Comparative Effectiveness of Some Novel Fungicides Against Soil-Borne Pathogens of Chili
Research Article
Nabeel Akhtar1, Owais Iqbal1,2*, Imtiaz Ahmed Nizamani1, Rehana Naz Syed1 and Abdul Mubeen Lodhi1
1Department of Plant Protection, Sindh Agriculture University Tandojam, Pakistan-70060; 2State Key Laboratory for Conservation and Utilization of Bio-Resources in Yunnan, Yunnan Agricultural University, Kunming, 650201, China.
Abstract | Soil-borne fungal pathogens are considered the most critical pathogens responsible for the enormous losses in chili crops. The diseases caused by them are often challenging to control due to different incidence level and limited knowledge of their epidemiological features. Advanced accessibility and a simple application process for chemical control systems have made it practical and widely accepted. In the present study, ten different fungicides viz., Shincar, Antracol, Alliete, Ridomil Gold, Moncut, Systhane, Kocide, Evito, Nativo, and Topsin M at 200, 400, 600, 800, and 1000 ppm concentrations were tested in in vitro by poisoned food method against Rhizoctonia solani, Verticillium albo-atrum and Macrophomina phaseolina. Our results showed that Nativo and Evito at 800-1000 ppm and Systhane and Kocide at 1000 ppm can significantly inhibit the mycelial growth of R. solani leading to 100% growth inhibition. Additionally, three fungicides, namely Systhane, Shincar, and Topsin M, were highly effective at all concentrations and caused 100% growth inhibition of M. phaseolina, followed by Antracol (87.22%) at 800 ppm. The Nativo, Evito and Moncut were moderately effective at 1000 ppm, causing more than 78% mycelial growth inhibition of M. phaseolina. Moreover, the ten fungicidal treatments were considerably effective against V. albo-atrum, with Moncut and Shincar being the most effective at all concentrations. This was followed by Evito (86.66%), Kocide (80%), and Systhane (79.94%) at 1000 ppm. While Topsin M and Systhane caused the slightest inhibition, i.e., 17.52% and 10% at 200 ppm. The chemicals demonstrated an escalating inhibitory trend as the concentration increased further.
Received | August 22, 2023; Accepted | February 26, 2024; Published | May 06, 2024
*Correspondence | Owais Iqbal, State Key Laboratory for Conservation and Utilization of Bio-Resources in Yunnan, Yunnan Agricultural University, Kunming, 650201, China; Email: owais.iqbal.918@gmail.com
Citation | Akhtar, N., O. Iqbal, I.A. Nizamani, R.N. Syed and A.M. Lodhi. 2024. Comparative effectiveness of some novel fungicides against soil-borne pathogens of Chili. Sarhad Journal of Agriculture, 40(2): 470-482.
DOI | https://dx.doi.org/10.17582/journal.sja/2024/40.2.470.482
Keywords | Capsicum annum, In vitro, Fungicides, Rhizoctonia solani, Verticillium albo-atrum, Macrophomina phaseolina
Copyright: 2024 by the authors. Licensee ResearchersLinks Ltd, England, UK.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Introduction
Chili (Capsicum annum L.) is an economically important and widely grown crop worldwide (Olatunji and Afolayan, 2018). Both green and dry chili and peppers cover an area of 3.69 million hectares and produce 4.03 million tonnes (dry + green) worldwide (FAOSTAT, 2022). It is commonly used in many food items worldwide as well as cosmetics, pharmaceuticals, and ornaments (Bashir et al., 2018; Tripodi and Kumar, 2019). It serves as a vital reservoir of human nutrients, abundant in Vitamins A, B, C, E, and P, while also being high in potassium, magnesium, and iron. In contrast to red chili, fresh green chili possesses a higher amount of vitamin C than citrus fruits (Kongkachuichai et al., 2015). Chili production faces various constraints, including fungal, bacterial, viral infection, and abiotic stress (Abdel-Fattah and Al-Amri, 2012; Zulfitri et al., 2020). These pathogens cause many diseases, from sowing seeds in the nursery to the field till harvesting (Kavitha et al., 2005; Goldberg, 2010; Divya and Sudini, 2013). Damping-off, root rots, and wilts are common and devastating diseases and affect the crop in either pre or post-harvest stage, which causes significant losses (Pakdeevaraporn et al., 2005). Soil-borne fungal pathogens, including Phytophthora capsici, Rhizoctonia solani, Macrophomina phaseolina, Phytophthora nicotianae, Fusarium solani, Fusarium oxysporum, Verticillium dahliae, Verticillium albo-atrum, and Pythium spp. has been reported for causing these diseases (Abdel-Monaim and Ismail, 2010; Abdel-Monaim et al., 2014). Soil-borne pathogens, especially Macrophomina phaseolina, Rhizoctonia solani, and Verticillium albo-atrum, attack chili plants on roots, leaves, and stems and cause 70% yield losses in the field (Fradin and Thomma, 2006; Jabeen et al., 2016). M. phaseolina (Tassi) Goid is responsible for diseases in elevated temperatures (30-35°C) and limited moisture conditions (Ghosh et al., 2018). It impacts over 500 cultivated and wild plant species globally (Cohen et al., 2022). M. phaseolina produces microsclerotia in the soil and affect various foods crops and their physiological process such as chlorophyll, proline, and sugar content under the favorable environmental conditions (Jadon and Shah, 2012). Root rot and damping-off caused by R. solani is a significant threat to nursery and young chili plants. R. solani is soil and seedborne pathogen (Sivalingam et al., 2006). The pathogen is challenging to control because of its ability to survive long-term in the soil and organic debris and cause disease incidence of 33.2% in seedlings and 40.2% in the field (Varma et al., 2020). R. solani is responsible for wilting and death of mature chili plants (Ashwini and Srividya, 2016). Several practices are available to avoid the disease, such as cultural practices, biological control, resistant varieties, crop rotation, and soil solarization (Van et al., 2016), but these methods contain limitations. Like non-availability of resistant varieties and/or biocontrol-based products for large-scale field application (Amini and Sidovich, 2010). Chemical control is the most broadly used and preferred method for controlling pathogens. Despite all the health and environment risks of fungicides, it has been proven effective in managing strategies (Dahal and Shrestha, 2018). Some agro pesticides and fungicides have been reported against many pathogens, such as carbendazim, carboxin, chlorothalonil, companion, copper oxychloride, mancozeb, metalaxyl, thiram, propiconazole azoxystrobin and iprodion (Hao et al., 2020). Out of these two fungicides carbendazim and mancozeb are considered as the most effective control agents. Typically, fungicides function by employing a shared mechanism centered on microtubule polymerization. This approach effectively regulates fungal cell division, contributing to an efficient crop protection strategy (Abrar Ul Hassan et al., 2021). Therefore, the objective of the present study was to evaluate the ten fungicides at different doses against soil-borne fungal pathogens associated with the chili plants.
Materials and Methods
Collection of specimen, isolation and identification of pathogens
To extract soil-borne fungi affecting chili plants, roots were gathered from diverse fields within the Mirpurkhas district of Sindh province, Pakistan. A total of three chili field selected in Mirpurkhas district and five infected plants were collected from each field. These collected plants were placed into individual paper bags, each labeled with its corresponding location, and transported to the laboratory for the purpose of isolating root-associated fungi. The plants were carefully washed with tap water to eliminate any adhering soil particles from the roots. Subsequently, the infected segments of the roots were dissected into small 1 cm pieces, using sterilized scissors. These root segments were subjected to surface sterilization using a 5% NaClO solution for 30 seconds. Following this, they underwent three washes with sterile distilled water and were then arranged on Petri dishes containing PDA medium. Streptomycin sulfate and penicillin were added to the PDA medium at a concentration of 1 ml/L. A five root samples were put in each Petri dish and seal with parafilm. The plates were incubated at 27°C ± 2°C for 3 days. The resulting fungal colonies exhibited a variety of colors, sizes, and shapes on the PDA medium. For more comprehensive analysis, these fungal colonies were subjected to purification through hyphal tip transfer onto fresh PDA plates. The identification of isolated fungi was accomplished by assessing their morphological characteristics, utilizing identification keys provided by Booth (1971), Ellis (1971), Barnett and Hunter (1972), and Singh (1982).
In vitro screening of fungicides against roots infecting fungi:
Ten fungicides viz., Shincar, Antracol, Aliette, Ridomil Gold, Moncut, Systhane, Kocide, Evito, Nativo, and Topsin M at 200, 400, 600, 800, and 1000 ppm were evaluated against Rhizoctonia solani, Verticillium albo-atrum, and Macrophomina phaseolina by food poisoned technique (Maitlo et al., 2013). The details of fungicides, including brand names, chemical names, chemical groups, active ingredients, and formulations, are shown in Table 1. Before pouring, the concentrations of fungicides were mixed in a PDA with the help of sterilize glass pippete. Fungicide-free mediums were used as control. After solidification of PDA, a 5 mm disk of 8 days old culture was transferred to the center of the Petri plate. All treatments were repeated three time with four replications. Mean values were calculated of each treatment. The inoculated plates were incubated at 27ºC for seven days. Two perpendicular lines were drawn on the back of the Petri plates and crossed in the middle of the plate to measure the radial colony growth of the tested fungi. The colony growth (mm) was measured using a scale every 24 hours until the control plate was filled in any treatment. The mycelial growth inhibition percentage was recorded as per given by formula (Vincent, 1947):
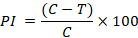
Where; PI= Percent inhibition of fungal mycelial growth; C= Fungal mycelial growth in control plates; T= Fungal mycelial growth in treated plates.
Data analysis
The experiment was conducted using a completely randomized design (CRD) with four replications. To compare the mean values, the least significant difference (LSD) test was performed at a significance level of p = 0.05. Additionally, Duncan’s Multi-Range Test was employed for comparing the means at the same significance level. All statistical analyses were carried out using Statistix version 8.1.
Results and Discussion
Isolation of fungi from infecting roots
A total of 18 fungi, namely, Aspergillus flavus, A. fumigatus, A. niger, Alternaria alternata, A. solani, Curvularia lunata, Colletotrichum capsici, C. gloeosporioides, Fusarium solani, F. oxysporum, Macrophomina phaseolina, Pythium dilense, P. aphanidermatum, Phytophthora capsici, Rhizoctonia solani, Rhizopus oryzae, R. stolonifer, and Verticillium albo-atrum were isolated from the roots with different frequency. Among all pathogens, F. solani appeared with the highest frequency (9.37%) isolated from chili roots, followed by R. solani (9.11%), F. oxysporum (8.85%), A. flavus (6.5%), A. fumigatus (6.45%), M. phaseolina (6.25%), P. aphanidermatum (5.77%), V. albo-atrum (5.4%), P. dilense (5.29%), A. solani (4.55%), A. alternata (4.5%), A. niger (4.3%), R. stolonifer (3.23%), C. gloeosporioides (3.12%), P. capsici (2.61%), R. oryzae (2.61%), C. capsici (2.5%) and C. lunata (2.12%) (Figure 1).
Table 1: Details of fungicides tested against M. phaseolina, R. solani and V. albo-atrum.
|
Trade name |
Active ingredient |
Chemical Group |
Manufacturer/Distributor in Pakistan |
|
Alliete |
Fosetyl Aluminium |
Ethyl Phosphonate |
Bayer Crop Science |
|
Antracol |
Propine |
Dithiocarbamates |
Bayer Crop Science |
|
Evito |
Fluoxastrobin |
Dihydro-dioxazines |
Arysta Life Science Pakistan |
|
Kocide |
Copper hydroxide |
Inorganic |
FMC Corporation |
|
Moncut |
Flutolanil |
Benzamides |
Arysta Life Science Pakistan |
|
Nativo |
Tebuconazole + Trifloxystrobin |
Oximino acetates and Triazoles |
Bayer Crop Science |
|
Ridomil Gold |
Mancozeb + Mefenoxam |
Dithiocarbamates and Acylalanines |
Syngenta Pakistan Limited |
|
Systhane |
Myclobutanil |
Triazole |
FMC Corporation |
|
Shinar |
Carbendazim |
benzimidazole |
FMC Corporation |
|
Topsin M |
Thiophanate-methyl |
Thiophanates |
Arysta Life Science Pakistan |
Effect of different fungicides on colony growth of fungi
Under in-vitro conditions, ten different fungicides viz., Antracol, Aliette, Evito, Kocide, Moncut, Nativo, Ridomil Gold, Systhane, Shincar, and Topsin M were tested for their effects on colony growth of the R. solani, V. albo-atrum, and M. phaseolina. Each fungicide was tested with five concentrations viz., 200, 400, 600, 800, and 1,000 ppm. All fungicides produced a significant reduction in the growth of fungi compared to control. An increase in the concentration of all the ten fungicides in the medium showed a significant gradual reduction in the growth of pathogenic fungi. The LD50 values were calculated according to the inhibition percentage of the tested fungus. For this purpose, the inhibition percentages are converted to probit units.
Effect of different fungicides on Macrophomina phaseolina
Ten different fungicides were employed at various doses to assess their impact on the growth inhibition of Macrophomina phaseolina. Among the investigated fungicides, Systhane, Shincar, and Topsin M appeared highly effective at all doses (200-1000 ppm), which yielded 100% inhibition, followed by Antracol 1000 ppm caused 100% inhibition of Macrophomina phaseolina. Antracol at 600 and 800 ppm causes 87.22% and 70.55% inhibition of mycelial growth. Among all concentrations, the highest concentration (1000 ppm) of Nativo, Evito, Moncut, and Aliette caused more than 70% inhibition of M. phaseolina. Kocide and Ridomil Gold were ineffective at all doses (Figure 2).
Based on LD50 values, M. phaseolina was found to be the most sensitive pathogen against Shincar with an LD50 value of 87.1065, followed by Topsin M (91.2182), Systhane (97.4625), Nativo (323.2536), Antracol (384.4707), Evito (510.8925), Moncut (557.9459) and Alliete (652.02). The Kocide and Ridomil Gold fungicide were infective with LD50 value Kocide (871.5312) and Ridomil Gold (874.0793), respectively (Figure 3).
Effect of different fungicides on Rhizoctonia solani
Out of ten fungicides, only four fungicides resulted in 100% inhibition of Rhizoctonia solani at higher concentrations; these include Systhane at 1,000 ppm, Kocide at 800-1000 ppm, Nativo at 800-1000 ppm, and Evito at 800-1000 ppm. In comparison, Shincar at 1000 ppm caused 61.66% inhibition. More than 50% inhibition of R. solani has been observed in Nativo at 600 ppm, Evito at 600 ppm, Topsin M at 1000 ppm, and Aliette at 1000 ppm. All the remaining fungicides produced either no or less than a 50% reduction in colony growth compared to the control (Figure 4). In the present study, it was proved that R. solani was most sensitive against four different fungicides at higher concentrations. However, 50% inhibition of R. solani was calculated with different fungicides. The lowest LD50 value was recorded with Nativo (538.8762), followed by Evito (588.4488), Moncut (616.6486), Antracol (636.5374), Topsin M (639.1904), Kocide (669.9019), Shinar (682.1744), Ridomil Gold (735.9251), Systhane (759.8890) and Allie (819.613), respectively (Figure 5).
Effect of different fungicides on Verticillium albo-atrum
Against Verticillium albo-atrum, all tested fungicides caused a significant reduction in the colony growth. At all concentrations, two fungicides, Moncut and Shincar, resulted in 100% inhibition, followed by Evito 1000 ppm, which caused 86.6% inhibition. While Systhane at 800-1000 ppm, Kocide at 600-1000 ppm, Ridomil Gold at 1000 ppm, Antracol at 600-1000 ppm, Aliette at 600-1000 ppm, Nativo at 600-1000 ppm, Evito at 600-800 ppm, and Topsin M at 800-1000 ppm exhibited more than 60% inhibition. Additionally, Ridomil Gold at 800 ppm, Nativo at 400 ppm, Alliete at 400 ppm, Antracol at 400 ppm, and Evito at 400 ppm reduced the growth of V. albo-atrum by 50-57%. Other concentrations were less effective (Figure 6). However, the LD50 values of ten different fungicides for the 50% inhibition of V. albo-atrum were determined. The significantly minimum LD50 value was recorded with Moncut (107.5457), followed by Shinar (118.298), Alliete (457.8515), Evito (487.7099), Kocide (489.4386), Antracol (518.9959), Ridomil Gold (520.5342), Systhane (575.7236), Nativo (593.2692), and Topsin M (612.6594), respectively (Figure 7).
Soil-borne fungal pathogens are considered the most critical pathogens responsible for the enormous losses in chili crops. The use of fungicides against soil-borne diseases can help to manage the crop. In the present study, ten fungicides were used for their effectiveness against three soil-borne fungal pathogens. Several chemical pesticides and fungicides are available in the market and also reported in previous studies for reduce the mycelial growth of different fungal pathogens, such as carbendazim, carboxin, chlorothalonil, companion, copper oxychloride, mancozeb, metalaxyl, thiram, propiconazole azoxystrobin and iprodion (Hao et al., 2020). In addition, among all fungicides, two fungicides carbendazim and mancozeb have been reported highly effective control agents. Typically, fungicides function by employing a shared mechanism centered on microtubule polymerization. This approach effectively regulates fungal cell division, contributing to an efficient crop protection strategy (Abrar Ul Hassan et al., 2021). Among all fungicides, Nativo, Evito at 800-1000 ppm, and Systhane and Kocide at 1000 ppm proved to be the most effective against R. solani. Other workers are also found Nativo highly effective for R. solani (Persaud et al., 2019; Karkee and Mandal, 2020; Rashid et al., 2020). Evito and myclobutanil also successfully inhibited the growth of R. solani (Eliwa et al., 2021; Daniels and Latin, 2013; Davis et al., 1997). Carbendazim is one of the most influential and broad-spectrum systemic fungicides that inhibit a wide range of pathogens (Amini and Sidovich, 2010). In present studies, Systhane, Shincar (carbendazim), and Topsin M were highly effective against M. phaseolina. In another study, carbendazim caused the remarkable inhibition of M. phaseolina and F. oxysporum under in vitro conditions (Karibasappa et al., 2020; Dahal and Shrestha, 2018). Similarly, Topsin M (thiophanate methyl) is also well recognized broad-spectrum fungicide, which effective against large number of fungal pathogens (Nasir et al., 2012; Khanzada et al., 2005). Myclobutanil effectively controls soybean rust, anthracnose, and bacterial pustule (Sangawongse, 1991). Against V. albo-atrum, two fungicides, Moncut (Flutolanil) and Shincar (carbendazim), at all concentrations, proved excellent performance and caused complete inhibition of fungus. Carbendazim also found to check the growth V. chlamydosporium (De et al., 2009). It inhibited the conidial germination and colony growth
(Bhat et al., 2017). Siilarly, Hussain et al. (2020), found carbendazim is the most effective fungicides against alterneria solani, leading complete inhibition. Moncut is the most commonly used fungicide to control soil fungal diseases. Moncut fungicide is widely used for controlling rice sheath blight and other plant diseases caused by fungal pathogens (Motoba et al., 1988). Hirooka et al. (1990) reported that moncut fungicides reduced the complete mycelial growth of R. solani.
Conclusions and Recommendations
Significant variability in the effectiveness of fungicides has been noted. Only specific fungicides demonstrate efficacy against M. phaseolina, R. solani and V. albo-atrum. In areas experiencing high disease prevalence, substances such as Systhane, Shincar, Nativo, Antracol, Evito, and Topsin M are commonly employed. In the present study, 50% growth inhibition also check through LD50 formula and found that R. solani was highly sensitive against Nativo fungicides. Therefore, M. phaseolina was found to be the most sensitive pathogen against Shincar and Topsin M fungicides, while V. albo-atrum was noted with Moncut and Shincar fungicides.
Acknowledgements
We are deeply grateful to Dr. Abdul Mubeen Lodhi for supervising us and help in the experiments.
Novelty Statement
The present study reveals the response of three different soil-borne pathogens viz., Macrophomina phaseolina, Rhizoctonia solani and Verticillium albo-atrum to 10 old and novel synthetic fungicides.
Author’s Contribution
Nabeel Akhtar: Conducted this research and prepared the manuscript for publication.
Owais Iqbal: Reanalyze the data, revised and finalize the manuscript writing.
Imtiaz Ahmed Nizamani: Supervised and helped in experimental setup.
Rehana Naz Syed: Evaluated and revised the final version of manuscript.
Abdul Mubeen Lodhi: Analyzed, edited and approved the manuscript.
All authors have read and agreed to the published version of the manuscript.
Conflicts of interest
The authors have declared no conflict of interest.
References
Abdel-Fattah, G.M. and S.M. Al-Amri. 2012. Induced systemic resistance in tomato plants against Fusarium oxysporum f. sp. lycopersici by different kinds of compost. Afr. J. Biotechnol, 11(61): 12454-2463. https://doi.org/10.5897/AJB12.924
Abdel-Monaim, M.F., M.A. Abdel-Gaid and S.A. Zayan. 2014. Effectiveness of organic compounds in controlling root rots/wilts diseases, growth, and yields parameters of pepper. Int. J. Agric. Sci., 4(4): 143-150.
Abdel-Monaim, M.F. and M.E. Ismail. 2010. The use of antioxidants to control root rot and wilt diseases of pepper. Not. Sci. Biol., 2(2): 46-55. https://doi.org/10.15835/nsb223699
Abrar Ul Hassan, M., I. Butt, I.H., Khan, A. Javaid and N. Shad. 2021. Comparative efficacy of three fungicides for in vitro control of Curvularia lunata. Mycopath, 18(2): 47-52.
Amini, J., and D. Sidovich. 2010. The effects of fungicides on Fusarium oxysporum f. sp. lycopersici associated with Fusarium wilt of tomato. J. Plant Prot. Res., 50(2): 172–178. https://doi.org/10.2478/v10045-010-0029-x
Arain, M.S., 2015. Comparative efficacy of some synthetic insecticides against chili red spider mite, Tetranychus urticae (Koch) under field condition. TESK Pak., 30(1): 37-44.
Ashwini, N. and S. Srividya. 2016. Biocontrol of Rhizoctonia solani root rot of chilli by Bacillus subtilis formulations under pot conditions. Biol. Contr., 30(2): 109-118. https://doi.org/10.18641/jbc/30/2/91995
Baloch, M.S., N.A. Rajput, M. Atiq, A. Rehman, S.M. Khan, K. Naveed, S. Ullah, B. Khan and N.M. Baloch. 2018. Effect of different plant activators against Rhizoctonia solani causing root rot of chili. Pak. J. Phytopathol., 30(1): 45-51. https://doi.org/10.33866/phytopathol.030.01.0441
Barnett, H.L. and B.B. Hunter. 1972. Illustrated genera of imperfect fungi. Illustrated genera of imperfect fungi., (3rd ed).
Bashir, M.R., M. Atiq, M. Sajid, M. Mohsan, W. Abbas, M.W. Alam and M. Bashair. 2018. Antifungal exploitation of fungicides against Fusarium oxysporum f. sp. capsici causing Fusarium wilt of chilli pepper in Pakistan. Environ. Sci. Pollut. Res., 25(7): 6797-6801. https://doi.org/10.1007/s11356-017-1032-9
Bhat, M.A., Z.A. Bhat, N.A. Khan, F.A. Mohiddin and G.H. Mir. 2017. Response of fungicides against Verticillium fungicola and the host fungus agaricus bisporus under in vitro conditions. Pestic. Res. J., 29(1): 6-11.
Booth, C., 1971. The genus fusarium. Farnham: Commonwealth Agricultural Bureaux, Kew, Surrey, England pp. 237.
Cohen, R., M. Elkabetz, H.S. Paris, S. Freeman and A. Gur. 2022. Charcoal rot (Macrophomina phaseolina) across melon diversity: Evaluating the interaction between the pathogen, plant age and environmental conditions as a step towards breeding for resistance. Eur. J. Plant Pathol., 163(3): 601-613. https://doi.org/10.1007/s10658-022-02500-2
Dahal, N. and R.K. Shrestha. 2018. Evaluation of efficacy of fungicides against Fusarium oxysporum f. sp. lentis in vitro at Lamjung, Nepal. J. Inst. Agric. Anim., 35(1): 105-112. https://doi.org/10.3126/jiaas.v35i1.22520
Daniels, J.P. and R. Latin. 2013. Residual efficacy of fungicides for controlling brown patch on creeping bentgrass fairways. Plant Dis., 97(12): 1620-1625. https://doi.org/10.1094/PDIS-12-12-1130-RE
Davis, R.M., J.J. Nunez and K.V. Subbarao. 1997. Benefits of cotton seed treatments for the control of seedling diseases in relation to inoculum densities of Pythium species and Rhizoctonia solani. Plant Dis., 81(7): 766-768. https://doi.org/10.1094/PDIS.1997.81.7.766
De, S., P.K. Sanyal, A.K. Sarkar, N.K. Patel, S. Pal and S.C. Mandal. 2009. Effect of heavy metals and carbendazim on the in vitro growth of Paecilomyces lilacinus (Thom.) Samson and Verticillium chlamydosporium Goddard. Proc. Natl. Acad. Sci., 79(4): 393-398.
Divya Rani, V. and H. Sudini. 2013. Management of soilborne diseases in crop plants: An overview. Int. J. Plant Anim. Environ. Sci., 3(4): 156-164.
Eliwa, M.A., M.M.E.S. Aly and S.M. Saber. 2021. Control of root rot disease of sugar beet using certain antioxidants and fungicides. J. Phytopathol. Pest Manage., 8(1): 1-14.
Ellis, M.B., 1971. Dematiaceous hyphomycetes. Commonwealth Mycological Institute, Kew, Surrey, England. p. 608. ISBN 85198-027-9. https://doi.org/10.1079/9780851986180.0000
FAOSTAT. 2022. Statistics Division, Food and Agriculture Organization of the UN. Food and Agriculture Organization of the UN. https://www.fao.org/faostat/en/#data
Fradin, E.F. and B.P. Thomma. 2006. Physiology and molecular aspects of Verticillium wilt diseases caused by V. dahliae and V. albo-atrum. Mol. Plant Pathol., 7(2): 71-86. https://doi.org/10.1111/j.1364-3703.2006.00323.x
Ghimire, R., R.K. Shrestha and J. Shrestha. 2021. In vitro evaluation of fungicides against Fusarium solani, the causative agent of brinjal root rot. Indonesian J. Agric. Res., 4(03): 187-193. https://doi.org/10.32734/injar.v4i3.6275
Ghosh, T., M.K. Biswas, C. Guin and P. Roy. 2018. A review on characterization, therapeutic approaches and pathogenesis of Macrophomina phaseolina. Plant Cell Biotechnol. Mol. Biol., 19(3-4): 72-84.
Goldberg, N.P., 2010. Verticillium wilt of chile peppers. Cooperative Extension Service, College of Agricultural, Consumer and Environmental Sciences, New Mexico State University.
Hao, J.J., W.L. Zhu, Y.Q. Li, J.Z. Liu, S.N. Xie, J. Sun, Z.D. Dong. 2020. Efficacy and profitability of fungicide use to manage curvularia leaf spot of maize. Crop Protec., 132: 105126.
Hirooka, T., K. Haruo M. Yukio and K. Hitoshi. 1990. Effects of the systemic fungicide flutolanil on morphology of Rhizoctonia solani following inhibition of succinate oxidation. J. Pestic. Sci, 15(1): 47-53. https://doi.org/10.1584/jpestics.15.47
Husain, A., M.M. Rashid, N. Akhtar, A. Muin and G. Ahmad. 2020. In-vitro evaluation of fungicides at different concentrations against Alternaria solani causing early blight of potato. J. Pharma. Phytochem., 9(4): 1874-1878. https://doi.org/10.22271/phyto.2020.v9.i4z.12026
Hussain, F. and M. Abid. 2011. Pest and diseases of chilli crop in Pakistan: A review. Int. J. Biol. Biotech., 8(2): 325-332.
Hussain, F., S.S. Shaukat, M. Abid, F. Usman and M. Akbar. 2013. Pathogenicity of some important root rot fungi to the chilli crop and their biological control. Int. J. Biol. Biotechnol., 10(1): 101-108.
Jabeen, K., A. Younas, S. Iqbal and S. Javed. 2016. Effect of Macrophomina phaseolina on germination, growth and physiology of Capsicum frutescens L. Pak. J. Phytopathol., 28(2): 207-211.
Jacobsen, B.J., N.K. Zidack and B.J. Larson. 2004. The role of Bacillus-based biological control agents in integrated pest management systems: Plant diseases. Phytopathology, 94(11): 1272-1275. https://doi.org/10.1094/PHYTO.2004.94.11.1272
Jadon, K.S. and R. Shah. 2012. Effect of Drechslera bicolor infection on physiology of bell pepper. J. Plant Pathol. Microb., 3(4): 1-4. https://doi.org/10.4172/2157-7471.1000126
Karibasappa, C.S., B.N. Bhat and S.C. Rao. 2020. Evaluation of potential fungicides, botanicals, biocontrol agents and their combination against M. phaseolina inciting root rot of sesame. Int. J. Chem. Stud., 8(2): 1765-1771. https://doi.org/10.22271/chemi.2020.v8.i2aa.9016
Karkee, A. and D.L. Mandal. 2020. Efficacy of fungicides against Rhizoctonia solani inciting rhizome rot diseases on large cardamom (Amomum subulatum Roxb.). Int. J. Appl. Sci. Biotechnol., 8(1): 61-64. https://doi.org/10.3126/ijasbt.v8i1.27240
Kavitha, K., S. Mathiyazhagan, V. Senthilvel, S. Nakkeeran and G. Chandrasekar. 2005. Development of formulations of antagonistic bacteria for the management of damping-off of Chilli (Capsicum annum L). Arch. Phytopathol., 38(1): 19-30. https://doi.org/10.1080/03235400400008382
Khanzada, M.A., A.M. Lodhi and S. Shahzad. 2005. Chemical control of Lasiodiplodia theobromae, the causal agent of mango decline in Sindh. Pak. J. Bot., 37(4): 1023-1030.
Kongkachuichai, R., R. Charoensiri, K. Yakoh, A. Kringkasemsee and P. Insung. 2015. Nutrients value and antioxidant content of indigenous vegetables from Southern Thailand. Food Chem., 173: 838-846. https://doi.org/10.1016/j.foodchem.2014.10.123
Maitlo, W.A., G.S. Markhand, A. Abul-Soad, A.M. Lodhi and M. Jatoi. 2013. Chemical control of sudden decline disease of date palm (Phoenix dactylifera L.) in Sindh, Pakistan. Pak. J. Bot., 45(S1): 7-11.
Mihajlović, M., E. Rekanović, J. Hrustić, M. Grahovac and B. Tanović. 2021. In vitro and in vivo toxicity of fungicides and biofungicides for the control of Verticillium and Fusarium wilt of pepper. Pestic. Fitomed., 36(1): 23-34. https://doi.org/10.2298/PIF2101023M
Motoba, K., M. Uchida and E. Tada. 1988. Mode of antifungal action and selectivity of flutolanil. Agric. Biol. Chem., 52(6): 1445-1449. https://doi.org/10.1080/00021369.1988.10868900
Nahar, M.N. and S. Shamsi. 2020. In vitro screening of fungicides and plant extracts against six pathogenic fungi isolated from cotton (Gossypium arboreum L.) seed. Bangl. J. Bot., 49(2): 197-204. https://doi.org/10.3329/bjb.v49i2.49292
Nasir, A.R., A.P. Mumtaz, M.L. Abdul, D. Daolong, L. Tingli, S.A. Muhammad and U.R. Faheem. 2012. In vitro evaluation of various fungicides against Fusarium solani isolated from Dalbergia sissoo dieback. Afr. J. Microbiol. Res., 6(27): 5691-5699. https://doi.org/10.5897/AJMR12.795
Olatunji, T.L. and A.J. Afolayan. 2018. The suitability of chili pepper (Capsicum annuum L.) for alleviating human micronutrient dietary deficiencies: A review. Food Sci. Nutr., 6(8): 2239-2251. https://doi.org/10.1002/fsn3.790
Orobiyi, A., M. Dansi, P. Assogba, L.Y. Loko, R.S. Vodouhe, A. Akouegninou and A. Sanni. 2013. Chili (Capsicum annuum L.) in southern Benin: Production constraints, varietal diversity, preference criteria and participatory evaluation. Int. Res. J. Agric. Sci. Soil Sci., 3(4): 107-120.
Pakdeevaraporn, P., S. Wasee, P.W.J. Taylor and O. Mongkolporn. 2005. Inheritance of resistance to anthracnose caused by Colletotrichum capsici in Capsicum. Plant Breed, 124(2): 206-208. https://doi.org/10.1111/j.1439-0523.2004.01065.x
Persaud, R., A. Khan, W.A. Isaac, W. Ganpat and D. Saravanakumar. 2019. Plant extracts, bioagents and new generation fungicides in the control of rice sheath blight in Guyana. Crop Prot., 119: 30-37. https://doi.org/10.1016/j.cropro.2019.01.008
Rashid, M.M., M.R. Bhuiyan, H.A. Dilzahan, M.A. Hamid, N. Hasan, M.A.I. Khan and M.A. Latif. 2020. Biological control of rice sheath blight disease (Rhizoctonia solani) using bio-pesticides and bio-control agents. Bangladesh Rice J., 24(1): 47-58. https://doi.org/10.3329/brj.v24i1.53239
Sangawongse, P., 1991. Efficacy of certain fungicide treatments for the control of seed-borne diseases of soybean. Wichai lae songsoem wichakan kaset.
Singh, R.S., 1982. Plant pathogens. The fungi.
Sivalingam, P.N., S.N. Vishwakarma and U.S. Singh. 2006. Role of seed-borne inoculum of Rhizoctonia solani in sheath blight of rice. Ind. Phytopathol., 59(4): 445.
Tripodi, P. and S. Kumar. 2019. The capsicum crop: An introduction. In: The capsicum genome. Springer, Cham. pp. 1-8. https://doi.org/10.1007/978-3-319-97217-6_1
Van Bruggen, A.H., A. Gamliel and M.R. Finckh. 2016. Plant disease management in organic farming systems. Pest Manage. Sci., 72(1): 30-44. https://doi.org/10.1002/ps.4145
Varma, S., D.R. Kumhar and A.K. Meena. 2020. Integrated disease management of rhizoctonia root rot of chilli (Capsicum annum L.) incited by Rhizoctonia solani Kuhn in vivo. Int. J. Curr. Microbiol. App. Sci., 9(4): 1635-1642. https://doi.org/10.20546/ijcmas.2020.904.192
Vincent, J.M., 1947. Distortion of fungal hyphae in the presence of certain inhibitors. Nature, 159(4051): 850-850. https://doi.org/10.1038/159850b0
Zulfitri, A., N.P.R.A. Krishanti, A.S. Lestari, D. Meisyara and D. Zulfiana. 2020. Efficacy of several entomopathogenic microorganism as microbial insecticide against insect pest on chili (Capsicum annum L.). In: IOP Conf. Ser. Earth Environ. Sci., 572(1): 012020. https://doi.org/10.1088/1755-1315/572/1/012020
To share on other social networks, click on any share button. What are these?








