus fertilization on seed yield of okra. Alex. J. Agric. Res., 59(1): 27-41.
Farrag, M.M. 1995. Yield of 23 mung bean accessions as affected by planting date under El-Menia conditions. Assiut J. Agric. Sci., 26(2): 49-62.
Getzin, L.W. 1983. Damage to inflorescence of cabbage seed plants by the pale legume bug (Heteroptera miridae). J. Econ. Entom., 76: 1083-85. https://doi.org/10.1093/jee/76.5.1083
Ghadir, M., E.M. Khah, S.A. Petropoulos, G. Yarsiand and A. Vlasakoudis. 2013. Effect of fertilizer and drying methods on seed germination of okra (Abelmoschus esculentus L.) cultivars at different harvesting times. J. Agric. Sci., 5(4): 201-214. https://doi.org/10.5539/jas.v5n4p1
Ghanti, P., G. Sounda and P.K. Verma. 1991. Effect of sowing dates on growth and yield of different bhindi (Abelmoschus esculentus L. Moench) varieties. Environ. Ecol., 9: 176-179.
Godon, I.L., L.N. Balaam and N.F. Derera. 1979. Selection against sprouting damage in wheat. 111. Harvest ripeness, grain maturity and germinability. Aust. J. Agric. Res., 30:1-17. https://doi.org/10.1071/AR9790001
Gupta D.R. 2005. Effect of number of pods per plant, stage of harvesting and drying techniques on yield and quality of okra seed. MS thesis, department of Horticulture, Bangladesh Agricultural University, Mymensingh. Page 1-58.
Gwathmey, C.O., M.P. Bange and R. Brodrick. 2016. Cotton cropmaturity: A compendium of measures and predictors. Field Crops Res., 191:41-53. https://doi.org/10.1016/j.fcr.2016.01.002
Hampton, J.G., B. Boelt, M.P. Rolston, T.G. Chastain. 2013. Effects of elevated CO2 and temperature on seed quality. J. Agric. Sci., 151(2): 154-162 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3594839/ https://doi.org/10.1017/S0021859612000263
Hedau, N.K., G. Singh, V. Mahajan, S.R.K. Singh and A. Gahlain. 2010. Seed quality and vigour in relation to nodal position and harvesting stage of okra under mid hills of North-western Himalayas. Indian J. Hort., 67 (Special Issue): 251-253.
Helengeson E.A. 1932. Impermeability in mature and immature sweet clover seed s as infected by condition of storage Wisconsin Academy of Science. Cited by Crocker and Barton, 1957. Physiol. Seed, 27: 193-206.
I.S.T.A., 2017. International Rules for Seed Testing. Seed Sci. & Technol. International Rule for Seed Testing. Chapter 9, Determination of moisture content. https://doi.org/10.15258/istarules.2017.09
I.S.T.A., 2017. International Rules for Seed Testing. Seed Sci. & Technol. International Rule for Seed Testing. Chapter 9, Germination.
Ibrahim, H and J.A. Oladiran. 2011. Effect of fruit age and position on mother-plant on fruit growth and seed quality in okra (Abelmoschus esculentus L. Moench). Int. J. Sci. Nat., 2(3): 587- 592.
Incalcaterra, G., F. Vetrano, P.J. Stoffella, D.J. Cantliffe and G. Damato. 2000. Effect of two sowing dates and plastic mulch on okra production. Acta-Horticulturae, 533: 329-336. https://doi.org/10.17660/ActaHortic.2000.533.40
Iremiren, G.O. and D.A. Okiy. 1986. Effect of sowing date on the growth, yield and quality of okra (Abelmoschus esculentus (L.) Moench. in Southern Nijeria. J. Agric. Sci., 106 (1): 21- 26. https://doi.org/10.1017/S0021859600061669
Khan, F. 2019. The influence of packing materials and storage durations on seed quality of okra variety “Arka anamika”. Ph.D Thesis. The University of Agriculture.
Marcos-Filho, J. 1999. Accelerated aging test. In: Krzyzanowski, F.C., Vieira, R.D., France-Neto, J.B. (Ed.). Seed vigor: concepts and tests. Londrina: ABRATES, pp .3.1-3.24.
Mistry, K.K. and T. Hossain. 2008. Determination of physiological maturity in okra [Abelmoschus esculentus (L.) Moench] seed. Int. J. Sustain. Agric. Tech., 4(2): 25-30.
Mohamed, M.S., H.M.I. Ahmed and A.I. Ismail. 2016. Seed yield and quality of okra (Abelmoschus esculentus (L.) Moench) as influenced by sowing dates, harvest date and pod position. J. Plant Prod., 7(11): 1137-1145. https://doi.org/10.21608/jpp.2016.46953
Mugnisjah W.O. and S. Nakamura. 1984. Vigour of soyabean seed production from different harvest dates and phosphorus fertilizer application. Seed Sci. Tech., 12: 483-491.
Nonnecke, I.B.L. 1989. Vegetable Production. Avi Book Publishers. New York, USA. pp. 200-229.
Nonnecke, I.L. 1989. Vegetable Production. Van Nostrand Reinhold AVI Publishing, pp: 608-609.
Oladiran, J.A. and S.A. Agunbiade. 2000. Germination and seedling development from pepper (Capsicum annuum L.) seeds following storage in different packaging materials. Seed Sci. Tech., 28: 413 – 419.
Passam, H.C., K. Akoumiankis and A. Sarigiannidi. 1998. The effect of time of sowing on the production of okra (Hibiscus esculentus L.) seed in the Mediterranean region. Plant Varieties Seeds., 11:145-150.
Powell, A.A. 2006. Seed vigour and its assessment. In Handbook of seed science and technology; Basra, A.S., Ed.; Food Products Press: New York, NY, USA, 2. pp. 603–648.
Prabhakar, R.S., D.M. Hegde, K. Srinivas and S.D. Doijode. 1985. Seed quality and productivity of okra in relation to nodal position of pod. South Indian Hort., 33: 115-117.
Samnotara, R.K., Tripathi, V. and Kaul, R. 2002. Seed developmet in okra (Abelmoschus esculentus (L.) Moench) cv. Parbhani Kranti. Environ. Econ., 20(1): 230-232.
Setubal, J.W., A.C.W. Zanin and J. Nakagawa. 1994. Effects of harvesting methods and fruit position in plant on hard seed occurrency in okra (Abelmoschus esculentus L.). Sci. Agric., 51(3): 490-493. https://doi.org/10.1590/S0103-90161994000300019
Shahid, M.A., Rehman, A.A. Malik, M.S. Khan and Zakaria. 2015. Effect of sowing dates on the yield and seed production of okra cultivars in Mansehra. J. Biol. Agric. Healthcare, 5(9): 172-176.
Silva, L.J., D.C.F.S. Dias, G.L. Oliveira and R.A.S. Júnior. 2017. The effect of fruit maturity on the physiological quality and conservation of Jatropha curcas seeds. Agron. Sci., 48(3): 487-495. https://doi.org/10.5935/1806-6690.20170057
Singh, R.P., P.V.V. Prasad and K.R. Reddy. 2013. Impacts of changing climate and climate variability on seed production and seed industry. Adv. Agron., 118: 49-110. https://doi.org/10.1016/B978-0-12-405942-9.00002-5
Steel, R.G.D. and J.H. Torrie. 1980. Principles and procedures of statistics. 2nd ed. McGraw Hill, New York.
Sultana, R., M. Salahuddin M. Chowdhury, Md. R. Islam and K. Akhter. 2016. Effects of container and duration of storage on the quality of okra (Abelmoschus esculentus) Seeds. The Agriculturists, 14(1): 63-72. https://doi.org/10.3329/agric.v14i1.29101
Tekrony, D.M. and D.B. Egli. 1991. Accumulation of seed vigour during development and maturation. p. 369-384. In: [Ellis, R.H.; Black, M.; Murdock, A.J.; Hong, T.H., eds]. Basic and applied aspects of seed biology. Kluwer Academic, Boston, MA, USA. https://doi.org/10.1007/978-94-011-5716-2_41
Thuzar, M., A.B. Puteh, N.A.P. Abdullah, M.B.M. Lassim and K. Jusoff. 2010. The effects of temperature stress on the quality and yield of soyabean [(Glycine max L.) Merrill.]. J. Agric. Sci., 2: 172-179. https://doi.org/10.5539/jas.v2n1p172
Verma, M.K., B.K. Srivastava and M.P. Singh. 2004. Effect of fruit position and stage of harvesting on the seed quality of okra. Veg. Sci., 31 (1): 73-74.
Verma, S.S.U. and R.P.S. Tomer. 2003. Studies on seed quality parameters in deteriorating seeds in Brassica (Brassica campestris). Seed Sci. Technol., 31:389-396. https://doi.org/10.15258/sst.2003.31.2.15
Vossen, H.A.M.B. 1994. Controlling and certifying the quality of seed in Bangladesh Paper presented on the implementation of the seed policy for the development of seed industry. Ministry of Agriculture, Dhaka, Bangladesh. pp: 56-60.
Yilmaz, D.D. and A. Aksoy. 2007. Physiological effects of different environmental conditions on the seed germination of Rumex scutatus L (Polygonaceae). Erciyes Üniversitesi Fen Bilimleri Enstitüsü Dergisi., 23(1-2): 24-29.
To share on other social networks, click on any share button. What are these?







