Effect of Dietary Inclusion of Soybean Hull on Production Performance and Nutrient Digestibility During Peak Egg Production Period with Different Phases in Laying Hens
Effect of Dietary Inclusion of Soybean Hull on Production Performance and Nutrient Digestibility During Peak Egg Production Period with Different Phases in Laying Hens
Muhammad Shuaib1*, Abdul Hafeez1, Naila Chand1 and Muhammad Tahir2
1Department of Poultry Science, Faculty of Animal Husbandry and Veterinary Science, The University of Agriculture, Peshawar, Pakistan
2Department of Animal Nutrition, Faculty of Animal Husbandry and Veterinary Science, The University of Agriculture, Peshawar, Pakistan
ABSTRACT
The aim of the present study was to investigate the effect of soybean hull in the diet of laying hens on the production performance and nutrient digestibility during peak egg production period with different phases (phase-1=week 29 to 32, phase-2=week 33 to 36, and phase-3=week 37 to 40). One hundred and sixty golden misri (Brown) laying hens of age 28 weeks were brought for the experimental purpose and reared up to 40 weeks of age. All birds were divided into 4 groups with 4 replicate per group containing 10 birds per replicate and offered the corn-soybean meal diet with soybean hull (3,%, 6 %, and 9 %) respectively. Results showed that during all phases, feed intake and weight gain were (P<0.05) higher in the control group, while feed conversion ratio (FCR) was non-significant among all groups. Water intake during phase-1 was (P<0.05) higher in group D as compared to other treated groups while nonsignificant during phase-2 and 3. The average daily eggs production on weekly basis during phase-1 was calculated as non-significant while during phase-2 and 3 higher for the control group than all other groups. Hen day egg production (HDEP), mortality, and ash digestibility were recorded as non-significant, while nutrient digestibility of dry matter (DM), crude protein (CP), crude fiber (CF), and fat were recorded higher in the control group during all the three phases. It is concluded that the different levels of soybean hull in the basal diet resulted in lower production performance and nutrient digestibility than the control group.
Article Information
Received 05 November 2021
Revised 12 January 2022
Accepted 04 February 2022
Available online 30 March 2022
(early access)
Published 10 November 2022
Authors’ Contribution
SM animal trial, laboratory experiment, statistical analysis, study design and writing. HA study design, feed formulation, data evaluation, manuscript review. CN data evaluation, manuscript review. TM data analysis, manuscript review.
Key words
Soybean hulls, Nutrient digestibility, Performance parameters, FCR, Laying hens
DOI: https://dx.doi.org/10.17582/journal.pjz/20211105091115
* Corresponding author: shoaibwzr@gmail.com
0030-9923/2023/0001-397 $ 9.00/0
Copyright 2023 by the authors. Licensee Zoological Society of Pakistan.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
INTRODUCTION
The poultry feed sector has become one of the most profitable businesses in recent years due to its numerous prospects and integral possibilities of earning money and services. Feed accounts for more than 70% of total production costs, so any effort to minimize feed costs could result in a large reduction in overall costs (Rojas et al., 2014). Aside from all of the benefits, merits, and vast investment capacity, the feed sector has several issues. Seasonal unavailability of some products is also a factor, resulting in the compelled usage of pricey items in the feed. As a result, there is an increase in the cost of production (Khurshid et al., 2017). The aim of this study was to develop feed formulation which could enhance egg production of golden laying hens. Animal nutritionists are constantly on the lookout for alternative ingredients that would provide a cushion for low-cost formulation without compromising the performance of birds and animals. To reduce feed costs, it is required to enhance scientific information for evaluating low-cost locally prevailing agro-industrial by-products in chicken feed (Thirumalaisamy et al., 2016).
Protein is an essential component for poultry and other animals’ growth and regular physiological functions. During the starting phase, layers require 20-22% protein, 14-16% during the grower phase, and 15-18% during the finisher phase. Animal and plant protein are the most common in poultry feed (Rojas et al., 2014). Fish meal, meat and poultry products as animal-derived protein while soybean meal, cottonseed meal, alfalfa meal, and sunflower meal are often used as plant-based protein (Khurshid et al., 2017). Soybean meal is a common plant protein source among plant sources of protein. For soybean meal approximately 8% of the seed coat or hull, 90% of the cotyledons, and 2% of the hypocotyl axis or germ are used (Ravindran, 2013). Soybean hulls are one of the ingredients that may be found in large quantities and at very inexpensive prices on the market. Soybean hulls are shells of soybeans that fall off during processing as a byproduct of the production of oil from soybean seeds. Soybean hulls are a valuable feed for cattle and other livestock, including poultry birds, and are accessible for on-farm feeding.
Due to the efficiency of the de-hulling process (Rojas et al., 2014), the chemical composition of soybean hull vary, and thus the soybean hulls may contain varying amounts of celluloses (29-51%), hemicelluloses (10-25%), proteins (11-15 %), lignin (1-4 %), and pectins (4-8 %) (Mielenz et al., 2009). As a result, soybean hulls are primarily lignocellulose physical entities. Soybean hulls, in contrast to many other lignocellulose materials such as switchgrass or hardwood, degrade quickly (Mielenz et al., 2009; Yoo et al., 2011). Soybean hulls are not typically used in chicken diets due to their high fiber content; however, positive incorporation of soybean hulls in poultry rations has been documented (Newkirk, 2010). Till now, the use of soybean hulls in layer diet has not been thoroughly investigated particularly during the peak production phase in laying hens. So the present study was designed with the objective to determine the effect of dietary inclusion of soybean hull on production performance and nutrient digestibility during peak egg production period with different phases in the golden misri (brown) laying hens.
MATERIALS AND METHODS
Availability of experimental diets
The poultry farm of The University of Agriculture Peshawar was used for the experimental trial. Four types of experimental feed were prepared by Sadiq Brother Company Rawalpindi. Control group (A) contained corn-soybean meal (basal diet), while group B, C and D contained 3%, 6%, and 9% soybean hull+basal diet respectively (Table I).
Experimental birds
A total of 160 golden misri (brown) laying hens, aged 28 weeks, were purchased from local market. All birds were raised together under the same environmental and managemental condition at 23.8°C, and enough light (17 h each day). A vaccination program was established for the flock. Birds were divided into 4 groups (A, B, C, D) each of 40 birds and fed on feed formulation comprising of corn-soybean meal (basal diet) and soybean hull (3%, 6% and 9% for groups B, C, D, respectively). The control group (A) was fed on corn-soybean meal. The experimental diet was fed to the birds and data was collected in three phases (1, 2 and 3, where phase-1 was ranging from 29 to 32 weeks, phase-2 from 33 to 36 weeks and phase-3 ranged from 37 to 40 weeks of age).
Table I. Experimental diet compostion.
|
Nutrient (%) |
Control |
3% SH |
6% SH |
9% SH |
|
Corn 12 M |
53.120 |
52.120 |
51.120 |
49.960 |
|
Canola meal 34 |
4.000 |
4.000 |
4.000 |
5.000 |
|
Soybean meal 44 |
24.340 |
24.340 |
22.340 |
20.340 |
|
Guar meal |
1.00 |
00 |
00 |
00 |
|
Soy hull |
00 |
3.000 |
6.000 |
9.000 |
|
PBM Hi fat |
2.000 |
1.020 |
1.020 |
00 |
|
Poultry oil/ fat |
2.790 |
2.790 |
2.790 |
2.970 |
|
Salt |
0.320 |
0.320 |
0.320 |
0.320 |
|
Sodium bicarbonate/ Soda |
0.100 |
0.100 |
0.100 |
0.100 |
|
Limestone/ chips |
11.190 |
11.190 |
11.190 |
11.190 |
|
DCP |
0.770 |
0.770 |
0.770 |
0.770 |
|
DLM |
0.080 |
0.080 |
0.080 |
0.080 |
|
Choline chloride 70 |
0.100 |
0.100 |
0.100 |
0.100 |
|
Vitamin premix broiler |
0.070 |
0.070 |
0.070 |
0.070 |
|
Mineral premix |
0.060 |
0.060 |
0.060 |
0.060 |
|
Phytase |
0.010 |
0.010 |
0.010 |
0.010 |
|
Enramycin |
0.020 |
0.020 |
0.020 |
0.020 |
|
Ethoxyquin/ antioxidant |
0.010 |
0.010 |
0.010 |
0.010 |
|
NSPs |
0.020 |
00 |
00 |
00 |
To provide one kg of diet: Retinyl acetaste, 4400 IU; DL-α-tocopheryl acetate, 12 IU; Cholecalciferol, 118µg; Thiamine, 2.5mg; Menadione sodium bisulphite, 2.40 mg; Niacin, 30mg; vit.B2, 4.8 mg; D-pantothenic acid, 10 mg; vit. B6, 5 mg; vit. B7, 130 µg; Cyanocobalamine, 19 µg; vit.B9, 2.5 mg; Mn, 85 mg; Zinc, 75 mg; Fe, 80 mg; Iodin, 1 mg; Selenium, 130 µg; Copper, 6 mg.
Performance parameters
On daily basis, egg production, water intake and feed intake was recorded. Hen day egg production (HDEP) was calculated as follows:
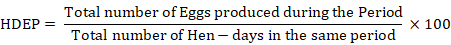
The feed conversion ratio (FCR) was calculated on weekly basis by dividing total feed intake by a dozen eggs. The body weight gain (BW) was calculated by subtracting the initial weight from the end weight of the body every week. The experiment’s mortality rate was tracked daily, along with the reason for death determined after a postmortem examination.
Nutrient digestibility
For ileal nutrient digestibility determination, Celite (Sigma Aldrich) was added at 1 % as an indigestible marker to the basal diet. At the end of the experiment, three birds per pen were randomly selected and slaughtered. The carcass was dissected and ileal digesta was collected and stored at −20 °C for chemical analysis. After freeze-drying, all the feed and ileal digesta samples were analyzed for dry matter, crude protein, crude fiber, fat, and ash by the method described by (Hafeez et al., 2020) using the formula.
Apparent digestibility(%)= 100 − ((concentration of marker in feed/ concentration of marker in digesta) × (concentration of nutrient in digesta/ concentration of nutrient in feed) × 100)
RESULTS
Data on feed intake (FI), weight gain (WG), feed conversion ratio (FCR), water intake and mortality are are shown in Table II. During the experimental period (phase 1, 2 and 3), feed intake and weight gain were recorded (P<0.05) higher in control group as compared to other treated groups. The FCR was non-significant on weekly basis among all the groups during different phases. The water intake was (P<0.05) higher in group-D as compared to other treated groups in phase1, though non significant during phase-2 and 3. Mortality rate during all phases were calculated as non-significant for all treated groups. Table III shows average daily egg production percentage on weekly basis and HDEP at different phases. During the experimental periods (phase 1, 2 and 3), at phase-2 and phase-3 total average daily egg production were recorded (P<0.05) higher in control group than all other treated groups. Among all the treatment groups, HDEP on phase-1, 2, and 3 were recorded as non-significant. The nutrient digestibility is shown in Table IV. The nutrients digestibility of DM, CP, CF and fat during all phases had (P<0.05) higher value in control group as compared to other treated groups. Ash digestibility during all phases was not affected among all groups.
Table II. Effect of dietary inclusion of soybean hull (SH) in the diet on feed intake and weight gain during different phases.
|
Parameters |
Phase |
Groups weeks |
Control (A) 0% |
B 3% SH |
C 6% SH |
D 9% SH |
p-value |
|
Feed intake |
1 |
Wk29 |
112.6a±0.32 |
110.9ab±0.946 |
108.9b±1.24 |
107.8b±0.63 |
<0.008 |
|
Wk30 |
114±0.37 |
112.5±1.52 |
112.3±1.03 |
110.9±1.83 |
0.456 |
||
|
Wk31 |
111.4a±0.21 |
107.5b±0.48 |
107.6b±0.40 |
105.7b±1.53 |
<0.003 |
||
|
Wk32 |
113.4a±0.65 |
112ab±1.08 |
110.7ab±0.87 |
108.7b±1.30 |
<0.034 |
||
|
Total |
3161.6a±8.38 |
3101.9b±10.06 |
3078.1bc±16.41 |
3033.4c±10.12 |
<0.0041 |
||
|
2 |
Wk33 |
115.1±0.31 |
112.3±1.96 |
110.4±3.30 |
107.6±0.98 |
0.1068 |
|
|
Wk34 |
112.1a ±0.45 |
108.8ab ±2.52 |
106.6ab ±1.51 |
104.7b ±1.59 |
<0.049 |
||
|
Wk35 |
109.3a±0.30 |
109.1ab±1.32 |
107.5ab±0.21 |
106.3b±0.23 |
<0.030 |
||
|
Wk36 |
113.1a±0.48 |
111.6ab±1.46 |
109b±0.70 |
102.9c±0.41 |
<0.004 |
||
|
Total |
3148.7a ±5.60 |
3093.3ab±34.22 |
3035.5bc±29.06 |
2952.1c±11.89 |
<0.025 |
||
|
3 |
Wk37 |
108.6a±0.67 |
105.6ab±1.91 |
101.7ab±2.41 |
98.5b±2.11 |
<0.0143 |
|
|
Wk38 |
105.6±0.67 |
103.6±1.91 |
100.2±2.41 |
99.1±2.11 |
0.1126 |
||
|
Wk39 |
112.2a±2.52 |
108.2ab±1.38 |
106.5ab±1.60 |
103.7b±1.38 |
<0.0363 |
||
|
Wk40 |
110.7±2.52 |
110±1.38 |
107±1.60 |
105.7±1.38 |
0.2066 |
||
|
Total |
3060.7a ±39.75 |
2992.7ab±39.51 |
2909.5ab±46.99 |
2850.9b±31.67 |
<0.0143 |
||
|
Weight gain |
1 |
Wk29 |
17.2a±2.567 |
15ab±1.77 |
12.2ab±1.31 |
9.7b±0.85 |
<0.014 |
|
Wk30 |
14±2.708 |
12±1.22 |
9.7±1.54 |
10±0.81 |
0.31 |
||
|
Wk31 |
15.5±3.27 |
14±1.29 |
14±2.041 |
12.5±1.25 |
0.801 |
||
|
Wk32 |
12.2±1.25 |
10±1.22 |
9.5±1.93 |
8±1.29 |
0.140 |
||
|
Total |
59a±2.97 |
51ab±1.29 |
45.5bc±2.59 |
40.2c±2.81 |
<0.004 |
||
|
2 |
Wk33 |
8.2±1.10 |
10.5±2.10 |
12±1.47 |
11.5±2.25 |
0.1166 |
|
|
Wk34 |
10±2.50 |
8±1.22 |
10.5±1.65 |
7.5±4.92 |
0.2938 |
||
|
Wk35 |
9.5±2.10 |
7.3±0.75 |
6.7±1.37 |
6.8±1.19 |
0.0954 |
||
|
Wk36 |
13.7a ±2.68 |
12 a ±3.61 |
7.2ab ±0.47 |
6ab±0.91 |
<0.0181 |
||
|
Total |
41a±1.47 |
37.50b±5.31 |
34.50c±2.90 |
30.50d±4.40 |
0.0109 |
||
|
3 |
Wk37 |
17a±1.22 |
18.2a±1.84 |
15.5b±0.50 |
14.5b±1.19 |
<0.0001 |
|
|
Wk38 |
15±2.54 |
13.2±0.75 |
13.2±0.75 |
15.4±2.97 |
0.7215 |
||
|
Wk39 |
17.7±2.65 |
15±1.22 |
18±3.76 |
16.2±1.10 |
0.6666 |
||
|
Wk40 |
9.5b±2.66 |
9.2b±0.94 |
8.7a±2.32 |
6.5c±1.19 |
0.0025 |
||
|
Total |
59.2ab±4.71 |
55.7a±3.52 |
53.5b±4.73 |
50.2c±3.14 |
0.0288 |
Means in the same row with different superscripts are significantly different (P<0.05).
Table III. Effect of dietary inclusion of soybean hull (SH) in the diet on feed conversion ratio (FCR) during different phases.
|
Parameters |
Phases |
Groups weeks |
A (0% Hull) |
B (3% Hull) |
C (6% Hull) |
D (9% Hull) |
p-value |
|
FCR |
1 |
Wk29 |
1.73±9.95 |
1.71±0.02 |
1.73±0.03 |
1.75±0.01 |
0.69 |
|
Wk30 |
1.72±0.01 |
1.73±0.02 |
1.75±0.03 |
1.76±0.02 |
0.65 |
||
|
Wk31 |
1.71±0.02 |
1.68±0.01 |
1.70±0.01 |
1.72±0.03 |
0.70 |
||
|
Wk32 |
1.70±0.01 |
1.72±0.02 |
1.73±0.02 |
1.75±0.02 |
0.54 |
||
|
Total |
1.72±0.01 |
1.71±0.01 |
1.73±0.02 |
1.75±0.01 |
0.38 |
||
|
2 |
Wk33 |
1.84±0.02 |
1.86±0.05 |
1.86±0.06 |
1.88±0.03 |
0.9410 |
|
|
Wk34 |
1.77±0.02 |
1.80±0.05 |
1.82±0.06 |
1.84±0.04 |
0.8184 |
||
|
Wk35 |
1.73±0.02 |
1.75±0.04 |
1.76±0.02 |
1.79±0.02 |
0.6305 |
||
|
Wk36 |
1.79±0.02 |
1.83±0.02 |
1.85±0.02 |
1.87±0.01 |
0.1145 |
||
|
Total |
1.78±0.01 |
1.81±0.03 |
1.82±0.02 |
1.84±0.04 |
0.3701 |
||
|
3 |
Wk37 |
1.77±0.01 |
1.77±0.05 |
1.76±0.06 |
1.79±0.04 |
0.9823 |
|
|
Wk38 |
1.76±0.01 |
1.75±0.04 |
1.77±0.05 |
1.79±0.02 |
0.9148 |
||
|
Wk39 |
1.84±0.05 |
1.79±0.01 |
1.80±0.03 |
1.87±0.02 |
0.3863 |
||
|
Wk40 |
1.85±0.05 |
1.82±0.03 |
1.84±0.03 |
1.90±0.03 |
0.6205 |
||
|
Total |
1.80±0.03 |
1.78±0.02 |
1.79±0.04 |
1.83±0.02 |
0.6903 |
Means in the same row with different superscripts are significantly different (P<0.05).
Table IV. Effect of dietary inclusion of soybean hull (SH) in the diet on water intake and mortality during different phases.
|
Parameter |
Phases |
Groups weeks |
A (0% Hull) |
B (3% Hull) |
C (6% Hull) |
D (9% Hull) |
p-value |
|
Water intake |
1 |
Wk29 |
170.5±2.90 |
174.2±2.81 |
177.7±3.03 |
181.2±2.13 |
0.0820 |
|
Wk30 |
173.2a±1.70 |
177.7ab±2.78 |
182.2ab±2.01 |
185b±2.38 |
<0.01 |
||
|
Wk31 |
178.7a±1.37 |
184ab±2.73 |
189.5bc±1.93 |
193.7c±1.88 |
<0.001 |
||
|
Wk32 |
176.5a±3.37 |
180ab±2.34 |
185.2ab±3.49 |
190.7b±2.65 |
<0.0210 |
||
|
Total |
176.6a±0.82 |
179ab±1.48 |
183.6bc±2.05 |
187.6c±1.20 |
<0.0008 |
||
|
2 |
Wk33 |
178.4±2.73 |
181.4±4.20 |
175.8±6 |
177.3±3.68 |
0.9984 |
|
|
Wk34 |
184.1±0.66 |
186.2±5.40 |
181.2±2.84 |
185.9±2.68 |
0.9758 |
||
|
Wk35 |
180.6±1.03 |
183.5±5.37 |
174.6±1.57 |
178.9±4.64 |
0.8971 |
||
|
Wk36 |
189.0±0.77 |
190±2.35 |
183.3±3.31 |
186±4.29 |
0.8559 |
||
|
Total |
183±0.86 |
185.2±2.23 |
178.7±1.86 |
182.2±2.83 |
0.8422 |
||
|
3 |
Wk37 |
186.9±1.07 |
184.5±3.06 |
183.9±3.86 |
180.9±3.39 |
0.8083 |
|
|
Wk38 |
180.1±0.57 |
177.9±2.66 |
175.3±3.61 |
173.8±5.17 |
0.9933 |
||
|
Wk39 |
183.6±4.04 |
181.5±3.53 |
179.7±2.56 |
176.2±2.21 |
0.9295 |
||
|
Wk40 |
193.7±1.74 |
190.9±1.33 |
187.8±3.64 |
185.1±4.87 |
0.9429 |
||
|
Total |
186.1±0.83 |
183.7±0.56 |
181.6±2.60 |
179.5±1.41 |
0.6737 |
||
|
Mortality |
1 |
0.25±0.25 |
0.25±0.25 |
0.50±0.28 |
0.50±0.28 |
0.831 |
|
|
2 |
0.00±0.00 |
0.00±0.00 |
0.25±0.25 |
0.25±0.25 |
0.5885 |
||
|
3 |
0.00±0.00 |
0.00±0.00 |
0.25±0.25 |
0.25±0.25 |
0.5885 |
Means in the same row with different superscripts are significantly different (P<0.05).
Table V. Effect of dietary inclusion of soybean hull (SH) in the diet on average daily egg production on weekly basis and Hen day egg production during different phases.
|
Parameter |
Phases |
Groups weeks |
A (0% Hull) |
B (3% Hull) |
C (6% Hull) |
D (9% Hull) |
p-value |
|
Avg daily egg production (%) |
1 |
Wk29 |
76.8a±0.41 |
76.3a±0.92 |
75.3ab±0.68 |
74.9b±0.68 |
<0.0039 |
|
Wk30 |
77.2a±0.41 |
76.8ab±0.41 |
76.7bc±0.68 |
75.3c±0.68 |
<0.0225 |
||
|
Wk31 |
76.2a±0.89 |
75.4ab±0.41 |
75.7ab±0.58 |
74.5b±0.92 |
<0.0062 |
||
|
Wk32 |
77.6a±0.35 |
77.4ab±0.41 |
76.2b±0.68 |
74.2c±0.58 |
<0.0301 |
||
|
Phase1Total |
76.2±0.42 |
76.5±0.29 |
75.4±0.51 |
75.2±0.52 |
0.0631 |
||
|
2 |
Wk33 |
75a±0.92 |
74.5a±1.07 |
74c±1.07 |
73.5c±0.58 |
<0.0030 |
|
|
Wk34 |
75.7a±1.01 |
74.5ab±0.89 |
74.3b±1.58 |
73.2b±1.07 |
<0.0444 |
||
|
Wk35 |
75.4±0.82 |
74.6±1.21 |
73.2±1.21 |
73±1.21 |
0.0656 |
||
|
Wk36 |
75.7a ±1.16 |
73.8ab±0.82 |
73.5ab±1.23 |
72c±0.68 |
<0.0022 |
||
|
Phase2 total |
75.5a±0.53 |
74.1b±0.74 |
73.8c±0.26 |
73.5c±0.08 |
<0.042 |
||
|
3 |
Wk37 |
74.5a±0.92 |
73.4ab±1.30 |
73.2ab±0.92 |
72c±0.68 |
<0.0011 |
|
|
Wk38 |
74.7a±1.07 |
73ab±1.21 |
73.8ab±0.71 |
72.4b±0.71 |
<0.0052 |
||
|
Wk39 |
74.2a±1.07 |
73.5a±1.07 |
72.7b±0.92 |
71.4b±0.41 |
<0.0009 |
||
|
Wk40 |
73.4a±0.89 |
72.5a±1.21 |
71.6a±1.21 |
70.7b±0.35 |
<0.0065 |
||
|
Phase3total |
74.5a±0.76 |
73.8ab±0.33 |
72.7b±0.89 |
72.4c±0.38 |
<0.046 |
||
|
Hen day egg production % |
1 |
80.9±1.88 |
79.6±2.17 |
80.3±2.44 |
78.4±2.55 |
0.877 |
|
|
2 |
75.5±0.53 |
73.1±0.74 |
73.3±2.15 |
70.3±1.93 |
0.179 |
||
|
3 |
72.5±0.76 |
71.8±0.33 |
71.3±2.20 |
68.2±1.61 |
0.2046 |
Means in the same row with different superscripts are significantly different (P<0.05).
Table VI. Effect of dietary inclusion of soybean hull (SH) in the diet on nutrient digestibility during different phases.
|
Groups parameters (%) |
Phases |
A (0% SH) |
B (3% SH) |
C (6% SH) |
D (9% SH) |
P-value |
|
DM |
1 |
77.96a±0.79 |
76.37ab±0.46 |
75.69ab±0.39 |
75.250b±0.75 |
<0.0469 |
|
CP |
67.88a±0.38 |
65.69ab±0.50 |
64.62b±0.38 |
64.24b±1.32 |
≤0.0223 |
|
|
CF |
70.82a±0.65 |
69.15a±1.27 |
66.72b±0.52 |
64.18c±1.00 |
<0.0005 |
|
|
Fat |
76.62a±0.81 |
75.83ab±0.43 |
74.58ab±0.97 |
72.57b±1.17 |
<0.0367 |
|
|
Ash |
57.12±0.76 |
57.69±0.85 |
59.12±0.90 |
60.58±1.22 |
0.3145 |
|
|
DM |
2 |
79.96a±0.79 |
78.87ab±0.46 |
77.69ab±0.39 |
76.75b±0.75 |
<0.0043 |
|
CP |
70.88a±0.38 |
68.69ab±0.50 |
66.42b±0.38 |
66.24b±1.32 |
<0.0223 |
|
|
CF |
72.12a±0.65 |
71.15a±1.27 |
68.22b±0.52 |
66.18c±1.01 |
<0.0003 |
|
|
Fat |
78.12±0.81 |
77.13±0.43 |
75.98±0.97 |
75.57±1.17 |
0.0944 |
|
|
Ash |
60.32±0.76 |
61.89±0.85 |
62.22±0.90 |
62.68±1.22 |
0.2813 |
|
|
DM |
3 |
81.46ab±0.73 |
80.37ab±0.59 |
78.64b±0.58 |
77.52b±0.44 |
<0.0068 |
|
CP |
72.01a±0.98 |
71.48ab±1.24 |
69.25b±0.98 |
68.29b±1.21 |
0.0154 |
|
|
CF |
74.14a±1.06 |
72.13ab±0.98 |
70.19bc±0.50 |
68.38c±0.58 |
<0.0020 |
|
|
Fat |
79.12±0.87 |
78.13±0.91 |
76.88±1.13 |
76.72±0.94 |
0.0927 |
|
|
Ash |
58.39±0.77 |
58.64±0.58 |
60.12±0.64 |
61.23±1.05 |
0.6662 |
Means in the same row with different superscripts are significantly different (P<0.05).
DISCUSSION
The effect of soybean hull in different levels (3, 6, and 9%) in the diet of golden misri (brown) laying hens were determined on the production performance and nutrient digestibility during peak egg production period with different phases (phase-1= week 29 to 32, phase-2= week 33 to 36, and phase-3= week 37 to 40). FI and WG were lower in the soybean hulls group than the control group during all three phases. Similar to the present study result Tejeda and Kim (2020) reported lower FI and WG in the broiler on dietary supplementation of soybean hulls in broiler at different levels in feed. The result is also in agreement with the finding of Esonu et al. (2005) who presented lower WG in laying hens for 10, 20, and 30% soybean hull in the diet while lower FI for 10% soy hull in feed as compared to control. Jiménez-Moreno et al. (2011) also described linearly reduced average daily WG in broiler from 1 to 12 days with an increased level of the fiber sources from 2.5 to 7.5%. High fiber diets usually mean relatively low energy density that may decrease FI, FCR, and BWG of poultry (Gonzalez-Alvarado et al., 2007). Soybean hull contains both soluble and insoluble fiber, and there are a variety of reasons why adding more than 4% crude fiber to a diet can reduce growth performance, especially when soluble dietary fibers are included (Gonzalez-Alvarado et al., 2007), which is similar to the findings in the present study. Contrary to the present results Saraee et al. (2014) reported no effect on weight gain in broiler when provided oil and tea leaves in feed with different levels. Present results showed (p<0.05) higher average daily egg production in the control group during the experimental phases (phase-2 and 3) than soybean hull treatment groups. Similar to present findings Roberts et al. (2007) recorded decreased egg production percentage in laying hen during different phases in the group containing 4.8 % soybean hull. Contrary to the present result Lumpkins et al. (2005) recorded no effect on egg production when fed laying hens high-fiber (low CP) diets. The addition of high-fiber feed components to pig or poultry diets reduce nutritional digestion (Dilger et al., 2004; Hogberg and Lindberg, 2004), and similarly in the present study low egg production in the soybean hull containing diet groups is mainly due to low FI and poor nutrient digestibility.
Whether the water intake increases or decreases depends on the nature of the dietary fiber. The present study results showed that the (WI) was (P˃0.05) higher in soybean hull groups during phase1, which is similar to the finding of Jiménez-Moreno et al. (2016) who reported an increase in water intake in broilers from day 18 to 20 on feeding oat hulls, rice hulls, and sunflower hulls as dietary fiber both in mash and pellet form at the level of 2.5 % and 5% in feed. Water use is strongly linked to feeding consumption (Schoorlemmer and Evered, 2002; Jiménez-Moreno et al., 2016). Under mild temperature conditions, broiler water intake is around twice as much as feed consumption on a weight basis (NRC, 1994). However, environmental temperature, feed composition, and the physico-chemical properties of the various components and ingredients of the diet all have an impact on this relationship (Francesch and Brufau, 2004; Carré et al., 2013). According to Garca et al. (2008), 21-day-old broilers fed barley had a 10% higher water intake (92 vs. 102 g/d) than broilers fed corn, indicating that the higher soluble fiber content of the barley was responsible for the increase observed, which is similar to the findings of the present study and the soluble fiber portion of soybean hulls is responsible for increasing water consumption in the soy hulls treated groups.
When fibers are administered in excessive quantities, they can interfere with nutritional absorption, resulting in lower performance (Cao et al., 2003). Hetland et al. (2004), Gonz’alez-Alvarado et al. (2008), Svihus (2011) and Rezaei et al. (2018) found that having more dietary fiber in the gastrointestinal tract increases organ size (i.e., gizzard, intestines) to compensate for the increased volume (i.e., bulky diets) of feed moving through the intestines. Changes in organ growth may increase maintenance requirements associated with increases in tissue synthesis and protein turnover, resulting in more nutrients being directed toward maintaining such tissues and less toward muscle protein accretion and growth performance even when adequate nutrient absorption is occurring in the gastrointestinal tract (Nyachoti et al., 2000). According to Cao et al. (2003) when fed 10% cellulose, laying hens exhibited poorer nitrogen digestion and absorption. Both soluble and insoluble fiber components can be found in soybean hull. The viscous components of soluble fibers have been shown to lower dry matter apparent digestibility coefficients. According to Silva et al. (2013) broilers given pectin in increasing levels from 10 to 50 g/kg had a quadratic and linear response in the starter and grower stages, respectively; increased pectin resulted in poorer dry matter digestibility, which is similar to the current study’s findings. Another study by Shakouri et al. (2009) found that birds given grains containing soluble and viscous non-starch polysaccharides had decreased apparent dry matter digestibility, which they attributed to the soluble fraction of the fiber components. Sklan et al. (2003) observed reduced digestibility of crude protein, fat, and gross energy in turkeys fed 8 to 9 percent CF in diets using sunflower meal as the primary source of dietary fiber which is similar to the findings in the current study. Soluble dietary fiber (DF) is hypothesized to enhance intestinal viscosity, which is linked to changes in intestinal microbiota, as well as decreased nutritional absorption and digestibility (Tejeda and Kim, 2021). Because of their impact on passage rate in the small intestines and fermentability in the hindgut, solubility and fermentability are two of the most notable parameters impacting nutrient digestion efficiency in the presence of soluble fiber (Davir et al., 2000; Kheravii et al., 2018).
CONCLUSION
With current soybean hull levels in feed, growth performance and nutrient digestibility were not favored during different phases, which could be linked to an increase in nutrient requirements for maintaining a higher epithelial cell turnover. The 3% soybean hulls group had a better result for production performance parameters and nutrient digestibility as compared to 9% soybean hull containing group. Fiber type and level of inclusion are important variables in controlling growth, intestinal development, and nutrient digestion, and further research is needed to understand how different fiber components alter layer performance from a physiological and nutritional viewpoint. This will lay the foundation for the feed industry to create cost-effective diets using low-cost fibrous feedstuffs for poultry industry.
ACKNOWLEDGMENTS
We gratefully acknowledge the assistanc of staff of the Department of Poultry Science, The University of Agriculture, Peshawar Pakistan for making technical and laboratory facilities avaliable to us.
Financial disclosure
This study was financially supported by Higher Education Comession (HEC) of Pakistan through (HEC Indigenous Scholarship) grant.
Statement of conflict of interest
The authors have declared no conflict of interest.
REFERENCES
Cao, B., Zhang, X., Guo, Y., Karasawa, Y., and Kumao, T., 2003. Effects of dietary cellulose levels on growth, nitrogen utilization, retention time of diets in digestive tract and caecal microflora of chickens. Asian Austral. J. Anim. Sci., 16: 863-866. https://doi.org/10.5713/ajas.2003.863
Carré, B., Lessire, M., and Juin, H., 2013. Prediction of metabolisable energy value of broiler diets and water excretion from dietary chemical analyses. Animal, 7: 1246-1258. https://doi.org/10.1017/S1751731113000359
Dilger, R., Sands, J., Ragland, D., and Adeola, O., 2004. Digestibility of nitrogen and amino acids in soybean meal with added soyhulls. J. Anim. Sci., 82: 715-724. https://doi.org/10.2527/2004.823715x
Dvir, I., Chayoth, R., Sod-Moriah, U., Shany, S., Nyska, A., Stark, A.H., Madar, Z., and Arad, S.M., 2000. Soluble polysaccharide and biomass of red microalga Porphyridium sp. alter intestinal morphology and reduce serum cholesterol in rats. Br. J. Nutr., 84: 469-476. https://doi.org/10.1017/S000711450000177X
Esonu, B., Izukanne, R., Inyang, O., 2005. Evaluation of cellulolytic enzyme supplementation on production indices and nutrient utilization of laying hens fed soybean hull-based diets. Int. J. Poul. Sci., 4: 213-216. https://doi.org/10.3923/ijps.2005.213.216
Francesch, M., and Brufau, J., 2004. Nutritional factors affecting excreta/litter moisture and quality. Worlds Poult. Sci. J., 60: 64-75. https://doi.org/10.1079/WPS20035
García, M., Lázaro, R., Latorre, M., Gracia, M., and Mateos, G., 2008. Influence of enzyme supplementation and heat processing of barley on digestive traits and productive performance of broilers. Poult. Sci., 87: 940-948. https://doi.org/10.3382/ps.2007-00266
González-Alvarado, J., Jiménez-Moreno, E., Lázaro, R., and Mateos, G., 2007. Effect of type of cereal, heat processing of the cereal, and inclusion of fiber in the diet on productive performance and digestive traits of broilers. Poult. Sci., 86: 1705-1715. https://doi.org/10.1093/ps/86.8.1705
González-Alvarado, J., Jiménez-Moreno, E., Valencia, D., Lázaro, R., and Mateos, G., 2008. Effects of fiber source and heat processing of the cereal on the development and pH of the gastrointestinal tract of broilers fed diets based on corn or rice. Poult. Sci., 87: 1779-1795. https://doi.org/10.3382/ps.2008-00070
Hafeez, A., Sohail, M., Ahmad, A., Shah, M., Din, S., Khan, I., and Khan, R.U., 2020. Selected herbal plants showing enhanced growth performance, ileal digestibility, bone strength and blood metabolites in broilers. J. appl. Anim. Res., 48: 448-453. https://doi.org/10.1080/09712119.2020.1818569
Hetland, H., Choct, M., and Svihus, B., 2004. Role of insoluble non-starch polysaccharides in poultry nutrition. Worlds Poult. Sci. J., 60: 415-422. https://doi.org/10.1079/WPS200325
Högberg, A., and Lindberg, J.E., 2004. Influence of cereal non-starch polysaccharides on digestion site and gut environment in growing pigs. Live Prod. Sci., 87: 121-130. https://doi.org/10.1016/j.livprodsci.2003.10.002
Jiménez, M.E., Romero, C., Berrocoso, J., Frikha, M., and Gonzalez M.G., 2011. Effects of the inclusion of oat hulls or sugar beet pulp in the diet on gizzard characteristics, apparent ileal digestibility of nutrients, and microbial count in the ceca in 36 day old broilers reared on floor. In: 100 th Annual Meeting Poultry Science Association (16/07/2011 - 19/07/2011) St. Louis, EEUU. https://oa.upm.es/12589/
Jiménez-Moreno, E., de Coca-Sinova, A., González-Alvarado, J., and Mateos, G., 2016. Inclusion of insoluble fiber sources in mash or pellet diets for young broilers. 1. Effects on growth performance and water intake. Poult. Sci., 95: 41-52. https://doi.org/10.3382/ps/pev309
Kheravii, S., Morgan, N., Swick, R.A., Choct, M., and Wu, S.-B., 2018. Roles of dietary fiber and ingredient particle size in broiler nutrition. Worlds Poult. Sci. J., 74: 301-316. https://doi.org/10.1017/S0043933918000259
Khurshid, H.D., Baig, S., Ahmad, J., Muhammad, A., and Khan, M.A., 2017. Miracle crop: The present and future of soybean production in Pakistan. MOJ Biol. Med., 2: 189-191. https://doi.org/10.15406/mojbm.2017.02.00042
Lumpkins, B., Batal, A., and Dale, N., 2005. Use of distillers dried grains plus solubles in laying hen diets. J. appl. Poult. Res., 14: 25-31. https://doi.org/10.1093/japr/14.1.25
Mielenz, J.R., Bardsley, J.S., and Wyman, C.E., 2009. Fermentation of soybean hulls to ethanol while preserving protein value. Bioresour. Technol., 100: 3532-3539. https://doi.org/10.1016/j.biortech.2009.02.044
Newkirk, R., 2010. Soybean: Feed industry guide. 1st edition, Canadian International Grains Institute. (http://www.cigi.ca/pdfs/2010%20Soybean%20Feed%20Industry%20Guide.pdf).
NRC (National Research Council), 1994. Nutrient requirements of poultry: Ninth edition. Washington, DC: The National Academies Press. https://doi.org/10.17226/2114
Nyachoti, C.M., de Lange, C.F., McBride, B.W., Leeson, S., and Gabert, V.M., 2000. Endogenous gut nitrogen losses in growing pigs are not caused by increased protein synthesis rates in the small intestine. J. Nutr., 130: 566-572. https://doi.org/10.1093/jn/130.3.566
Ravindran, V., 2013. Main ingredients used in poultry feed formulations. Poultry development review (ed.) FAO, 67-69. https://scholar.cu.edu.eg/wafaaabdelghany/files/book_41.pdf#page=66
Rezaei, M., Karimi Torshizi, M., Wall, H., and Ivarsson, E., 2018. Body growth, intestinal morphology and microflora of quail on diets supplemented with micronised wheat fiber. Br. Poult. Sci., 59: 422-429. https://doi.org/10.1080/00071668.2018.1460461
Roberts, S.A., Xin, H., Kerr, B.J., Russell, J.R., and Bregendahl, K., 2007. Effects of dietary fiber and reduced crude protein on nitrogen balance and egg production in laying hens. Poult. Sci., 86: 1716-1725. https://doi.org/10.1093/ps/86.8.1716
Rojas, M.J., Siqueira, P.F., Miranda, L.C., Tardioli, P.W. and Giordano, R.L., 2014. Sequential proteolysis and cellulolytic hydrolysis of soybean hulls for oligopeptides and ethanol production. Ind. Crops Prod., 61: 202-210. https://doi.org/10.1016/j.indcrop.2014.07.002
Saraee, M.H.A., Seidavi, A., Dadashbeiki, M., Laudadio, V., and Tufarelli, V., 2014. Effect of dietary supplementation with different levels of green tea powder and fish oil or their combination on carcass characteristics in broiler chickens. Pakistan J. Zool., 46: 1767-1773. http://www.zsp.com.pk/pdf46/1767-1773.
Schoorlemmer, G.H., and Evered, M.D., 2002. Reduced feeding during water deprivation depends on hydration of the gut. Am. J. Physiol. Regulat. Integ. Comp. Phys., 283: R1061-R1069. https://doi.org/10.1152/ajpregu.00236.2002
Shakouri, M., Iji, P., Mikkelsen, L.L., and Cowieson, A., 2009. Intestinal function and gut microflora of broiler chickens as influenced by cereal grains and microbial enzyme supplementation. J. Anim. Physiol. Anim. Nutr., 93: 647-658. https://doi.org/10.1111/j.1439-0396.2008.00852.x
Silva, V.K., Morita, V.D.S., and Boleli, I.C., 2013. Effect of pectin extracted from citrus pulp on digesta characteristics and nutrient digestibility in broilers chickens. Rev. Brasil. Zoot., 42: 575-583. https://doi.org/10.1590/S1516-35982013000800007
Sklan, D., Smirnov, A., and Plavnik, I., 2003. The effect of dietary fibre on the small intestines and apparent digestion in the turkey. Br. Poult. Sci., 44: 735-740. https://doi.org/10.1080/00071660310001643750
Svihus, B., 2011. The gizzard function, influence of diet structure, and effects on nutrient availability. Worlds Poult. Sci. J., 67: 207-224. https://doi.org/10.1017/S0043933911000249
Tejeda, O., and Kim, W., 2020. The effects of cellulose and soybean hulls as sources of dietary fiber on the growth performance, organ growth, gut histomorphology, and nutrient digestibility of broiler chickens. Poult. Sci., 99: 6828-6836. https://doi.org/10.1016/j.psj.2020.08.081
Tejeda, O., Kim, K.W., 2021. Role of dietary fiber in poultry nutrition. Animal, 11: 461. https://doi.org/10.3390/ani11020461
Thirumalaisamy, G., Muralidharan, S., Senthilkumar, R., Hema, S., and Priyadharsini, M., 2016. Cost-effective feeding of poultry. Int. J. Sci. environ. Technol., 5: 3997-4005.
Yoo, J., Alavi, S., Vadlani, P., and Amanor-Boadu, V., 2011. Thermo-mechanical extrusion pretreatment for conversion of soybean hulls to fermentable sugars. Bioresour. Technol., 102: 7583-7590. https://doi.org/10.1016/j.biortech.2011.04.092
To share on other social networks, click on any share button. What are these?










