Dietary Habits of Altai Weasel (Mustela altaica) in Bunjosa Game Reserve Rawalakot, Azad Jammu and Kashmir, Pakistan
Dietary Habits of Altai Weasel (Mustela altaica) in Bunjosa Game Reserve Rawalakot, Azad Jammu and Kashmir, Pakistan
Nausheen Irshad1, Maria Akhter1, Tariq Mahmood2*, Faraz Akrim3, Muhammad Rafique Khan1 and Muhammad Sajid Nadeem4
1Department of Zoology, The University of Poonch, Rawalakot, Azad Jammu and Kashmir, Pakistan.
2Department of Wildlife Management, PMAS Arid Agriculture University, Rawalpindi 46300, Pakistan.
3Department of Zoology, University of Kotli, Azad Jammu & Kashmir, Pakistan
4Zoology Department, PMAS Arid Agriculture University, Rawalpindi 46300, Pakistan.
ABSTRACT
We investigated the occurrence and diet of the Altai weasel (Mustela altaica), a “Near Threatened” mammal, in Bunjosa Game Reserve, Azad Jammu and Kashmir, Pakistan. The species occurrence was confirmed by direct field sightings (live and kill), and indirect signs (pugmarks, burrows, fecal droppings, and reported sightings by local community). During one-year study period (September 2015-August 2016), a total of 28 individuals of the species were sighted in the field including 18 live and 10 dead specimens. In addition, 93 fecal samples of the species were collected and analyzed for investigating its diet composition and identification of its prey species. Analysis of scats revealed its diet comprising of both vertebrates and invertebrates along with some proportion of vegetation. The weasel most frequently consumed arthropods (26%), followed by mammals (21%), birds (9 %) and least reptiles (2 %), along with consuming vegetation (23 %). The prey species in the overall diet of the species were five different orders of arthropods, two species of mammals (rodents) and birds (domestic hen and pigeon), while among plant food it consumed seeds and twigs of wild mulberry, Japanese fruit, wild fig and cucumber. The study concludes that Altai weasel in Bunjosa Game Reserve occurs at an elevation range between 1773 m to 1875 m, and consumes most frequently insects, followed by small mammals, birds and reptiles, along with seeds and twigs of some wild plant species.
Article Information
Received April 06 2021
Revised June 18 2021
Accepted July 09 2021
Available online 01 December 2021
(early access)
Published 10 June 2022
Authors’ Contribution
NI and MA designed the study and collected field data. MRK helped in lab analysis. TM, FA and MSN analysed the data and wrote the manuscript.
Key words
Altai weasel, Bunjosa Game Reserve, Diet, Field signs, Scats
DOI: https://dx.doi.org/10.17582/journal.pjz/20210406060437
* Corresponding author: tariqjajua75@uaar.edu.pk
0030-9923/2022/0005-2259 $ 9.00/0
Copyright 2022 by the authors. Licensee Zoological Society of Pakistan.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
INTRODUCTION
Altai weasel (Mustela altaica) also known as alpine weasel, or mountain weasel belongs to order Carnivora, family Mustelidae and genus Mustela (Graphodatsky et al., 1976). This genus consists of 17 subspecies distributed in Pakistan (Roberts, 1997), India, Nepal, Bhutan, Eastern Kazakhstan, Tajikistan, Mongolia, Russia, northern Korea (Wozencraft, 2005; Harris and Loggers, 2004), China (Won and Smith, 1999) and Afghanistan (Bischof et al., 2014). Pakistan has only two species of this kind namely Ermine weasel (Mustela ermine) and Altai weasel (Mustela altaica) (Roberts, 1997). However, there are scanty or whatsoever no scientific reports about occurrence of the species in different areas of the country.
The Altai weasel is found in a wide range of habitats including forests, mountains, scrublands and alpine
meadows (Bischof et al., 2014). The species excavates burrows for its living purpose (Roberts, 1997). In its dietary habits, Altai weasel is reportedly carnivorous (Roberts, 1997), but nothing is known about its dietary components and prey species in Pakistan. It also consumes invertebrates (Wilson and Mittermeier, 2009). It plays a positive role in ecosystem by feeding on insects and small rodents that are harmful for human vegetation. However, on the other side, it is also responsible for extinction of many small vertebrates in some parts of the world (Baldwin et al., 1952; Pimentel, 1955a, b).
The Altai weasel has been categorized as “Near Threatened” (Harris and Loggers, 2004) and it is placed in appendix-III of the Convention on International Trade in Endangered Species of Flora and Fauna (CITES). However, it is among one of the data deficient species in Pakistan (Sheikh and Molur, 2004), and data on its ecology and food habits is scanty. Its only existence or occurrence in some parts of Pakistan was reported by Roberts (1997) earlier on, and then by Bishof (2014) in northern parts of Pakistan and Indian occupied Kashmir. Therefore, the current study is the first of its kind in terms of investigating its occurrence and feeding habits in a least studied region of Bunjosa Game Reserve AJ and K, Pakistan.
MATERIALS AND METHODS
Study area
The current study was conducted in Banjosa Game Reserve (33.8100°N, 73.8164°E), located in Poonch district covering an area of 855 km2, including “Bunjosa Game Reserve (BGR)” comprising of 5.604 km2, situated 18 km away from Rawalakot city, Azad Jammu and Kashmir, Pakistan (Fig. 1A). The Game Reserve contains vital flora and fauna but has not been properly explored and there is a deficiency of base line data on wildlife species occurring here. The geographical coordinates are with an elevation of approximately 1981 m above sea level. The summer temperature ranges from 16 oC to -25 oC and drops below -3̊ C in snowy winter months (Rawlakot Azad Kashmir climate chart. Retrieved 14 June 2013). The study area was divided into four sampling sites: Site I-Kamal Wali Jandali, Site II-Munjagran, Site III-Kahalotian Bunjonsa and Site IV-Khunn Bunjonsa) (Table I, Fig. 1B).
Collection of data
The field surveys were conducted fortnightly from dawn to dusk for data collection about Altai weasel (Mustela altaica) in the study area.
All individuals of Altai weasel, field observed, either killed or alive, were recorded and identified (Roberts, 1997). The indirect signs like burrows and feces were also traced out. The geographical coordinates and elevation of the positive sites were recorded using a handheld Geographical Positioning System (GPS) device (Garmin eTrax Vista). Moreover, 200 local people including students, teachers, shopkeepers, and farmers were approached for their knowledge about the species occurrence and feeding.
Diet composition-fecal analysis
The scat samples of weasel collected during field surveys were analyzed in the laboratory following procedures of William (1992) and Ward (1970). The scats were broken into small pieces using piston martin and segregated into different identifiable groups and components. Hairs recovered from scats were processed for preparing light microscopic slides (whole mount). At least, three slides of each sample were prepared, examined under light microscope, and compared to the reference slides for identification of mammalian prey species. The seeds were identified physically and confirmed by sowing in the pots and observing the seedlings germinated from them. While recovered chitinous body parts of arthropods were identified by seeking expert help from Entomologists.
From the analyzed diet of the Altai weasel the percentage occurrence was calculated as:

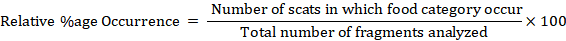
Reference material collection
During different seasons of the year, the vegetation and other reference material (hair, bones) were also collected from the study area and processed as above to prepare reference slides for identification of dietary components recovered from the weasel scats.
Statistical analysis
The fecal segregates collected in different seasons were statistically compared by using SPSS version 19.
RESULTS
Occurrence of weasels in BGR
A total of 139 burrows of Altai weasel were identified in the study area at four different sampling sites. The burrows were confirmed based on the presence and use by Altai weasel and / or presence of fecal droppings inside or around the burrows. A total of 28 individuals of the species (18 alive and 10 dead bodies) and its 93 scats were found in the study area during the current study period (Table I). Moreover, 70% of the local community confirmed its presence over there. The species was found occurring in the study area at an elevation range between 1773 m to 1875m (Table I, Fig. 1).
Table I. Direct and indirect signs of occurence of Altai weasel (Mustela altaica) in Bunjonsa Game Reserve (BGR).
|
Sampling sites |
Geographical coordinates |
Elevation (m) |
No. of burrows |
Fecal droppings/ scat |
Reported sightings |
Direct field- sightings |
Kill records |
|
Site-1 (Kamal Wali Jandali) |
33° 48.109 N 73° 48.413 E |
1875 |
28 |
29 |
19 |
11 |
1 |
|
Site-11 (Munjagran) |
33° 48.035 N 73° 47.726 E |
1773 |
60 |
32 |
32 |
7 |
2 |
|
Site-111 (Kahalotian) |
33° 47.651 N 73° 47.236 E |
1836 |
40 |
23 |
17 |
1 |
5 |
|
Site-1V (Khunn Bunjonsa) |
33° 48.611 N 73° 49.192 E |
1782 |
11 |
9 |
3 |
0 |
2 |
|
Total |
- |
139 |
93 |
72 |
18 |
10 |
Table II. Body measurements of three males and one female dead specimens of the Altai weasel, recovered from different sampling sites of BGR during current study period.
|
Specimen number |
Gender |
Body weight (g) |
Body girth (cm) |
Head-body length (cm) |
Tail length (cm) |
Total body length (cm) |
Head length (cm) |
Neck length (cm) |
Limb length (cm) |
|
|
Fore limbs |
Hind limbs |
|||||||||
|
1 |
Female |
435 |
10.8 |
26 |
21 |
47 |
6.5 |
5 |
7.5 |
6 |
|
2 |
Male |
420 |
9.9 |
25 |
20 |
45 |
6.3 |
4.8 |
7.3 |
6 |
|
3 |
Male |
479 |
9.9 |
23 |
19 |
42 |
6.3 |
4.9 |
7.3 |
6 |
|
4 |
Male |
250 |
9 |
14 |
9 |
23 |
3 |
2.5 |
6 |
5 |
|
Mean±SE |
390 ± 10 |
9.9 ± 0.7 |
22 ± 5.4 |
17.25 ± 5.5 |
39.25 ±11.02 |
5.52 ± 1.6 |
4.3 ± 1.2 |
7 ± 0.6 |
5.75 ±0.5 |
|
Species identification
The morpho-physical characteristics of the dead specimens recovered were used to identify the Altai weasel. The brownish body with white (face and underside of neck) (Fig. 2A) and black patches (face) (Fig. 2B) were its distinguishing feature, which confirmed it to be the Altai weasel. Average tail length was about 17.25±5.5 cm, that constituted about half of its total body length. The average body weight, length and girth were 390±10 g, 39.25±11.02 cm and 9.9±0.7 cm, respectively. Forelimbs were slightly larger (7±0.6 cm) than the hind limbs (5.75±0.5 cm) (Table II).
Diet composition
A total of 93 scat samples of the species collected from the study area were analyzed. Average length, diameter and weight of scats were 4.06 cm, 0.93 cm and 2.2 g, respectively (Table III). The weight and diameter of fecal samples collected from different sites of BGR showed no significant difference whereas a significant difference was found among the length of collected scat samples (p < 0.05).
Analysis of scat samples of Altai weasel revealed maximum contribution in its diet of insect (26%), followed by rodents (21%), and birds (9%), with least contribution of reptiles (2%) in its diet. Its diet also contained vegetation (23%) (Fig. 3). Relative frequency of food items in the fecal analysis revealed that most frequently consumed prey were insects (22.61%), followed by plant material (21%), and rodents (17%). The birds (6.82%) and reptiles (7.265%) constituted less in the diet of weasel (Table IV).
Insects
The arthropods in the diet of Altai weasel comprised of five different insect orders including Isoptera (termites), Orthoptera (crickets and grasshoppers), Blattodae (cockroaches), Lepidoptera (moths and butterflies) and Diptera (true flies) (Table IV). The body parts of these arthropods were clearly visible in the fecal droppings while body parts of blattodae (such as hard wings) were also found inside the feeding burrows of the species.
Rodents
Small mammals identified from scat analysis included two species of rodents: black rat (Rattus rattus) and house mouse (Mus musculus) (Table IV). These rodents were the second most frequently consumed food items by weasel and were preyed upon during all four seasons.
Birds
The feathers of birds recovered from analyzed fecal samples, were identified as those of two bird species viz., domestic hen (Gallus gallus domesticus) and pigeons (Columba livia domestica) (Table IV).
Reptiles
Reptile body parts could not be identified to the species level.
Vegetation
Vegetation component of the diet included seeds, leaves and twigs of different plants. The seeds of mulberry (Morus nigra), Japanese fruit (Japanese persimmon), wild fig (Fiscus carica) and cucumber (Cucumis sativus) were recovered from the fecal samples of Altai weasel while those of apricot (Prunus armeniaca), peach (Prunus persica), walnut (Juglans regia), and Phulai (Acacia modesta) were found near the burrows of Altai weasel.
Seasonal variation in diet composition
Table IV shows the dietary composition of weasles during different seasons. Arthropods were more frequently consumed during spring and summer seasons, with the highest consumption in summer. Mammals were consumed during all the four seasons. Birds were more frequently preyed upon in summer season but less frequently in spring and fall, however, during winter season no birds were consumed by Altai weasel. Reptiles were more frequently consumed in spring and summer compared to fall while no reptiles were preyed upon in winter. Seeds, leaves and twigs of vegetation were consumed by weasel during all four seasons of the year.
A highly significant difference was found between arthropods and reptiles (< 0.005) and arthropods and birds (< 0.005). However, there was no significant difference in consumption between arthropods and mammals (> 0.005), arthropods and vegetation (> 0.005), arthropods and unidentified material (> 0.005) eaten by Altai weasel in different seasons (Table V).
DISCUSSION
Globally, ecological data on Altai weasel is scanty, where it is incidentally mentioned in papers on other subjects such as by Harris and Loggers (2004). Likewise, little published information exists on this particular “Near Threatened” species (Abramov, 2016) in Pakistan, as no formal scientific studies have been focused on it yet. According to an estimate, Altai weasel is facing a probable threat to enter in to the “Vulnerable” category in immediately preceding times due to its significant decline in past years (Abramov, 2016). Therefore, the baseline data regarding its distribution, population estimation, food habits and other biological parameters are prerequisite for its conservation.
Table III. Physical characteristics of scat samples of Altai weasel (Mustela altaica) collected from different sampling sites of BGR, AJK, Pakistan.
|
Site-1 |
Site -11 |
Site -111 |
Site -1V |
ANOVA p-value |
|
|
Sample size |
21 |
34 |
29 |
9 |
93 |
|
Weight (g) |
1.93±0.56 |
2.39±0.71 |
2.33 ±0.65 |
2.31± 0.58 |
> 0.05 |
|
Length (cm) |
4.42± 0.59 |
3.99±0.27 |
3.875±0.36 |
3.98±0.40 |
< 0.05 |
|
Diameter (cm) |
0.93±0.23 |
1.02±0.21 |
1.00±0.20 |
0.91±0.39 |
> 0.05 |
Table IV. Percent occurrence (F) of dietary components of Altai weasel (Mustela altaica) in different seasons by fecal analysis in BGR, AJK, Pakistan.
|
Prey items |
Spring |
Summer |
Autumn |
Winter |
|||||
|
F |
RF |
F |
RF |
F |
RF |
F |
RF |
||
|
Insects Isoptera Orthoptera Blattodea Lepidoptera Diptera |
17.39 39.13 13.04 8.69 0 |
4.93 11.11 3.70 02.46 0 |
22.22 18.51 14.81 18.51 7.40 |
6.45 5.37 4.30 5.37 2.15 |
40.74 14.81 7.40 0 14.81 |
10.57 3.84 1.92 0 3.84 |
25 12.5 25 0 0 |
9.75 4.87 9.75 0 0 |
|
|
Sub-total |
78.26 |
22.22 |
81.48 |
23.65 |
77.77 |
20.19 |
62.5 |
24.30 |
|
|
Reptiles |
34.78 |
13.58 |
33.33 |
9.67 |
18.51 |
5.81 |
0 |
0 |
|
|
Aves Pigeons Domestic hen |
4.34 17.39 |
1.23 4.93 |
7.40 33.33 |
2.15 9.67 |
7.40 22.22 |
1.92 6.97 |
0 0 |
0 0 |
|
|
Sub-total |
21.73 |
6.17 |
40.74 |
11.82 |
29.62 |
9.30 |
- |
- |
|
|
Rodents Rattus rattus Mus musculus |
52.17 52.17 0 |
14.81 8.64 0 |
59.25 44.44 13.81 |
17.20 12.90 4.30 |
66.66 59.25 7.40 |
17.30 15.38 1.92 |
43.75 48.75 0 |
17.07 17.07 0 |
|
|
Sub-total |
52.17 |
14.81 |
59.25 |
17.20 |
66.66 |
17.30 |
43.75 |
17.07 |
|
|
Vegetation Seeds Leaves, twigs |
43.47 34.78 |
9.07 13.58 |
37.03 29.62 |
10.75 8.60 |
40.74 18.51 |
10.57 480 |
43.75 25 |
17.07 9.75 |
|
|
Sub-total |
78.26 |
22.22 |
66.66 |
19.35 |
59.25 |
15.38 |
68.75 |
26.82 |
|
|
Unidentified |
82.64 |
23.45 |
62.96 |
18.27 |
66.66 |
17.30 |
81.25 |
31.70 |
|
*F, frequency of occurrence; *RF, Relative frequency.
Table V. Multiple comparisons (p values) among dietary components of the Altai weasel.
|
Insects |
Reptiles |
Aves |
Rodents |
Vegetation |
|
|
Insects |
- |
0.000 |
0.003 |
0.007 |
0.030 |
|
Reptiles |
0.000 |
- |
0.857 |
0.719 |
0.415 |
|
Aves |
0.003 |
0.857 |
- |
1.000 |
0.216 |
|
Rodents |
0.007 |
0.719 |
1.000 |
- |
0.345 |
|
Vegetation |
0.644 |
0.010 |
0.216 |
0.345 |
- |
In Pakistan, Altai weasel is found in the northern parts of higher altitudes. But the species has rarely been studied in its natural habitat for its ecology and dietary habits. Reportedly, it occurs in a wide variety of habitats ranging from the wide valleys having an elevation up to 1500 m, humid alpine slopes ranging up to 5200 m and in dry coniferous forest bearing an altitude in between 2450-3000m (Roberts, 1997). It is reported to be found along 1500 m to 4500 m in India (Choudhury, 2013) and up to 3970 - 4890 m in Nepal (Ghimirey and Acharya, 2014; Hornskov and Foggin, 2007) reported the occurrence of the particular species at much higher elevations of about 7000 m in Tibetan plateau.
Although Altai weasel is generally considered a nocturnal mammal but during the current study, it was frequently field observed during daytime. Ghimirey and Acharya (2014) also described it to be diurnal, where they reported that individuals started searching prey species after sun rise by digging and scratching ground to explore insect’s larvae or by chasing rodents/ reptiles. Similar dietary components have been revealed during current investigation, as recovered food items from fecal analysis mainly belonged to arthropods, reptiles, birds, and mammals, along with some plant species. Wilson and Mittermeier (2009) also reported the identical food components of Altai weasel including crabs, scorpions, earthworms, lizards, birds, and small mammals in South Africa. On the contrary, the species was described to be exclusively carnivorous in its feeding habits by Pocock (1941). The food habits of the Altai weasel studied in the Tibetan plateau by Hornskov and Foggin (2007) showed that the species primarily fed upon pikas Ochotona roylei. Its main prey item recorded was also Pika (Ochotona species) by Bischof et al. (2014) from the only reported study from Pakistan. However, the results of the current study showed no consumption of pika, probably this species does not occur in Bunjosa GR. In a correlation to the feeding habits of this species, Roberts (1997) concluded that it was an opportunistic predator; predominantly feeding on small mammals and insects and occasionally on reptiles and birds. It is also known for eating muskrats, rabbits, ground squirrels, small birds, lizards, frogs, and fish (Allen, 1938).
The results of the current study are somewhat different from some previous studies since a significant proportion of plant material has also been recovered from feces of the species. All the fecal droppings had some vegetation materials including seeds, leaves and twigs. Previously published studies did not report its feeding on vegetation.
The Altai weasel, in the current study, consumed small mammals including two rodent species viz., black rat and house mouse. The occurrence of reptiles in the diet of Altai weasel is justified from earlier published studies conducted using stomach contents analysis in South Africa where reptiles were the second most abundantly consumed vertebrate prey species (Wilson and Mittermeier, 2009).
The predation on rodents and insects by Altai weasel is amply beneficial to the human beings, especially in agricultural plantations, due to its ability to reduce great numbers of many destructive pests. Moreover, it also plays a significant role in biological diversity and controlling the insect population. On the other hand, it also inhibits harmful impacts of feeding on the eggs of domestic and wild birds as reported by local community. The fecal analysis showed occurrence of domestic hen and pigeons by Altai weasel in the current study. Roberts (1997) had also supported the fact by reporting hen’s egg and chicken in diet of the species. The birds are considered as supplementary food items of weasel diet (Sumption and Flowerdew, 1985).
Limited knowledge and data deficiency about Altai Weasel makes it difficult to determine its present status in the country. However, the results of the current study will be helpful in highlighting prey species of the weasel which will serve as baseline data for developing conservation strategy for the species. We recommend more scientific studies to be conducted on various ecological aspects of Altai weasel in the country.
Statement of conflict of interest
The authors have declared no conflict of interest.
References
Abramov, A.V., 2016. Mustela altaica. The IUCN Red List of Threatened Species 2016: e.T41653A45213647.
Allen, G.M., 1938. Mammals of China and Mongolia. American museum of the natural history, natural history of Central Asia, Vol. 11, pt.1-2. New York, USA. Cyranoski, D., 2005. The long-range forecast. Nature, 438: 275–276. https://doi.org/10.1038/438275a
Baldwin, P.H., Schwartz, C.W. and Schwartz, E.R.,1952. Life history and economic status of the mongoose in Hawaii. J. Mammal., 33: 335- 356.
Bischof, R., Ali, H., Kabir, M., Hameed, S. and Nawaz, M.A., 2014. Being the part to undergo an elusive small carnivore uses space with prey and time without enemies. J. Zool., 293: 40- 48. https://doi.org/10.1111/jzo.12100
Choudhury, A., 2013. The mammals of north east India. Gibbon Books, Guwahati.
Ghimirey, Y. and Acharya, R., 2014. Status and ethnobiology of mountain weasel (Mustela altaica) in Humla district, Nepal. Small Carniv. Conserv., 51: 64–67.
Graphodatsky, A.S., Yang, F., Perelman, P.L., O’Brien, P.C.M., Serdukova, N.A., Milnem B.S., Biltueva, L.S., Fu, B., Vorobieva, N.V., Kawada, S.I., Robinson, T.J. and Ferguson-Smith, M.A., 1976. G-banding of the chromosomes in seven species of Mustelidae (Carnivora). Russian J. Zool., 55: 1704-1709.
Harris, R.B. and Loggers, C.O., 2004. Status of Tibetan Plateau mammals in the Yeniugou, China. Wildl. Biol., 10: 91-99. https://doi.org/10.2981/wlb.2004.013
Hornskov, J. and Foggin, M., 2007. Brief notes on the Altai weasel (Mustela altaica) on the Tibetan Plateau. Small Carniv. Conserv., 36: 48 - 49.
Pimentel, D., 1955a. Biology of Indian mongoose in Puerto Rico. J. Mammal. 36: 62-68. https://doi.org/10.2307/1375723
Pimentel, D., 1955b. The control of mongoose in Puerto Rico. Am. J. Trop. Med. Hyg., 4: 147-151. https://doi.org/10.4269/ajtmh.1955.4.147
Pocock, R.I., 1941. The fauna of British India, including Ceylon and Burma. J. Mammal., 2: 997.
Roberts, T.J., 1997. The mammals of Pakistan. Oxford University Press, London. UK. pp. 363-364.
Sheikh, K.M. and Molur, S., 2004. Status and red list of Pakistan’s mammals. Based on the conservation assessment and management plan. IUCN Pakistan. pp. 312
Sumption, K.J. and Flowerdew, J.R., 1985. The ecological effects of the decline of Rabbits (Oryctolagus cuniculus) due to myxomatosis. Mamm. Rev., 15: 151-186. https://doi.org/10.1111/j.1365-2907.1985.tb00396.x
Ward, A.L., 1970. Stomach content and fecal analysis: methods of forage identification. Rocky Mt. For. Range Exp. Stn. Misc. Publ., 1147: 220.
William, O., 1992. The techniques for studying microtone food habits. J. Mammal., 43: 365-368. https://doi.org/10.2307/1376945
Wilson, D.E. and Mittermeier, R.A., 2009. Handbook of mammals of the world. Volume 1: Carnivores. Lynx Edicions, Barcelona, Spain. pp. 556.
Won, C. and Smith, K.G., 1999. History and current status of mammals of the Korean peninsula. Mammal Rev., 29: 3-33.
Wozencraft, W.C., 2005. The mammalian species of the world, 3rd edition. Johns Hopkins Univ. Press, Baltimore, USA., 3: 532-628.
To share on other social networks, click on any share button. What are these?










