Dual Targeting of Janus Kinase and Bruton’s Tyrosine Kinase: A New Approach to Control the Pathogenesis of Rheumatoid Arthritis
Dual Targeting of Janus Kinase and Bruton’s Tyrosine Kinase: A New Approach to Control the Pathogenesis of Rheumatoid Arthritis
Neelam Pery1*, Fatima Ijaz1, Nayab Batool Rizvi1, Munawar Ali Munawar1 and Muhammad Imtiaz Shafiq2*
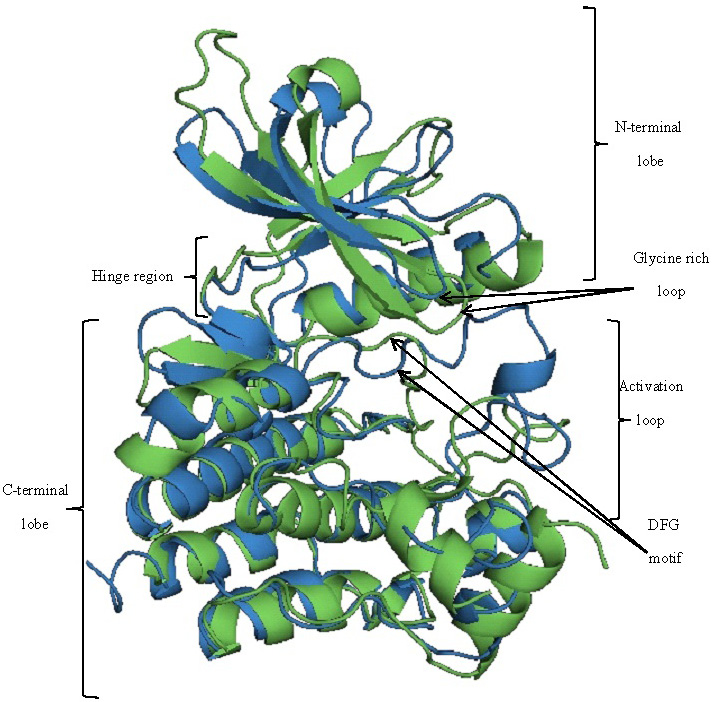
Structural alignment of JAK3 (green) and BTK (blue). Both the structures are aligned with a RMSD of 5.056 Å.
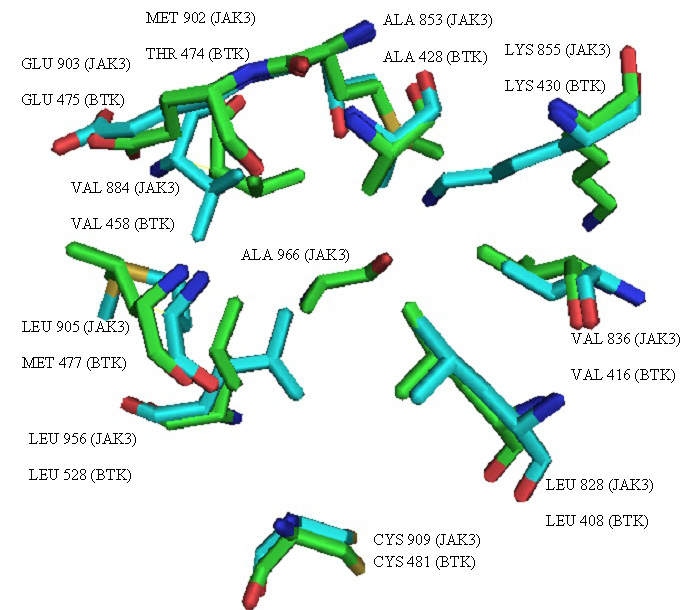
Overlay of active sites of JAK3 (green) and BTK (blue) representing the residues found interacting in the reported enzyme-inhibitor complexes.
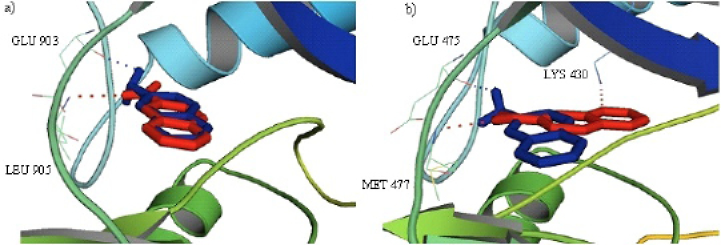
Cartoon representation of the JAK3 (a) and BTK (b) with an overlay of 6-nitroquinoxaline (Red) and quinoxalin-6-amine (Blue) in the binding cavity highlighting the hydrogen bonding residues. Dotted lines show hydrogen bonding.
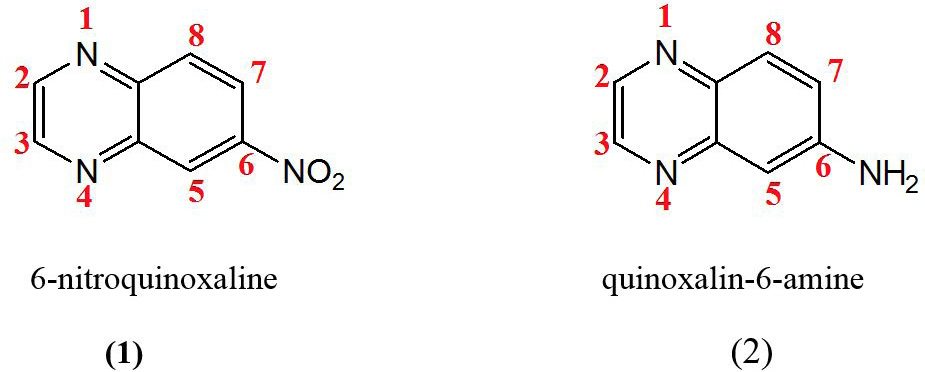
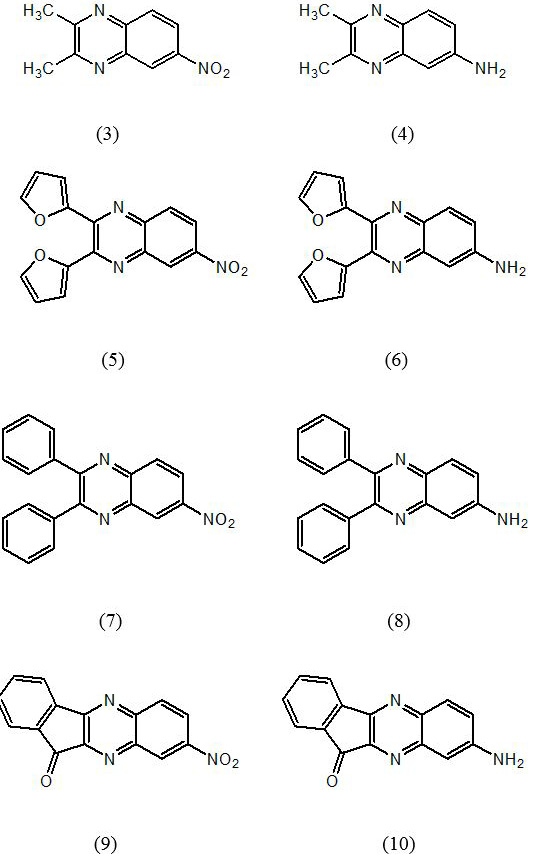
Structures of quinoxaline derivatives showing appreciable binding mode in both the enzymes (JAK3 and BTK).
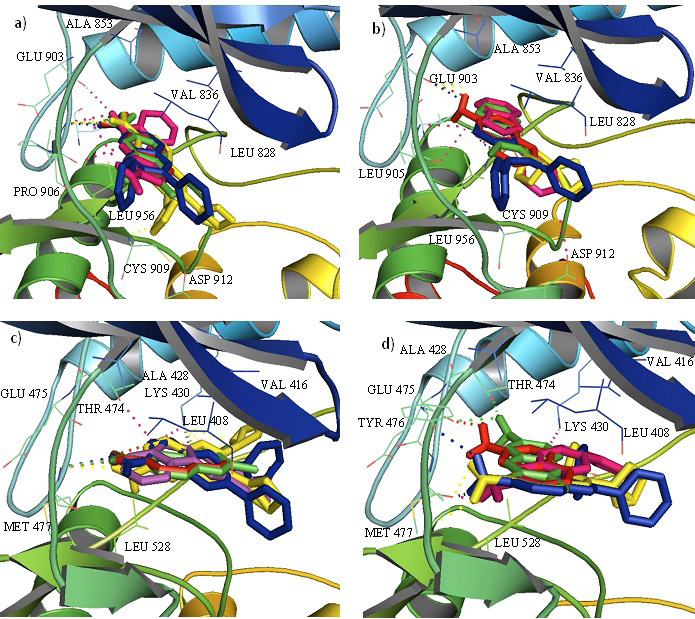
Cartoon representation of the JAK3 (upper) with an overlay of 6-nitroquinoxaline derivatives (a) and quinoxalin-6-amine derivatives (b) and BTK (lower) with an overlay of 6-nitroquinoxaline derivatives (c) and quinoxalin-6-amine derivatives (d) in the binding cavity highlighting the interacting residues. Dotted lines show hydrogen bonding.
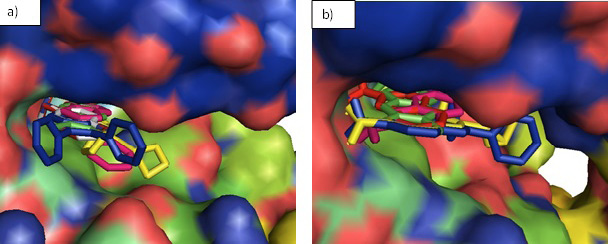
Surface representation of the designed inhibitors in the binding pocket of JAK3 (a) and BTK (b). The inhibitors fit well in the binding groove of both the enzymes and no clashes are seen.
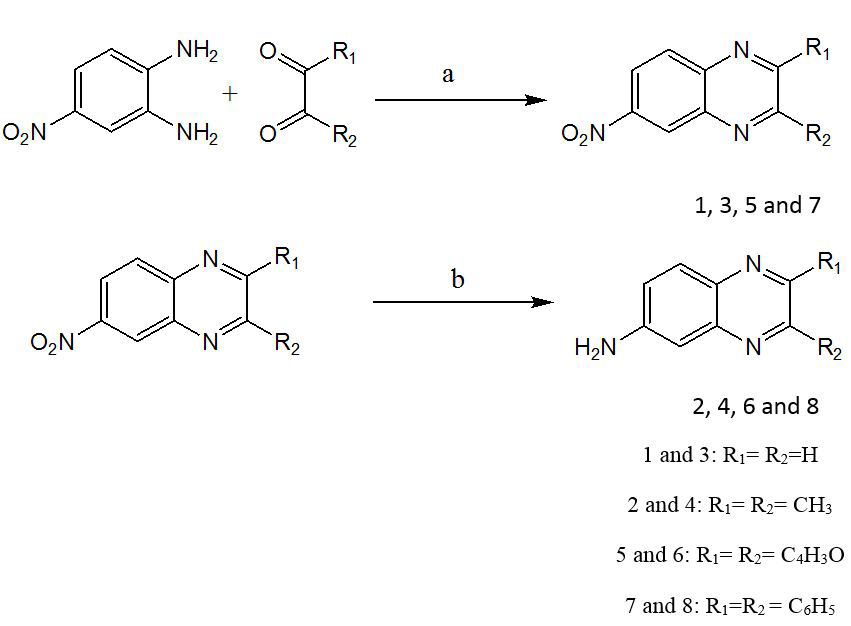
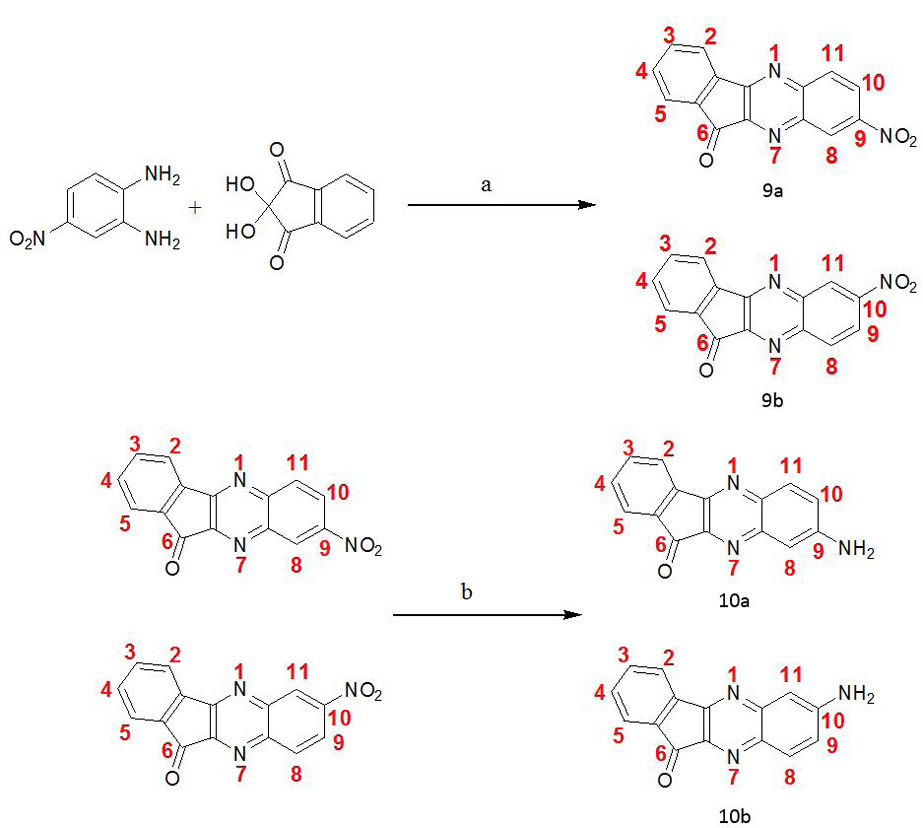
Synthetic scheme I: Reagents and conditions: (a) AcOH/ EtOH, Reflux 2-3 h, -H2O (b) NH2NH2.H2O, Pd/C, EtOH, reflux 2h.
Synthetic scheme II. Reagents and conditions: (a) AcOH/ EtOH, Reflux 2-3 hrs, -H2O (b) NH2NH2.H2O, Pd/C, EtOH, reflux 2.