Curcuma Longa Methanolic Extract and Losartan Improves Memory Impairment and Oxidative Stress induced by a High Caloric Diet in Wistar Rats
Research Article
Curcuma Longa Methanolic Extract and Losartan Improves Memory Impairment and Oxidative Stress induced by a High Caloric Diet in Wistar Rats
Sara Brikat, Mouloud Lamtai*, Miloud Chakit, Laila Ibouzine-Dine, Ilhame Fitah, Oumaima Abouyaala, Abdelhalem Mesfioui, Aboubaker El Hessni
Laboratory of Biology and Health, Department of Biology, Faculty of Sciences, Ibn Tofail University, Kenitra, Morocco.
Abstract | The objective of the current study was to assess the methanolic extract of Curcuma longa (Curc) and losartan on memory impairment induced by a high fructose diet (HFD) in Wistar rats. 36 female Wistar rats aged 2 months and weighing 74 to 100 g were randomly divided into 6 groups as follows: 2 groups would receive a standard diet which a group serve as control and a group receive 100 mg/kg/d of Curc and 30 mg/kg/d of Losartan (Curc + Los), 4 groups would receive the high HFD diet: one group serve as control (HFD), a group will receive 100 mg/kg/day of Curc, qualified (HFD + Curc), a group will receive 30 mg/kg/day of Losartan, qualified (HFD + Los) and a group will receive HFD + Curc + Los. After 8 weeks of experience, working and recognition memory were evaluated using the Y-maze and Object Recognition (OR) tests. The oxidative stress (OS) was assessed by measuring the levels of Thiobarbituric acid reactive substances (TBARS), nitric oxide (NO) and catalase activity (CAT) in the hippocampus. The administration of Curc and/or Los increased significantly the recognition index (p < 0.01) as compared with the HFD group, and were able to prevent this effect on the memory induced by the HFD (p < 0.01). Additionally, the levels of TBARS and NO in HFD group significantly exceeded the respective values in female animals when compared to control rats (p < 0.05). In contrast, the administration of HFD + Curc + Los lowered NO levels significantly in rats in comparison with the standard diet-treated rats (p < 0.01). In summary, our results suggest that treatments with Curc and/or Los could serve as possible therapeutic agents for memory impairment and OS provoked by HFD.
Keywords | Curcuma longa, Losartan, Memory, High caloric diet, Wistar rats
Received | January 14, 2024; Accepted | January 29, 2024; Published | February 15, 2024
*Correspondence | Mouloud Lamtai, Laboratory of Biology and Health, Department of Biology, Faculty of Sciences, Ibn Tofail University, Kenitra, Morocco; Email: mouloud-lamtai@hotmail.fr
Citation | Brikat S, Lamtai M, Chakit M, Ibouzine-Dine L, Fitah I, Abouyaala O, Mesfioui A, El Hessni A (2024). Curcuma longa methanolic extract and losartan improves memory impairment and oxidative stress induced by a high caloric diet in Wistar rats. Adv. Anim. Vet. Sci., 12(4):614-623.
DOI | https://dx.doi.org/10.17582/journal.aavs/2024/12.4.614.623
ISSN (Online) | 2307-8316
Copyright: 2024 by the authors. Licensee ResearchersLinks Ltd, England, UK.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
INTRODUCTION
Consumption of high amounts of fructose in excess of advised nutritional needs can be responsible of several metabolic diseases in animals and humans (El-Hasnaoui et al., 2015). The fructose intake has elevated in last years, particularly in underdeveloped nations. In the visceral adipose tissue, the accumulation of triglycerides resulting from transformation of fructose can lead to hepatic steatosis, obesity, cardiac diseases (Baataoui et al., 2023a, b; Benchelha et al., 2023a, b; Elmaleh-Sachs et al., 2023).
Fructose high diet constitutes a risk factor for metabolic disorders (Tovoli et al., 2023), in addition, it negatively affects the structure and functioning of the nervous system and mainly the brain. Neuropsychiatric symptoms are common in obesity and significantly influence the social functioning and quality of life of people with metabolic disease (El-Hamaoui et al., 2023; Fitah et al., 2023). Although the undergoing physiological processes of the origin of neuropsychiatric symptoms linked to obesity are still under study and require further study, they involve the oxidative stress (OS) pathway (Brikat et al., 2023). In addition, several studies have characterized gain weight as a moderate chronic inflammation in obese people, with a production deregulation of adipokines and cytokines by adipose tissue. A series of inflammatory cytokines are produced by this tissue, increasing the risk of the development and the complication of metabolic diseases. In brain cells, adipokine synthesis deregulation can lead to many changes, such as consequential neurodegeneration, cognitive and affective disorders, and changes in blood irrigation (Soczynska et al., 2011; Nassiri et al., 2023a; Brikat et al., 2023).
Diet is a determining factor responsible for several diseases, as it can be beneficial for health. Several studies have shown the beneficial effects of certain foods on the health of the body. In recent decades, many efforts have therefore been devoted to the use of different plants for therapeutic purposes due to their high medicinal effects, minimal adverse effects, and comparatively affordable price (Liu et al., 2012). The medicinal effects of these plants are linked to secondary metabolism molecules such as flavonoids, polyphenols, saponins, terpenoids, and alkaloids. Plant-derived phenols have therapeutic effects in the treatment of some metabolic diseases, like obesity, brain cell disorders, atherosclerosis, and urolithiasis (Aiboud et al., 2014; El-Hasnaoui et al., 2015; Nakache et al., 2017; Bahbiti et al., 2018; Chakit et al., 2022a, b).
Among medicinal plant species, Curcuma longa (Turmeric) is widely used as an herbal supplement by the world population including Moroccan people. One of the active constituents of Turmeric is curcumin (Curc) which is a polyphenol responsible for the main therapeutic properties of this plant, mainly its antimicrobial, antioxidant, and anti-inflammatory activities. Curc is characterized by its capacity to traverse the barrier blood-brain providing direct contact with neurons and possible neuroprotection (Guariglia et al., 2023).
The objective of this study is to ass the therapeutic activity of methanolic extract of the rhizome of Curcuma longa and losartan used as an antagonist of AT1R on memory impairment and OS provoked by a HFD, in adult female Wistar rats.
MATERIALS AND METHODS
Study design
36 female Wistar rats have free access to drinking water and standard food. Animals food consisted of crude proteins, fats, cellulose, and minerals. Certain groups of animals were fed a standard food and others with a rich fructose hypercaloric diet dissolved in beverage water (23%, 30 ml/animal) for eight weeks. For each group, fructose consumptions were recorded during the eight weeks of manipulation. A group has received Losartan (30 mg/kg) in their drinking water (30ml/day) for four weeks.
Chemical products
The methanolic extract of Curcuma longa was prepared by using Soxhlet apparatus and rotary evaporator. Animals were administered by gavage 0.45ml of Curcuma longa methanolic extract through a 50 mm copper feeding tool (100 mg/kg body weight) for 10 days. The daily dose of turmeric extract is 100 mg/kg of body weight per day. In the literature, the minimum and maximum doses of Curc were 5 and 480 mg/kg. Doses between 100 mg/kg and 300 mg/kg were recorded as the most common doses (Brikat et al. 2023). Curc was administered between 1 and 84 days (Sanei and Saberi-Demneh, 2019).
The group of animals treated with Los received a dose of 30 mg/kg/day of Los in their drinking water for one month (Brikat et al., 2023). The volume needed for each rat is 30 ml per day.
Animals
ALL experiments were conducted on adult female rats from the Animal House of Biology and Health Laboratory of the Sciences of Life Department, University of Ibn Tofail (Morocco). During all experiments, the rats were kept in 12-h/12-h photoperiod and a temperature of 23°C, and their body weight was regularly weighed. The breeding cages were regularly cleaned and the litter was renewed.
Over a period of 8 weeks, 36 female Wistar rats aged 2 months and weighing 74 to 100 g were randomly stratified into six groups: 2 groups would receive a standard diet and 4 groups would receive the high HFD diet which will induce an obese state in rats. By the end of two months, the phase of treatment will start according to follow diet categories:
- A group having received a standard diet will not receive any qualified treatment (Control).
- A group will not receive any qualified treatment (HFD).
- A group will receive 100 mg/kg/day of turmeric, qualified (HFD + Curc),
- A group will receive 30 mg/Kg/day of Losartan, qualified (HFD + Los)
- A group having received a standard diet will also receive 100 mg/kg/d of turmeric extract and 30 mg/kg/d of Losartan (Curc + Los).
- A group will receive 100 mg/kg of turmeric + 30 mg/kg/d of Losartan, qualified (HFD + Curc + Los).
Memory tests
Y-maze test
This Y-maze test examines working memory using three identical aisles arranged in an equilateral triangle. The dimensions of the aisle are 13 x 4.5 x 5.5 cm. Known as a “spontaneous alternation” test; the rat freely explores these aisles without external reinforcement. The rat starts in one aisle, facing the intersection, and is allowed 5 minutes of exploration, recording entries when all four legs are inside. Parameters include total visits and alternations, and the percentage of alternation is indicative of working memory capacities, showing an inverse correlation. The sequence of alternation of entries was analyzed to obtain the % correct alternation which is calculated as follows:
% of Spontaneous alternation = [(Number of alternations)/ (Total arms entries-2)] × 100
(El-Brouzi et al., 2021; Zghari et al., 2023b).
Object recognition test (ORT)
The object recognition test in rodents assesses recognition memory and the ability to discern between objects in familiar surroundings. It measures the behavioral reactions of rats upon introducing a new object. Relying on the natural inclination of rats to explore novel objects, the test employs an open field with a square translucent floor and white vertical walls. Over three days, including habituation and training, the test introduces two identical objects and the third is different. The discrimination index parameter reflects the proportion of exploration time of the new object, varying between -100% (familiar object) to 100% (new object). Deficits in short-term recognition memory are expressed by a reduction in the recognition index (% RI), which is calculated according to the following formula: [The total time spent exploring the novel object/ (The total time spent exploring the novel object + The total time spent exploring the familiar object)] x 100 (Nassiri et al. 2023b; Rhaimi et al., 2023).
Morris water maze test (MWMT)
MWMT was used to assess visual short-term memory and visual-spatial abilities in small rodents (Morris, 1984). In this test, rats navigate a circular pool filled with opaque water, aiming to reach a platform that may be visible or hidden. Despite no noticeable impairment in swimming ability, all groups successfully locate the full platform through exploration. Video tracking records successive swims, revealing reduced time and distance during repeated attempts. After 12 training trials, when the platform’s position is changed, knockout mice exhibit increased difficulty in learning the new location compared to normal littermates. This suggests a challenge in altering learned spatial strategies, potentially linked to the hippocampal function, as spatial memory heavily relies on it.
The rat is placed in the basin, with its head directed against the wall at the four cardinal points. The animal’s control times on the platform are recorded. Each trial lasts 60 seconds. If you cannot find the platform at the end of the test, it is a good time for the experimenter to wait 20 seconds. The test is carried out the day after the last day of learning. The platform is removed from the pool and the animal is successively placed on 4 poles. The times spent in the platform quadrant are during the acquisition phase (NO) and are measured in seconds of a 60-second session. After 2 hours of probe testing, the visible platform phase appears. The platform is placed in the middle of the NO quadrant and visible light (1 cm above the water surface). Four minutes of 60 seconds are conduits to place the rat successively at the 4 cardinal points. Bad times to check the platform are measured. The parameters calculated in our protocol are latency times (time spent to reach the platform) and times spent in the NO quadrant (probe test).
NO assay
Nitric oxide (NO) production from hippocampal samples can be determined by estimating the concentrations of the final products of NO production (nitrates and nitrites) (Chao et al., 1992). Similarly, the nitrates were used to prepare a standard range which serving in the determination of NO.
Lipid peroxidation assay
The principle of lipid peroxidation assay is the measure of Malondialdehyde (MDA) formed during the free radical-mediated breakdown of fatty acids. The assessment involves inducing the formation of a colored pigment in an acidic and hot environment (100°C) through the interaction between MDA and thiobarbiturate (TBA). This pigment absorbs at 530 nm and can be extracted by organic solvents like butanol.
To 0.5 ml of a homogenate from the two structures, 0.5 ml of 20% acid trichloroacetic acid (TCA) and 1 ml of 0.67% Thiobarbituric acid (TBA) were added. The mixture was heated at 100°C for 15 minutes and then cooled before adding 4 ml of n-butanol. After centrifugation for 15 minutes at 3000 rpm, the optical density was determined on the supernatant in a spectrophotometer at 530 nm (Draper and Hadley, 1990).
Catalase activity
In this work, we studied the activity of catalase (CAT), since it is one of the antioxidant enzymes that catalyze the disproportionation of hydrogen peroxide into dioxygen and water according to the following reaction: 2H2O2 ==> 2H2O + O2.
The determination of CAT activity is based on the measure of optical density related to H2O2 disappearance (Aebi 1984). In a quartz cuvette, 0.05 ml of phosphate buffer (for the blank) or 0.05 ml of hippocampus tissue extract was mixed to 1.95 ml of phosphate buffer (0.05 M, pH 7.4) and 1ml of H2O2 (0.05M). At 240 mm, the absorbance is measured after each 30 s, during two minutes in cells. The Catalase activity is expressed in IU/min/g. it’s calculated by using following formula:
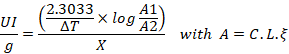
A: the absorbance at 240 nm of H2O2; L: length of the tank used (cm) = 1cm; ξ: molar extinction coefficient = 43.6 M-1 cm-1; X: Tissue concentration in mg/ml.
Statistical analysis
The value differences between experimental and control groups are determined by analysis of variance (Anova one way) via GraphPad Prism software (version 8.0). The post-hoc Tukey was performed in the case of significant difference variance. The used significant degrees are significant at p < 0.05 (*).
RESULTS and Discussion
Y-maze test
The results recorded for the state of working memory of female adult rats are shown in Figure 1. HFD-treated group had a lower % of alternation in females in comparison with rats treated with standard diet (–52%; p < 0.001). Additionally, the administration of Curc and Los elevated the percentage of spontaneous alternation no significantly (+35% and +59%, respectively; p > 0.05), when compared to the HFD group. Interestingly, the association between HFD, Curc and Los led to a substantial increase in the % of alternation by 114% in comparison with the HFD group (p < 0.01) (Figure 1A).
OR test
Recognition performances were tested using the OR test, as illustrated in Figure 2. In the ORT, our findings show that the recognition index did not exhibit a significant reduction in the HFD group when compared to control rats (–30%; p > 0.05). Also, the administration of Curc + Los increased significantly the recognition index (p < 0.01; +67%) in comparison with the HFD group. In contrast, the administration of Curc and/or Los did not improve the recognition index in comparison with the HFD group (p > 0.05).
Morris water maze test
The MWM test was used to examine the influence of different treatments on the spatial learning and memory performance of rats. Figure 3A shows that HF intake lengthened escape latency in adult female rats, especially during the first two days of the test, in comparison with rats treated with standard diet (p > 0.05). Furthermore, Curc, Los and Curc + Los treatments were able to prevent this HFD-induced rise in latency (p < 0.01) (Figure 3A).
During the probe test, the percentage of time spent in the correct quadrant by female rats significantly decreased in the HFD group compared to control animals (–63%, p < 0.01). Notably, Figure 3B illustrates that the administrations of Curc, Los, and their combination effectively counteracted this HFD-induced memory effect (p < 0.01). They achieved this by elevating the percentage of time spent in the correct quadrant by 165%, 160%, and 197%, respectively (Figure 3B).
Effects on oxidative stress parameters
As depicted in Figure 4A, the levels of TBARS exhibited a significant increase in female rats subjected to a HFD compared to control rats (+89%; p < 0.01). Moreover, the administration of Curc, Los, and their combination resulted in a noteworthy reduction of TBARS levels in the hippocampus when compared to HFD-treated rats (–72%, p < 0.001; –57%, p < 0.01; and –76%, p < 0.001, respectively).
In addition, our data indicates a significant increase in the mean level of NO in the HFD group, surpassing the corresponding values observed in female animals by 56%, as compared to control rats (p < 0.05). In contrast, the administration of HFD + Curc + Los lowered NO levels significantly in rats in comparison with the standard diet-treated rats (–83%, p < 0.01). Additionally, Curc, Los and Curc + Los treatments decreased significantly NO concentrations (–58%, p < 0.001; –59%, p < 0.01 and –88%, p < 0.001, respectively), as compared with the HFD group (Figure 4B).
As shown in Figure 4C, CAT activity was significantly elevated in the female rats treated with HFD in comparison with the control rats (+182%; p < 0.01). Interestingly, chronic Curc, Los and Curc + Los administration, supplementation was markedly noted to improve the activity of this antioxidant enzyme, by decreasing its activity by 39%, 68% and 63%, respectively, when compared to the HFD-treated rats (p < 0.01) (Figure 4C).
This experiment was undertaken to assess the impacts of methanolic extract of Curcuma longa (rhizome) and losartan on memory impairment provoked by a HFD, in adult female Wistar rats.
Memory impairment following a HFD
A key observation from this study is that a HFD had detrimental effects on working memory assessed in the Y maze, recognition memory evaluated in the ORT, and spatial learning and memory tested in the MWM in female Wistar rats. This present finding aligns with previous studies that demonstrated memory dysfunction resulting from hypercaloric diets. For example, the experiments carried out by Underwood and Thompson confirmed the adverse effects of HFD on spatial learning and memory (decline in the object recognition index and the % of alternation) in rats of both genders (Underwood and Thompson, 2016a, b). Also, Sangüesa et al. (2018) reported that the hypercaloric-fed group showed a reduced short-term memory in comparison with their controls (Sangüesa et al., 2018). Additionally, as assessed by the MWM, the hypercaloric-fed rats showed an altered spatial learning and memory (decreased long-term memory) (Ganji et al., 2017; Hyer et al., 2022; Chávez-Gutiérrez et al., 2022).
These detrimental impacts of high-caloric diets on memory function can be attributed to various mechanisms, such as diminished neurogenesis (Dias et al. 2014), alteration of the Hypothalamic Adrenal Pituitary (Harrell et al., 2018), and changes in the gut microbiota composition (Pyndt Jørgensen et al., 2014). Notably, oxidative stress (OS) is regarded as a crucial factor in the causation of memory impairments (Naïla et al., 2021; Zghari et al., 2023a, b). Hence, it is plausible that the impaired memory observed in rats treated with a HFD may be attributed to elevated OS in the hippocampus, a brain region recognized for its role in cognitive regulation (Jangra et al., 2014; Chahirou et al., 2018). In this context, several studies have identified elevated levels of OS in individuals experiencing memory dysfunction (Singh et al., 2022). Additionally, it is essential to highlight the increased susceptibility of this brain region to OS (Taniguti et al., 2018). Naturally, given the brain’s elevated oxygen demand, it is particularly prone to oxidative damage (Lovell and Markesbery, 2007). The increased oxygen metabolism can result in the excessive generation of free radicals such as NO, which, if produced in abundance, have the potential to harm numerous essential biomolecules crucial for the normal functioning of neuronal cells (Harman, 1956). In fact, reactive oxygen species (ROS) are recognized for their ability to readily combine with NO, giving rise to peroxynitrite (ONOO-). This free radical has been linked to diverse neurotoxic effects, ultimately leading to compromised memory function (Lamtai et al., 2020; Naïla et al., 2021; El-Brouzi et al., 2021). Supporting this view, our study revealed that a high-fructose diet impacted memory function, correlating with heightened OS in the hippocampus. This was evidenced by elevated levels of LPO and free radicals such as NO in the hippocampus of female rats. In line with our data, short-term fructose consumption has been shown to produce an increase in OS-specific markers (increased LPO) in the hippocampus of young and adult rats (Cigliano et al., 2018). The proper functioning of brain mitochondria is of paramount importance, and the overproduction of mitochondrial free radicals may be one of the main contributors to the oxidative changes in the brain associated with HFD. In this context, it is established that the metabolic state of an organism significantly influences mitochondria. When there is an excess of energetic substrates compared to demand, it has been demonstrated to negatively impact mitochondrial structure and function, leading to heightened fragmentation and an increased generation of free radicals (Picard and Turnbull, 2013). Furthermore, it is hypothesized that OS results from heightened activity of both nitric oxide synthase and nicotinamide adenine dinucleotide phosphate oxidase (NADPH), the latter catalyzing the production of superoxide radicals (Li et al., 2013). A study by Jiang et al. (2011) also indicated that hypercaloric diets can elevate NADPH activity (Jiang et al., 2011).
Additionally, an increased OS could also arise from changed antioxidant defenses. In agreement, we observed that CAT activity was elevated in the hippocampus of all female rats receiving fructose chronically. Also, a significant reduction in the activity of the antioxidant enzyme has been detected in the brain following high caloric diet administration (Mastrocola et al., 2016). In this context, it is crucial to emphasize that any alteration in the expression or activity of antioxidant enzymes, whether a decrease or increase, serves as an indication of OS (Peng, 2015). Taken together, the substantial production of NO and the alterations in CAT activity induced by a HFD contribute to OS, ultimately causing cellular damage in the hippocampus. These modifications may contribute to the observed memory impairment in the current study.
Neuroprotective effects of Curc and losartan
In females fed HFD, Curc significantly improves the memory function as assessed by Y-Maze, ORT and MWM tests. The memory-deficit-ameliorating impact of Curc in diverse animal models has been substantiated through various behavioral assessments, indicating a neuroprotective effect. The majority of these experiments have reported improved short and long-term memory (Borre et al., 2014; Liu et al., 2014). In a recent study by Kamali et al. (2019) Curc administration improved memory and neurological deficits in rats (Kamali et al., 2019). The mechanisms behind the positive enhancement of memory deficits remain to be elucidated. Importantly, these effects of Curc on memory are accompanied by a decrease in NO and LPO levels, and a reduction in CAT activity in the hippocampus. Curc, having the ability to traverse the blood-brain barrier, demonstrates interactions with various target molecules associated with OS. Additionally, it has been observed to regulate the OS response within the central nervous system (Hayder and Abdulwahid, 2023). In this regard, Curc is commonly employed to protect against OS and enhance antioxidant capability. This is attributed to its ability to reduce LPO induced by hydrogen peroxide (H2O2) and significantly decrease MDA levels upon administration. The antioxidant properties of Curc are ascribed to various mechanisms, encompassing the inhibition of LPO, heightened glutathione peroxidase activity, elevated expression of antioxidant enzymes, and enhanced removal of free radicals (Adibian et al., 2019). In this sense, Curc was identified as having the capacity to alleviate OS by reducing the activity inducible NO synthase (iNOS) enzymes and modulating the levels of antioxidant markers (Liao et al., 2020). Moreover, Curc’s well-known antioxidant properties are a result of its phenolic structure, which has properties that destabilize free radicals by collecting electrons. On the other hand, Curc has been discovered to have contradictory effects on the amount of intracellular ROS, which appears to be highly dependent on the type of cell, much like other antioxidants such carotenoids and vitamins (C and E) (Hodaei et al., 2019). Given the above attributes, Curc appears to be a possible candidate as a therapeutic agent to alleviate cognitive impairment caused by a HFD, as our research indicates.
Multiple data sources have substantiated the neuroprotective effects of Los and its therapeutic efficacy in addressing various neurological and psychological issues (Zhang et al., 2012). Our experiment’s results further demonstrated Los’s involvement in ameliorating memory deficits induced by HFD, aligning with numerous prior rodent experiments that have highlighted Los’s ability to protect cognitive function (Campos et al., 2020). Los can strengthen and improve memory and learning abilities in addition to its effects through the conversion of angiotensin II to IV and other pathways that have been demonstrated in earlier studies (Sharieh et al., 2014). Angiotensin II continues to circulate in the bloodstream and is changed by blood aminopeptidases into angiotensin III and IV when losartan and other blockers are present. Angiotensin IV improves memory by raising intracellular calcium levels, which are unrelated to NMDA receptors (Davis et al., 2006). This is confirmed by the abundance of AT4 receptors in the hippocampus, neocortex, basal glands, and amygdala, which play a role in regulating learning and cognitive processing (Chai et al., 2000). Also, Los causes a dose-dependent increase in the hippocampal depolarization-induced release of acetylcholine (Lee et al., 2001). This implies that Ang IV enhances memory through a mechanism involving potentiation of cholinergic transmission. Kramár et al. (2001) have shown that Ang IV also improves LTP, the neuronal underlying of learning and memory (Kramár et al., 2001). Additionally, one suggested explanation for how Los protects memory involves its ability to diminish OS in the brain through various concurrent mechanisms. These include the suppression of pro-inflammatory mediators like iNOS (Saavedra et al., 2011; Benicky et al., 2011). Remarkably, NOX2 deletion and SOD2 upregulation restored hippocampal-dependent and contextual memory deficits when using Los (Lin et al., 2018). Through the inhibition of the angiotensin-II receptor, Losartan has the potential to reduce oxidative stress and alleviate oxidative damage (Shirai et al., 2014). This is typically manifested by an elevation in the activity of antioxidant enzymes such as SOD or CAT and a reduction in the levels of certain peroxidation products like MDA. In agreement, our study demonstrates that chronic intake of Los prevents against HFD complications such as hippocampal OS by restoring NO and LPO levels and CAT activity.
Importantly, we noted that co-administration of methanolic extract of Curcuma longa (rhizome) and Los exhibited a more pronounced improvement in HFD-provoked memory alterations in comparison with treatment with Curc or Los only. This may be attributable to the possible synergistic interaction between Curc extract and Los. An investigation into the interplay between medicinal plants and synthetic antihypertensive medications revealed that Curc Longa exhibits positive impacts on the pharmacokinetics of antihypertensive drugs like Los (Liu et al., 2012). Administration of Curc Longa prior to treatment enhances the plasma concentrations of Losartan and its metabolite in Wistar rats, consequently boosting the bioavailability of Losartan (Liu et al., 2012), and subsequently enhancing its beneficial effects in the body, particularly in the brain.
CONCLUSIONs and Recommendations
In conclusion, the results of this study illustrated that persistent intake of HFD adversely impaired working memory, recognition memory and spatial learning and memory in female Wistar rats, which was linked to increased OS in the hippocampus. Furthermore, our findings indicate that treatments involving Curcuma Longa and/or Los may serve as promising therapeutic approaches for addressing memory impairment and OS induced by a HFD. Nevertheless, a notable limitation of our experiment may stem from the absence of further determinations of OS biomarkers, such as SOD, GSH, or carbonyl groups. In addition, the assessment of the effects of HFD, Curc and Los was carried out only on female rats. It is preferable to compare these effects in rats of both sexes in order to investigate the possible presence of sexual dimorphism. It is evident that further research is necessary to elucidate the specific mechanisms underlying these associations.
ACKNOWLEDGMENTS
We express our gratitude to the technical support provided by the staff of the Biology Department at the Faculty of Science, Ibn Tofail University.
NOVELTY STATEMENT
The novelty of this study is to evaluate the impacts of methanolic extract of Curcuma longa (rhizome) and/or losartan on memory impairment provoked by a HFD, in adult female Wistar rats.
AUTHOR’S CONTRIBUTION
All authors contributed equally to the manuscript.
Conflict of interest
The authors have declared no conflict of interest.
REFERENCES
Adibian M, Hodaei H, Nikpayam O, Sohrab G, Hekmatdoost A, Hedayati M (2019). The effects of curcumin supplementation on high‐sensitivity C‐reactive protein, serum adiponectin, and lipid profile in patients with type 2 diabetes: A randomized, double‐blind, placebo‐controlled trial. Phytother. Res., 33(5):1374–1383. https://doi.org/10.1002/ptr.6328
Aebi H (1984). Catalase in vitro. Methods Enzymol., 105: 121–126. https://doi.org/10.1016/S0076-6879(84)05016-3
Aiboud A, Moussaif A, El Abbadi N, Ettabia A, El Hessni A, Ouichou A, Chakit M, Mesfioui A (2014). In vitro antidermatophytic activity of Allium sativum L, Nicotiana tabacum and cade oil against Trichophyton rubrum. World J. Pharm. Res., 4(1): 414–423.
Baataoui S, Chakit M, Boudhan M, Ouhssine M (2023a). Assessment of vitamin D, calcium, cholesterol, and phosphorus status in obese and overweight patients in Kenitra city (Morocco). Res. J. Pharm. Technol., 16(7): 3405–3409. https://doi.org/10.52711/0974-360X.2023.00563
Baataoui S, Chakit M, Boudhan M, Ouhssine M (2023b). Effect of vitamin D supplementation on the response of phosphocalcic metabolism in Moroccan population. Int. J. Chem. Biochem. Sci., 24(5): 770–775.
Bahbiti Y, Ammouri H, Berkiks I, Hessni AE, Ouichou A, Nakache R, Chakit M, Bikjdaouene L, Mesfioui A (2018). Anticonvulsant effect of argan oil on pilocarpine model induced status epilepticus in wistar rats. Nutr. Neurosci., 21(2): 116–122. https://doi.org/10.1080/1028415X.2016.1228492
Benchelha H, Chakit M, Ahami AOT, Bikjdaouene L (2023a). Aerobic capacity, attention and well-being in obese and normal adolescents. Radiol. Onkol., 17(12): 859–865.
Benchelha H, Chakit M, Lotfi S, Ahami AOT, Bikjdaouene L (2023b). Perceptual and cardiorespiratory response to progressive running test in relation with puberty and weight status. Int. J. Chem. Biochem. Sci., 24(5): 664–673.
Benicky J, Sánchez-Lemus E, Honda M, Pang T, Orecna M, Wang J, Leng Y, Chuang D-M, Saavedra JM (2011). Angiotensin II AT1 receptor blockade ameliorates brain inflammation. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol., 36(4): 857–870. https://doi.org/10.1038/npp.2010.225
Borre YE, Panagaki T, Koelink PJ, Morgan ME, Hendriksen H, Garssen J, Kraneveld AD, Olivier B, Oosting RS (2014). Neuroprotective and cognitive enhancing effects of a multi-targeted food intervention in an animal model of neurodegeneration and depression. Neuropharmacology, 79: 738–749. https://doi.org/10.1016/j.neuropharm.2013.11.009
Brikat S, Chakit M, Lamtai M, Fitah I, Abouyaala O, Mesfioui A, El-Hessni A (2023). Effects of Curcuma longa methanolic extract and losartan on anxiety- and depression-like behaviors induced by a high caloric diet in adult female Wistar rats. Int. J. Chem. Biochem. Sci., 24(6): 886–895.
Campos GV, de Souza AMA, Ji H, West CA, Wu X, Lee DL, Aguilar BL, Forcelli PA, de Menezes RC, Sandberg K (2020). The angiotensin type 1 receptor antagonist losartan prevents ovariectomy-induced cognitive dysfunction and anxiety-like behavior in long evans rats. Cell Mol. Neurobiol., 40(3): 407–420. https://doi.org/10.1007/s10571-019-00744-x
Chahirou Y, Lamtai M, Mesfioui A, Ouichou A, Coulibaly M, Boussekkour R, El Hessni A (2018). Impact of the association of a high fructose diet and chronic mild stress on metabolic and affective disorders in male rat. J. Behav. Brain Sci., 8(4): 157–170. https://doi.org/10.4236/jbbs.2018.84010
Chai SY, Bastias MA, Clune EF, Matsacos DJ, Mustafa T, Lee JH, McDowall SG, Paxinos G, Mendelsohn FA, Albiston AL (2000). Distribution of angiotensin IV binding sites (AT4 receptor) in the human forebrain, midbrain and pons as visualised by in vitro receptor autoradiography. J. Chem. Neuroanat. 20(3–4): 339–348. https://doi.org/10.1016/S0891-0618(00)00112-5
Chakit M, Boussekkour R, El Hessni A, Bahbiti Y, Nakache R, Mustaphi HE, Mesfioui A (2022a). Antiurolithiatic Activity of Aqueous Extract of Ziziphus lotus on Ethylene Glycol-Induced Lithiasis in Rats. Pharmacogn. J., 14: 596–602. https://doi.org/10.5530/pj.2022.14.141
Chakit M, El Hessni A, Mesfioui A (2022b). Ethnobotanical study of plants used for the treatment of urolithiasis in Morocco. Pharmacogn. J., 14: 542–547. https://doi.org/10.5530/pj.2022.14.133
Chao CC, Hu S, Molitor TW, Shaskan EG, Peterson PK (1992). Activated microglia mediate neuronal cell injury via a nitric oxide mechanism. J. Immunol. Baltim Md. 1950, 149(8): 2736–2741. https://doi.org/10.4049/jimmunol.149.8.2736
Chávez-Gutiérrez E, Fuentes-Venado CE, Rodríguez-Páez L, Guerra-Araiza C, Larqué C, Martínez-Herrera E, Ocharan-Hernández ME, Lomelí J, Loza-Mejía MA, Salazar JR, Meneses-Ruiz DM, Gallardo JM, Pinto-Almazán R (2022). High fructose and high fat diet impair different types of memory through oxidative stress in a sex- and hormone-dependent manner. Metabolites, 12(4): 341. https://doi.org/10.3390/metabo12040341
Cigliano L, Spagnuolo MS, Crescenzo R, Cancelliere R, Iannotta L, Mazzoli A, Liverini G, Iossa S (2018). Short-term fructose feeding induces inflammation and oxidative stress in the hippocampus of young and adult rats. Mol. Neurobiol., 55(4): 2869–2883. https://doi.org/10.1007/s12035-017-0518-2
Davis CJ, Kramár EA, De A, Meighan PC, Simasko SM, Wright JW, Harding JW (2006). AT4 receptor activation increases intracellular calcium influx and induces a non-N-methyl-D-aspartate dependent form of long-term potentiation. Neuroscience, 137(4): 1369–1379. https://doi.org/10.1016/j.neuroscience.2005.10.051
Dias GP, Bevilaqua MC do N, da Luz ACDS, Fleming RL, de Carvalho LA, Cocks G, Beckman D, Hosken LC, de Sant’Anna Machado W, Corrêa-e-Castro AC, Mousovich-Neto F, de Castro Gomes V, Bastos G de NT, Kubrusly RCC, da Costa VMC, Srivastava D, Landeira-Fernandez J, Nardi AE, Thuret S, Gardino PF (2014). Hippocampal biomarkers of fear memory in an animal model of generalized anxiety disorder. Behav. Brain Res., 263: 34–45. https://doi.org/10.1016/j.bbr.2014.01.012
Draper HH, Hadley M (1990). Malondialdehyde determination as index of lipid peroxidation. Methods Enzymol., 186: 421–431. https://doi.org/10.1016/0076-6879(90)86135-I
El-Brouzi MY, Lamtai M, Zghari O, Ouakki S, Azizoun I, El Hessni A, Mesfioui A, Ouichou A (2021). Intrahippocampal effects of nickel injection on the affective and cognitive response in wistar rat: potential role of oxidative stress. Biol. Trace Elem. Res., 199(9): 3382–3392. https://doi.org/10.1007/s12011-020-02457-5
El-Hamaoui A, Chakit M, Saidi H, Fitah I, Khadmaoui A (2023). Psychological assessment of quality of life in a Moroccan population with chronic disease. Int. J. Chem. Biochem. Sci., 24(6): 121–129
El-Hasnaoui A, Mesfioui A, Berkiks I, Chakit M, Kribii A, Ali O, El-Hessni A (2015). Effects of the peroxisome proliferator-activated receptors alpha agonist and Cinnamon oil on obesity induced by high fructose diet. World J. Pharm. Res., 4(5): 23–38.
Elmaleh-Sachs A, Schwartz J, Bramante C, Nicklas J, Gudzune K, Jay M (2023). Obesity management in adults: A review. J. Am. Med. Assoc., 330(20). https://doi.org/10.1001/jama.2023.19897
Fitah I, Chakit M, El-Kadiri M, Brikat S, El Hessni A, Mesfioui A (2023). The evaluation of the social functioning of schizophrenia patients followed up in the health center My El-Hassan of Kenitra, Morocco. Egypt. J. Neurol. Psychiatry Neurosurg., 59(1): 125. https://doi.org/10.1186/s41983-023-00714-7
Ganji A, Salehi I, Nazari M, Taheri M, Komaki A (2017). Effects of Hypericum scabrum extract on learning and memory and oxidant/antioxidant status in rats fed a long-term high-fat diet. Metab. Brain Dis. 32(4): 1255–1265. https://doi.org/10.1007/s11011-017-0022-4
Guariglia M, Saba F, Rosso C, Bugianesi E (2023). Molecular mechanisms of curcumin in the pathogenesis of metabolic dysfunction associated Steatotic liver disease. Nutrients, 15(24): 5053. https://doi.org/10.3390/nu15245053
Harman D (1956). Aging: A theory based on free radical and radiation chemistry. J. Gerontol., 11(3): 298–300. https://doi.org/10.1093/geronj/11.3.298
Harrell CS, Zainaldin C, McFarlane D, Hyer MM, Stein D, Sayeed I, Neigh GN (2018). High-fructose diet during adolescent development increases neuroinflammation and depressive-like behavior without exacerbating outcomes after stroke. Brain Behav. Immun., 73: 340–351. https://doi.org/10.1016/j.bbi.2018.05.018
Hayder Khayoon E, Ahmed Abdulwahid A (2023). The neuroprotective effect of curcumin cumin against oxidative stress- induced by acrylamide in male rats. Adv. Anim. Vet. Sci., 12(2). https://doi.org/10.17582/journal.aavs/2024/12.2.226.232
Hodaei H, Adibian M, Nikpayam O, Hedayati M, Sohrab G (2019). The effect of curcumin supplementation on anthropometric indices, insulin resistance and oxidative stress in patients with type 2 diabetes: A randomized, double-blind clinical trial. Diabetol. Metab. Syndr., 11(1): 41. https://doi.org/10.1186/s13098-019-0437-7
Hyer MM, Dyer SK, Kovalchick LV, Targett I, Burns CM, Kloster A, Wegener AJ, Sanchez CS, Neigh GN (2022). Fructose-induced cognitive impairment co-occurs with morphological changes in dendrites and microglia of male rats. Psychoneuroimmunol. J., 3: 1–12. https://doi.org/10.32371/pnij/246142
Jangra A, Lukhi MM, Sulakhiya K, Baruah CC, Lahkar M (2014). Protective effect of mangiferin against lipopolysaccharide-induced depressive and anxiety-like behaviour in mice. Eur. J. Pharmacol., 740: 337–345. https://doi.org/10.1016/j.ejphar.2014.07.031
Jiang F, Lim HK, Morris MJ, Prior L, Velkoska E, Wu X, Dusting GJ (2011). Systemic upregulation of NADPH oxidase in diet-induced obesity in rats. Redox Rep. Commun. Free Radic. Res., 16(6): 223–229. https://doi.org/10.1179/174329211X13049558293713
Kamali Dolatabadi L, Emamghoreishi M, Namavar MR, Badeli Sarkala H (2019). Curcumin effects on memory impairment and restoration of irregular neuronal distribution in the hippocampal ca1 region after global cerebral ischemia in male rats. Basic Clin. Neurosci. J., pp. 527–540. https://doi.org/10.32598/bcn.9.10.365
Kramár EA, Armstrong DL, Ikeda S, Wayner MJ, Harding JW, Wright JW (2001). The effects of angiotensin IV analogs on long-term potentiation within the CA1 region of the hippocampus in vitro. Brain Res., 897(1–2): 114–121. https://doi.org/10.1016/S0006-8993(01)02100-X
Lamtai M, Ouakki S, Zghari O, Hamzaoui AE, Benmhammed H, Azirar S, Hessni AE, Mesfioui A, Ouichou A (2020). Neuroprotective effect of melatonin on nickel-induced affective and cognitive disorders and oxidative damage in rats. Environ. Anal. Health Toxicol., 35(4): e2020025. https://doi.org/10.5620/eaht.2020025
Lee J, Chai SY, Mendelsohn FA, Morris MJ, Allen AM (2001). Potentiation of cholinergic transmission in the rat hippocampus by angiotensin IV and LVV-hemorphin-7. Neuropharmacology 40(4): 618–623. https://doi.org/10.1016/S0028-3908(00)00188-X
Li J, O W, Li W, Jiang Z-G, Ghanbari H (2013). Oxidative Stress and Neurodegenerative Disorders. Int. J. Mol. Sci., 14(12): 24438–24475. https://doi.org/10.3390/ijms141224438
Liao D, Lv C, Cao L, Yao D, Wu Y, Long M, Liu N, Jiang P (2020). Curcumin attenuates chronic unpredictable mild stress-induced depressive-like behaviors via restoring changes in oxidative stress and the activation of Nrf2 Signaling Pathway in Rats. Oxid. Med. Cell Longev., 2020: 9268083. https://doi.org/10.1155/2020/9268083
Lin Y-T, Wu Y-C, Sun G-C, Ho C-Y, Wong T-Y, Lin C-H, Chen H-H, Yeh T-C, Li C-J, Tseng C-J, Cheng P-W (2018). Effect of resveratrol on reactive oxygen species-induced cognitive impairment in rats with angiotensin ii-induced early alzheimer’s disease. J. Clin. Med., 7(10): 329. https://doi.org/10.3390/jcm7100329
Liu A-C, Zhao L-X, Xing J, Liu T, Du F-Y, Lou H-X (2012). Pre-treatment with curcumin enhances plasma concentrations of losartan and its metabolite EXP3174 in rats. Biol. Pharm. Bull., 35(2): 145–150. https://doi.org/10.1248/bpb.35.145
Liu D, Wang Z, Gao Z, Xie K, Zhang Q, Jiang H, Pang Q (2014). Effects of curcumin on learning and memory deficits, BDNF, and ERK protein expression in rats exposed to chronic unpredictable stress. Behav. Brain Res., 271: 116–121. https://doi.org/10.1016/j.bbr.2014.05.068
Lovell MA, Markesbery WR (2007). Oxidative damage in mild cognitive impairment and early Alzheimer’s disease. J. Neurosci. Res., 85(14): 3036–3040. https://doi.org/10.1002/jnr.21346
Mastrocola R, Nigro D, Cento AS, Chiazza F, Collino M, Aragno M (2016). High-fructose intake as risk factor for neurodegeneration: Key role for carboxy methyllysine accumulation in mice hippocampal neurons. Neurobiol. Dis., 89: 65–75. https://doi.org/10.1016/j.nbd.2016.02.005
Morris R (1984). Developments of a water-maze procedure for studying spatial learning in the rat. J. Neurosci. Methods, 11(1): 47–60. https://doi.org/10.1016/0165-0270(84)90007-4
Naïla N, Makthar W, Lamtai M, Zghari O, Aboubaker EH, Abdelhalem M, Ali O (2021). Effect of intra-hippocampal lead injection on affective and cognitive disorders in male Wistar rats: Possible involvement of oxidative stress. E3S Web Conf., 319: 02017. https://doi.org/10.1051/e3sconf/202131902017
Nakache R, Touil T, El Hessni A, Ouichou A, Bahbiti Y, Berkiks I, Chakit M, Mesfioui A (2017). In vivo acute toxicity assessment of a novel quinoxalinone (6-nitro-2 (1H)-quinoxalinone) in Wistar rats. Cogent. Chem., 3(1): 1301242. https://doi.org/10.1080/23312009.2017.1301242
Nassiri A, Chakit M, Berkiks I, benmehammed H, Lamtai M, Chana L, Mesfioui A, El-Hessni A (2023a). Sex dimorphism of memory response to long-term effect lipopolysaccharide administration in Wistar rats. Int. J. Chem. Biochem. Sci., 24(5): 685–692.
Nassiri A, Lamtai M, Berkiks I, Benmhammed H, Coulibaly M, Chakit M, Mesfioui A, Hessni AE (2023b). Age and sex-specific effects of maternal deprivation on memory and oxidative stress in the hippocampus of rats.
Peng L (2015). Mice brain tissue injury induced by diisononyl phthalate exposure and the protective application of vitamin E. J. Biochem. Mol. Toxicol., 29(7): 311–320. https://doi.org/10.1002/jbt.21700
Picard M, Turnbull DM (2013). Linking the metabolic state and mitochondrial DNA in chronic disease, health, and aging. Diabetes, 62(3): 672–678. https://doi.org/10.2337/db12-1203
Pyndt Jørgensen B, Hansen JT, Krych L, Larsen C, Klein AB, Nielsen DS, Josefsen K, Hansen AK, Sørensen DB (2014). A possible link between food and mood: dietary impact on gut microbiota and behavior in BALB/c mice. PLoS One, 9(8): e103398. https://doi.org/10.1371/journal.pone.0103398
Rhaimi S, Brikat S, Lamtai M, Ouhssine M (2023). Acute oral toxicity and neurobehavioral effects of salvia officinalis essential oil in female Wistar rats. Adv. Anim. Vet. Sci., 11(4). https://doi.org/10.17582/journal.aavs/2023/11.4.654.662
Saavedra JM, Sánchez-Lemus E, Benicky J (2011). Blockade of brain angiotensin II AT1 receptors ameliorates stress, anxiety, brain inflammation and ischemia: Therapeutic implications. Psychoneuroendocrinology, 36(1): 1–18. https://doi.org/10.1016/j.psyneuen.2010.10.001
Sanei M, Saberi-Demneh A (2019). Effect of curcumin on memory impairment: A systematic review. Phytomed. Int. J. Phytother. Phytopharm., 52: 98–106. https://doi.org/10.1016/j.phymed.2018.06.016
Sangüesa G, Cascales M, Griñán C, Sánchez RM, Roglans N, Pallàs M, Laguna JC, Alegret M (2018). Impairment of novel object recognition memory and brain insulin signaling in fructose- but not glucose-drinking female rats. Mol. Neurobiol., 55(8): 6984–6999. https://doi.org/10.1007/s12035-017-0863-1
Sharieh Hosseini SG, Khatamsaz S, Shariati M (2014). The effects of losartan on memory performance and leptin resistance induced by obesity and high-fat diet in adult male rats. Iran J. Basic Med. Sci., 17(1): 41–48.
Shirai A, Yamazaki O, Horita S, Nakamura M, Satoh N, Yamada H, Suzuki M, Kudo A, Kawakami H, Hofmann F, Nishiyama A, Kume H, Enomoto Y, Homma Y, Seki G (2014). Angiotensin II dose-dependently stimulates human renal proximal tubule transport by the nitric oxide/guanosine 3’, 5’-cyclic monophosphate pathways. J. Am. Soc. Nephrol. JASN, 25(7): 1523–1532. https://doi.org/10.1681/ASN.2013060596
Singh P, Barman B, Thakur MK (2022). Oxidative stress-mediated memory impairment during aging and its therapeutic intervention by natural bioactive compounds. Front. Aging Neurosci., 14: 944697. https://doi.org/10.3389/fnagi.2022.944697
Soczynska JK, Kennedy SH, Woldeyohannes HO, Liauw SS, Alsuwaidan M, Yim CY, McIntyre RS (2011). Mood disorders and obesity: Understanding inflammation as a pathophysiological nexus. Neuromol. Med., 13(2): 93–116. https://doi.org/10.1007/s12017-010-8140-8
Taniguti EH, Ferreira YS, Stupp IJV, Fraga-Junior EB, Mendonça CB, Rossi FL, Ynoue HN, Doneda DL, Lopes L, Lima E, Buss ZS, Vandresen-Filho S (2018). Neuroprotective effect of melatonin against lipopolysaccharide-induced depressive-like behavior in mice. Physiol. Behav., 188: 270–275. https://doi.org/10.1016/j.physbeh.2018.02.034
Tovoli F, Stefanini B, Mandrioli D, Mattioli S, Vornoli A, Sgargi D, Manservisi F, Piscaglia F, Curti S, Bolondi L (2023). Exploring occupational toxicant exposures in patients with metabolic dysfunction-associated steatotic liver disease: A prospective pilot study. Dig. Liver Dis. Off. J. Ital. Soc. Gastroenterol. Ital. Assoc. Study Liver., S1590-8658(23)01097–6. https://doi.org/10.1016/j.dld.2023.12.007
Underwood EL, Thompson LT (2016a). High-fat diet impairs spatial memory and hippocampal intrinsic excitability and sex-dependently alters circulating insulin and hippocampal insulin sensitivity. Biol. Sex. Differ., 7: 9. https://doi.org/10.1186/s13293-016-0060-3
Underwood EL, Thompson LT (2016b). A high-fat diet causes impairment in hippocampal memory and sex-dependent alterations in peripheral metabolism. Neural. Plast., 2016: 7385314. https://doi.org/10.1155/2016/7385314
Zghari O, Azirar S, Lamtai M, El Hessni A, Ouichou A, Mesfioui A (2023a). Melatonin counteracts aluminum-induced affective and cognitive disorders and oxidative damage in male wistar rats. Neurosci. Behav. Physiol., 53: 917–928. https://doi.org/10.1007/s11055-023-01465-x
Zghari O, Lamtai M, Azirar S, El-Brouzi MY, Benmhammed H, El-Hessni A, Ouichou A, Mesfioui A (2023b). Neuroprotective Effects of melatonin against neurotoxicity induced by intrahippocampal injection of aluminum in male Wistar rats: Possible involvement of oxidative stress pathway. Adv. Anim. Vet. Sci., 11(5). https://doi.org/10.17582/journal.aavs/2023/11.5.711.719
Zhang T-L, Fu J-L, Geng Z, Yang J-J, Sun X-J (2012). The neuroprotective effect of losartan through inhibiting AT1/ASK1/MKK4/JNK3 pathway following cerebral I/R in rat hippocampal CA1 region. CNS. Neurosci. Ther., 18(12): 981–987. https://doi.org/10.1111/cns.12015
To share on other social networks, click on any share button. What are these?






