Combined Effect of Pseudomonas fluorescens and Arbuscular Mycorrhizal Fungi on Meloidogyne incognita Infecting Solanum melongena L.
Combined Effect of Pseudomonas fluorescens and Arbuscular Mycorrhizal Fungi on Meloidogyne incognita Infecting Solanum melongena L.
Wafaa M. A. El-Nagdi1*, Ahmed E.A. Mahgoob2, Mahmoud M.A. Youssef1, Mona M.S. Zayed3, Entesar H. Taha2 and Nora R.A. Saleh1
1Plant Pathology Department, Nematology Laboratory, National Research Centre, Dokki, 12622, Cairo, Egypt; 2Plant Protection Department, Faculty of Agriculture, Ain Shams Univ., P.O. Box 68, Hadayek Shoubra 11241, Cairo, Egypt; 3Microbiology Department, Faculty of Agriculture, Ain Shams Univ., P.O. Box 68, Hadayek Shoubra 11241, Cairo, Egypt.
Abstract | Sole and consortium treatments of four isolates of Pseudomonas fluorescens (Pf1, Pf2, Pf9 and Pf10) with arbuscular endomycorrhizal fungi (AMF) on root-knot nematode, Meloidogyne incognita (Ne) on eggplant (Solanum melongena L.) were evaluated. In general, the combined treatments by AMF and Pf isolates decreased significantly (P≤0.05) root-knot nematode as indicated by numbers of egg masses, eggs and galls per root system more than single treatments. Co-toxicity% for the two applied combined treatments of each bacterial isolate+ AMF, added at the same time with nematode (Ne) or added at 7 days before Ne inoculation, showed additive or synergistic interaction effects. The tested isolates increased significantly (p≤0.05) plant growth criteria according to the tested materials. The same isolates +AMF with Ne at the same time recorded less colonization percentage (35%), while 40% root colonization was achieved when added at 7 days before nematode inoculation. Also, when AMF alone was applied at 7 days before nematode inoculation, it caused the highest percentage of colonization (60%). It could be concluded that the consortium treatment with AMF and P. fluorescens significantly decreased M. incognita on eggplant more than single treatments.
Received | January 24, 2024; Accepted | April 12, 2024 ; Published | May 06, 2024
*Correspondence | Wafaa M. A. El-Nagdi, Plant Pathology Department of, Nematology Laboratory, National Research Centre, Dokki, 12622, Cairo, Egypt; Email: wafaaeolnagdi@yahoo.com
Citation | El-Nagdi, W.M.A., Mahgoob, A.E.A., Youssef, M.M.A., Zayed, M.M.S., Taha, E.H. and Saleh, N.R.A., 2024. Combined effect of Pseudomonas fluorescens and arbuscular mycorrhizal fungi on Meloidogyne incognita infecting Solanum melongena L. Pakistan Journal of Nematology, 42(1): 39-48.
DOI | https://dx.doi.org/10.17582/journal.pjn/2024/42.1.39.48
Keywords | Biocontrol, Pseudomonas fluorescens, Arbuscular endomycorrhizal fungi, Meloidogyne incognita, eggplant, Screenhouse trial
Copyright: 2024 by the authors. Licensee ResearchersLinks Ltd, England, UK.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Introduction
Eggplant (Solanum melongena L.) is an important vegetable crop in Egypt. It is highly susceptible to soil borne diseases. Root-knot nematode, Meloidogyne incognita infests eggplant and causes losses in yield and fruit quality (Sikora and Fernandez, 2005; Youssef and Korayem, 2008; El-Nagdi and El-Khair, 2008).
As reviewed by Youssef and El-Nagdi (2015) that the most cultivated plant species can be colonized by arbuscular mycorrhizal fungi (AMF) as obligate symbionts. The same area of the rhizosphere of host plants can be infected by plant-parasitic nematodes, especially endo-parasitic nematodes and AMF, often survive simultaneously and interact with each other. The effect of AMF on root-knot nematodes can be suggested on the bases, competition for the same space and nutrients and induced systemic resistance (ISR) in the host plant by these microorganisms through changing the biological and chemical status of the rhizosphere. Schouteden et al. (2015) reported the same mechanisms for AMF. The importance of ISR in biocontrol was clarified by recent studies. Hayder et al. (2014) showed that the efficacy of certain rhizobacteria (P. fluorescens, B. subtilis, Azotobacter spp.), and mycorrhizal fungus (Glomus fasciculatum) solely or in co-treatments on the reproduction of M. incognita and growth of brinjal, as they significantly reduced the number of galls as well as second stage juveniles (J2s). Also, plant growth was improved over control and sole treatments of rhizobacteria or mycorrhizal fungus. Utobo et al. (2016) studied the effect of AMF (Glomus intraradices), either alone or in combination with P. fluorescens (Pf) and M. incognita (Ne) on tomato plant. Root-knot nematode was added to AMF inoculated under ambient conditions. The results showed that shoot dry weight was significantly better in the treatments of (AMF + Pf +Ne) > (AMF+ Ne) than in the control and sole treatment. The results indicated that, the consortium application of AMF + Pf was better for reducing of root- knot nematode.
The aim of this investigation was to study effect of selected four P. fluorescens (Pf) isolates, when they were added as sole or in consortium with endo arbuscular mycorrhizal fungi (AMF) on root-knot nematode, Meloidogyne incognita reproduction, eggplant growth criteria and root colonization rate of AMF.
Materials and Methods
Isolation and identification of Pseudomonas fluorescens (Pf)
Four isolates of Pf (Pf1, Pf2, Pf9, Pf10) were isolated and identified according to Ghini et al. (2007); Schaad (1980); Lelliot and Stead (1987) and Goszczynska et al. (2000). Bacterial inoculum for each isolate was justified to 107-109 colony forming unit (CFU)/ml by turbidity method as described by Baird et al. (2000) and was used in the form of mixture of bacterial cells and culture filtrate as cited in previous study by Saleh et al. (2020).
Preparation of arbuscular endomycorrhizal fungi (AMF) inoculum
Endomycorrhizal spores were originally extracted from soil around roots of maize plants grown in the Experimental Field of Faculty of Agriculture, Ain Shams University, Shoubra El-Kheima, Cairo, Egypt. Spores were extracted by using the wet sieving and decanting technique as described by Gerdemann and Nicolson (1963). Spores were reared on eggplant roots as stock culture. To obtain the AMF inoculum, fresh roots of eggplant colonized with endomycorrhizae that exhibited 90% mycorrhizal infection were excised. Mycorrhizae- infected root extract (MIRE) was prepared using the method described by Sharma et al. (2005).
Pot experiment design
The experiment was carried out in pots at screenhouse of Plant Pathology Department, National Research Centre. Seeds of eggplant cv. Ice were sown in nursery tray for a month. After 15 days, 4- week old one eggplant seedling was transplanted in each pot (25-cm diameter) containing 2kg of solarized sandy loamy soil. Each pot was inoculated with 1,000 newly emerged juveniles (J2s) of M. incognita (Ne) in four holes made around the plant. Eggplants were treated with four isolates of P. fluorescens (Pf1, Pf2, Pf9 and Pf10) that proved more effect on root-knot nematode in previous study (Saleh et al., 2020). Each replicate contained a mixture of bacterial cells cultures and filtrates at the tested rate of 30 ml/pot (10-7-10-9 colony forming unit (CFU)/ml). Arbuscular mycorrhizal fungi (AMF) at the rate of 2.5ml/seedling (100 spores/ml) and the tested isolates either alone or in combination were divided into five groups according to type of treatments (Table 1).
Pots were arranged in a completely randomized design with 8 replicates per each treatment on a bench under screenhouse conditions maintained at 30 ± 5 °C. Then, the plants were irrigated as needed. Four months of nematode inoculation (at harvest stage), nematode parameters as numbers of egg masses and eggs and galls/root system were counted on roots. Also, at the same time, plant growth criteria of eggplant including shoot length (cm), fresh and dry shoot weights (g) and fresh root weight (g) were recorded. Plant measurements were compared to those of untreated check (Control 1) which received nematode only. Those plants without nematode inoculation were compared to healthy plants (Control 2).
Table 1: Effect of four isolates of Pseudomonas fluorescens (Pf1, Pf2, Pf9, Pf10) and arbuscular mycorrhizal fungi (AMF) on root-knot nematode, Meloidogyne incognita (Ne) infecting eggplant.
|
Treatments/type |
No. of nematode reproductive parameters/ root system |
No. of galls/ root system |
|
|
Egg masses |
Eggs |
||
|
Group 3 |
|||
|
Bacterial isolates at the same time with Ne |
|||
|
Pf1+ Ne |
20c |
142bc |
84d |
|
Pf2+ Ne |
35bc |
288b |
104cd |
|
Pf9 + Ne |
57b |
72c |
173b |
|
Pf10+ Ne |
35bc |
217bc |
120b-d |
|
Group 4 |
|||
|
Bacterial isolates + AMF at the same time with Ne |
|||
|
Pf1+AMF+ Ne |
17c |
167bc |
57d |
|
Pf2+AMF + Ne |
24c |
88bc |
92cd |
|
Pf9+AMF+ Ne |
27c |
86bc |
90cd |
|
Pf10+AMF+ Ne |
35bc |
153bc |
81d |
|
AMF only+ Ne |
9c |
59c |
72d |
|
Group 5 |
|||
|
Bacterial isolates at 7 days before Ne |
|||
|
Pf1+AMF+Ne |
60b |
65c |
151bc |
|
Pf2+AMF +Ne |
31bc |
146bc |
90cd |
|
Pf9+AMF+Ne |
25c |
110bc |
78d |
|
Pf10+AMF +Ne |
23c |
51c |
64d |
|
AMF only+ Ne |
26c |
143bc |
86d |
|
Nematode only (control 1) |
201a |
810a |
460a |
-Values are means of 8 replicates. Values followed by same letter(s) are not significantly (P ≤ 0.05) different according to duncan’s multiple range test.
For comparison among treatments, averages total percentage of nematode reduction and plant growth increase of all treatments were considered. Overall average percentages was calculated to compare among treatments in different groups by dividing sum average total percentages /number of treatments in each group.
Interaction and co-toxicity of Pf and AMF
Interaction of mixtures of Pf and AMF based on the total percentages nematode reduction at harvest stage according to Lempel’s formula was reported by Richer (1987).
The expected effect was compared with observed effect obtained experimentally for mixture to determine co-toxicity effect according to the equation given by Mansour et al. (1966) as follows:
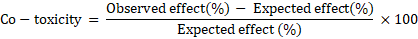
Co-toxicity was used to classify the results into three categories, where a positive factor 20 or more is considered potentiation, a negative factor -20 or more is considered antagonistic and immediate values between -20 to + 20 indicate additive.
Estimation percentage of mycorrhizal colonization on eggplant roots
Inoculated eggplant roots were collected after four months from endomycorrhizal infection. The percentage of root colonization with endomycorrhizae was estimated by the method described by Phillips and Hayman (1970). The root system per pot was washed thoroughly with tap water several times to remove adhering soil particles. The roots were cut into small segments and treated with 10% potassium hydroxide (KOH) in test tubes and heated in water path for 10 minutes at 80-90oC.Thereafter, the root segments were washed with tap water followed by 10% hydrochloric acid (HCL), then stained by trypan blue solution in lactic acid 0.5% and heated again at 80-90oC for 5 minutes. The root segments were picked up and placed on glass slides. Few drops of lactic acid were added. Mycorrhizal infection was recorded in each segment in order to calculate percentage of root colonization.
Statistical analysis
To statistically analyze data, analysis of variance (ANOVA) test was used for the obtained data and means were separated on the basis of Duncan’s Multiple Range test using MSTAT programme version 4.
Results and Discussion
Impact of AMF and/or Pf isolates on root-knot nematode, Meloidogyne incognita (Ne)
Table 1 illustrated the tested bacterial isolates of Pf and/or AMF to biocontrol root-knot nematode, M. incognita on eggplant. It was clearly noticed that, the tested isolates adversely affected reproduction of nematode as indicated by numbers of egg masses and eggs as well as number of galls per root system.
Table 2: % Reduction of Meloidogyne incognita (Ne) criteria on eggplant as affected by four isolates of Pseudomonas fluorescens (Pf) and Arbuscular endomycorrhizal fungi (AMF) either alone or in combinations.
|
Treatments/ Type |
% Nematode reproductive parameters reduction |
|||
|
Egg masses |
Eggs |
% Average total percentages reduction |
Galls |
|
|
Group 3 |
||||
|
Bacterial isolates only added at the same time with Ne |
||||
|
Pf1 + Ne |
90.0 |
82.5 |
86.3 |
81.7 |
|
Pf2 + Ne |
82.6 |
64.4 |
73.5 |
77.4 |
|
Pf9+ Ne |
71.6 |
91.1 |
81.4 |
62.4 |
|
Pf10+ Ne |
82.6 |
77.9 |
80.3 |
73.9 |
|
Overall average percentages |
- |
- |
80.4 |
73.9 |
|
Group 4 |
||||
|
Bacterial isolates +AMF added at the same time with Ne |
||||
|
Pf1+AMF + Ne |
91.5 |
79.4 |
85.5 |
87.6 |
|
Pf2+AMF+ Ne |
88.1 |
89.1 |
88.6 |
80.0 |
|
Pf9+AMF + Ne |
86.6 |
89.4 |
88.0 |
80.4 |
|
Pf10+AMF + Ne |
82.6 |
81.1 |
81.9 |
82.4 |
|
Overall average percentages |
- |
- |
86.0 |
82.6 |
|
AMF only+ Ne |
95.5 |
92.7 |
94.1 |
84.3 |
|
Group 5 |
||||
|
Bacterial isolates+ AMF added at 7 days before Ne |
||||
|
Pf1+AMF +Ne |
70.1 |
92.0 |
81.1 |
67.2 |
|
Pf2+AMF+Ne |
84.6 |
82.0 |
83.3 |
80.4 |
|
Pf9+AMF +Ne |
87.6 |
86.4 |
87.0 |
83.0 |
|
Pf10+AMF+Ne |
88.6 |
93.7 |
91.2 |
86.1 |
|
Overall average percentages |
- |
- |
85.7 |
79.2 |
|
AMF only+ Ne |
87.1 |
82.3 |
84.7 |
81.3 |
|
Nematode only (Control 1) |
0.0 |
0.0 |
0.0 |
0.0 |
In general, the combined treatments by AMF and Pf decreased the numbers of M .incognita and galls more than those of their single treatments. In Table 2, in single treatments with Pf+ Ne recorded 86.3, 73.5, 81.4 and 80.3% nematode reductions with an overall percentage reduction of 80.4% only. However, Pf1, Pf2, Pf9 and Pf10 alongside AMF, as combined treatments, when they were added at the same time with nematode inoculation achieved percentages nematode reduction, 85.5, 88.6, 88.0 and 81.9%, respectively with an overall average of 86.0% which were almost equal to the combined treatments of Pf +AMF at 7 days prior Ne, with an overall averages of 85.7%. On the other hand, AMF only, when inoculated with Ne at the same time recorded the highest average percentage nematode reduction of 94.1% as compared to untreated control. Whereas when AMF only was added, 7 days prior to Ne, registered nematode reduction of 84.7%. Number of galls followed the same trend, as the combined treatments of bacterial isolates + AMF when, either added at the same time or 7 days before Ne inoculation achieved higher overall percentages gall reductions (82.6% and 79.2%, respectively) than that of each bacterial isolate + Ne only (73.9%).
Impact on interaction between Pf and AMF
Co-toxicity% influence was based on the applied combined treatments of bacterial isolates+ AMF+ Ne added at the same time and bacterial isolates +AMF, added at gap 7 days before Ne inoculation. It showed additive or synergistic interaction effects for nematode reductions on eggplant (Table 3).
Table 3: Type of interactions of some bacterial isolates (Pf) and arbuscular endomycorrhizal fungi (AMF) mixtures on root-knot nematode, Meloidogyne incognita (Ne) infecting eggplant.
|
Treatment/Type |
Effect based on average total percentage nematode reductions |
Co- toxicity % |
Type of interaction |
|
|
Expected |
Observed |
|||
|
Group 4 |
||||
|
Bacterial isolates added at the same time with Ne |
||||
|
Pf1+AMF+Ne |
99.2 |
85.5 |
-13.8 |
Additive or synergistic |
|
Pf2+AMFN+e |
98.4 |
88.6 |
-10.0 |
Additive or synergistic |
|
Pf9+AMF+Ne |
98.9 |
88.0 |
-11.0 |
Additive or synergistic |
|
Pf10+AMF+Ne |
98.8 |
81.9 |
- 17.1 |
Additive or synergistic |
|
Group 5 |
||||
|
Bacterial isolates added at7days prior to Ne |
||||
|
Pf1+AMF+Ne |
97.9 |
81.1 |
-17.2 |
Additive or synergistic |
|
Pf2+AMF+Ne |
95.9 |
83.3 |
13.1 |
Additive or synergistic |
|
Pf9+AMF+Ne |
97.2 |
87.0 |
10.5 |
Additive or synergistic |
|
Pf10+AMF+Ne |
97.0 |
91.2 |
- 6. |
Additive or synergistic |
Impact of Pf isolates and AMF on the parameters of eggplant growth infected by Ne
Tables 4 and 5 showed the mean values of shoot length, fresh and dry weights and fresh weight of roots of eggplant and percentage of AMF colonization on eggplant roots affected by the Pf and AMF in absence or presence of root- knot nematode, M. incognita and mycorrhizal root colonization%. It was clearly noticed that, the tested isolates significantly (P ≤ 0.05) increased plant growth criteria as indicated by shoot length, fresh and dry weights and root fresh weight according to the tested materials.
Table 4: Growth parameters of eggplant infected with or without root-knot nematode, Meloidogyne incognita (Ne) as affected by four isolates of Pseudomonas fluorescens (Pf) and arbuscular endomycorrhizal fungi (AMF) either alone or in combinations.
|
Treatments/Type |
Plant growth criteria/plant |
AMF root colonization (%) |
|||
|
Shoot |
Root |
||||
|
Length (cm) |
Fresh weight (g) |
Dry weight(g) |
Fresh weight (g) |
||
|
AMF only |
26.2b-g |
11.1b-f |
5.4a |
10.4a-f |
40.0 |
|
AMF + Ne added at the same time |
24.0f-h |
10.4b-g |
2.8ij |
7.1d-i |
20.0 |
|
AMF added at 7days Prior to Ne |
25.7c-g |
12.6a-e |
5.1ab |
9.3a-h |
60.0 |
|
Group 1 |
|||||
|
Bacterial isolates only |
|||||
|
Pf1only |
29.0a-e |
11.4b-f |
3.6d-i |
10.1a-f |
- |
|
Pf2 only |
27.0b-g |
9.6b-g |
2.7ij |
7.4d-i |
- |
|
Pf9 only |
32.3a |
17.4a |
4.8a-d |
11.1a-d |
- |
|
Pf10only |
31.0ab- |
13.4a-c |
3.9b-h |
8.6b-h |
- |
|
Average percentages of colonization |
- |
- |
- |
- |
- |
|
Group 2 |
|||||
|
Bacterial isolates +AMF |
|||||
|
Pf 1+AMF |
28.5a-e |
13.7a-c |
3.0g-j |
9.6a-g |
60.0 |
|
Pf2+AMF |
29.1a-e |
14.6ab |
4.6a-e |
10.2a-f |
40.0 |
|
Pf9+AMF |
26.1c-g |
10.2b-g |
2.2j |
6.7e-i |
30.0 |
|
Pf10+AMF |
29.5a-d |
12.1b-f |
4.4a-f |
9.1a-h |
20.0 |
|
Average percentages of colonization |
- |
- |
- |
- |
37.5 |
|
Group 3 |
|||||
|
Bacterial isolates added at the same time with Ne |
|||||
|
Pf1+Ne |
27.2a-g |
13.7a-c |
3.8c-i |
12.6ab |
|
|
Pf2+Ne |
26.9b-g |
11.6b-f |
2.8h-j |
8.2c-I |
|
|
Pf9+Ne |
27.0b |
13.0a-c |
3.7d-i |
8.0c-i |
|
|
Pf10+Ne |
30.5a-c |
13.3a-c |
4.1a-h |
9.7a-f |
|
|
Group 4 |
|||||
|
Bacterial isolates +AMF added at the same time with Ne |
|||||
|
Pf1+AMF+Ne |
27.8a-f |
10.9b-g |
3.6d-i |
6.7e-i |
30.0 |
|
Pf2+AMF+Ne |
25.9c-g |
9.4c-g |
3.4d-j |
6.2f-k |
20.0 |
|
Pf9+AMF+ne |
22.8gh |
7.7e-h |
3.8ab |
5.6g-k |
20.0 |
|
Pf10+AMF+Ne |
25.3d-g |
8.2d-h |
3.2e-j |
5.3h-k |
70.0 |
|
Average percentages of colonization |
- |
- |
- |
- |
35.0 |
|
Group 5 |
|||||
|
Bacterial isolates +AMF added at 7 days prior to Ne |
|||||
|
Pf1+AMF +Ne |
28.81-f |
13.3a-c |
5.1ab |
10.6a-e |
50.0 |
|
Pf2+AMF +Ne |
29.8c-g |
12.6a-e |
4.2a-g |
11.6a-c |
50.0 |
|
Pf9+AMF+ Ne |
24.0f-h |
11.8b-f |
3.2f-j |
5.5g-k |
30.0 |
|
Pf10+AMF+ Ne |
28.3a-f |
12.6a-e |
5.1ab |
12.8a |
30.0 |
|
Average percentages of colonization |
- |
- |
- |
- |
40.0 |
|
Nematode only (Control 1) |
20.0h |
3.8h |
2.8ij |
2.6jk |
- |
|
Healthy plant (Control 2) |
26.8b-g |
5.9gh |
4.1a-h |
2.4k |
- |
-Values are means of 8 replicates. Values followed by same letters are not significantly (P ≤ 0.05) different according to duncan’s multiple range test.
Table 5: Increase% of eggplant growth infected with or without M. incognita (Ne) as affected by four isolates of P. fluorescens (Pf) and arbuscular endomycorrhizal fungi (AMF) alone or in combinations.
|
Treatment/ Type |
% Increase of plant growth parameters |
%Average total plant growth increases |
|||
|
Shoot |
Root |
||||
|
Length (cm) |
Fresh weight(g) |
Dry weight(g) |
Fresh weight (g) |
||
|
AMF only |
- |
88.1 |
32.0 |
333.3 |
113.4 |
|
AMF + Ne added at the same time with Ne |
20.0 |
173.7 |
00.0 |
173.1 |
91.7 |
|
AMF+ Ne added at,7days Prior to Ne |
28.5 |
231.6 |
82.1 |
257.7 |
214.1 |
|
Group 1 |
|||||
|
Bacterial isolates only |
|||||
|
Pf1*only |
8.0 |
93.0 |
- |
321.0 |
10 |
|
Pf2*only |
1.0 |
63.0 |
- |
208.0 |
68.0 |
|
Pf9*only |
21.0 |
195.0 |
17.1 |
363.0 |
149.0 |
|
Pf10*only |
16.0 |
127.0 |
- |
258.0 |
100.3 |
|
Overall average percentages |
- |
105.7 |
|||
|
Group 2 |
|||||
|
Bacterial isolates+ AMF |
6.0 |
132.0 |
- |
300.0 |
109.5 |
|
Pf1+AMF |
9.0 |
- |
|||
|
Pf2+AMF |
- |
147.5 |
- |
325.0 |
123.4 |
|
Pf3+AMF |
- |
73.0 |
12.0 |
179.0 |
63.0 |
|
Pf4+AMF |
10.1 |
105.0 |
- |
279.0 |
100.3 |
|
overall average percentages |
- |
- |
7.0 |
- |
99.1 |
|
Group 3 |
|||||
|
Bacterial isolates added at the same time with Ne |
|||||
|
Pf1+Ne |
36.0 |
260.5 |
35.7 |
384.6 |
179.2 |
|
Pf2+Ne |
34.5 |
205.3 |
00.0 |
215.4 |
113.9 |
|
Pf3+Ne |
35.0 |
242.1 |
32.1 |
207.7 |
129.2 |
|
Pf10+Ne |
52.5 |
250.0 |
46.4 |
273.1 |
155.5 |
|
Overall average percentages |
- |
- |
- |
- |
144.3 |
|
Group 4 |
|||||
|
Bacterial isolates+ AMF added at the same time with Ne |
|||||
|
Pf1+AMF +Ne |
39.0 |
186.4 |
28.6 |
157.7 |
102.9 |
|
Pf2+AMF +Ne |
29.5 |
147.4 |
21.4 |
138.5 |
84.2 |
|
Pf9+AMF+Ne |
14.0 |
102.6 |
35.7 |
115.4 |
38.1 |
|
Pf10+AMF+ Ne |
26.5 |
115.8 |
14.3 |
103.8 |
65.1 |
|
Overall average percentages |
- |
- |
- |
- |
72.6 |
|
Group 5 |
|||||
|
Bacterial isolates +AMF added at 7 days prior to Ne |
|||||
|
Pf1+AMF +Ne |
44.0 |
250.0 |
82.1 |
307.7 |
171.0 |
|
Pf2+AMF + Ne |
49.0 |
231.6 |
50.0 |
246.2 |
169.2 |
|
Pf9+AMF+Ne |
20.0 |
521.1 |
14.3 |
111.5 |
166.7 |
|
Pf10+AMF+Ne |
41.5 |
168.4 |
82.1 |
292.3 |
146.1 |
|
Overall average percentages |
- |
- |
- |
- |
163.2 |
|
Ne only(Control 1) |
0.0 |
0.0 |
0.0 |
0.0 |
0.0 |
|
Healthy plant(Control 2) |
0.0 |
0.0 |
0.0 |
0.0 |
0.0 |
-Values are means of 8 replicates. *Treatments without nematodes were compared to healthy plants.
Table 5 clarified the averages total percentages plant growth increases as affected by treatments of certain bacterial isolates of Pf and AMF either alone or in combination. These averages were used to compare different values among treatments. In single treatments, AMF alone recorded an average of total percentage increase of 113.4% compared to AMF+ Ne which caused 91.7% increase only. But AMF, added at 7 days prior Ne achieved the highest plant growth increase of 214.1%. Pf1, Pf2, Pf9 and Pf10 as single without Ne caused averages total percentages plant growth increases of 105.5, 68.0, 149.0, and 100.3%, respectively, with an overall averages percentage of 105.7%. The combined treatments of Pf1, Pf2, P9 and P10 + AMF without nematodes caused less percentages of increases of 109.5, 123.4, 63.0 and 100.3, respectively with an overall average percentage of 99.1% compared to healthy plants (Control 2). The respective bacterial isolates singly plus Ne recorded averages percentages of 179.2, 113.9, 129.2 and 155.5% in relation to plants inoculated with nematode only (Control 1) with an overall average percentage of increase of 144.3%. On the other hand, the combined treatments of Pf1,Pf2,Pf9 and Pf10 plus AMF, added at 7 days before nematode inoculation caused the highest averages percentage of increase of 171.0, 169.2, 166.7 and 146.1%., respectively with an overall average percentage increase of 163.2%. However, bacterial isolates + AMF + Ne inoculated at the same time caused the least averages total percentage of increase of 102.9, 84.2, 38.1 and 65.1%, respectively with an overall average percentage increase of 72.6% compared to plants inoculated with nematode only (Control 1).
Impact of Pf isolates on AMF root colonization with or without Ne
Regarding AMF root colonization, Data given in Table 4 indicated that, Pf isolates + AMF without nematode recorded an average percentage of root colonization of 37.5%. The same isolates +AMF with Ne recorded less average of 35%. However, bacterial isolates +AMF, inoculated at 7 days before nematode inoculation achieved higher root colonization of 40%. Also, when AMF alone was applied at 7 days before Ne inoculation, caused the highest percentages of colonization of 60% than that caused by AMF+ Ne together (20%). AMF alone without Ne caused an intermediate value.
Several microorganisms, such as AMF, parasitic fungi and plant growth promoting rhizobacteria (PGPR) have different action mechanisms, either sole or in consortium treatment and could protect the plants against nematodes, where they provide a stable and more effective rhizosphere community over a wide range of environmental conditions (Sikora et al., 2007). Plant nutrition, growth and protection of plants against pathogens can be improved by AMF and bacteria by interacting positively with each other. Thus, when co-applied, they became usually more effective than alone (Liu et al., 2012; Talavera et al., 2012). Vigorous growth and productivity due to positive synergistic interactions between AMF and PGPR were recorded by Siddique and Akhtar (2009) which was similar to the present results. Utobo et al. (2016) reported that, shoot dry weight of tomato was significantly better in the treatments of (AMF + Pf + M. incognita > AMF+ Ne) over the control and sole treatment. Disease incidence and index were lower significantly in AMF + Pf treatment compared to AMF sole treatment. Also, relative disease control was significantly improved in AMF + Pf + Ne > AMF + Ne only. However, no plant growth stimulation and nematode reduction were detected, when these microorganisms were combined together as shown by the results of Medina et al. (2003) and Vestberg et al. (2004). Hence, Wei et al. (1996) concluded that there is a need for a careful selection of AMF and PGPR species and strains to increase plant growth and reduce damage caused by plant pathogens, as the specific interactions among the microorganisms involved determine their beneficial effects. In this respect, it was noticed in the present study that treatments of bacterial isolates +AMF applied, 7 days before root-knot nematode inoculation recorded higher an overall average percentages of nematode reduction and consequently higher plant growth which may be attributed to treatment with Pf + AMF. Also, higher overall average percentages nematode reduction occurred, when Pf +AMF was inoculated with Ne at the same time which could be due to competition between the two microorganisms as symbionts for infection sites (Hajra et al., 2013). This was emphasized that overall percentages of gall reduction in the mycorrhizal plants was higher than in non-mycorrhizal ones. This could be due to the reduction in nematode penetration into the roots or the formation of giant cells may be affected by AMF and further reduction of the nematode. The parasitism of the nematode may be endured by the increase of mycorrhizal growth in mycorrhizal plants. This view agreed with those obtained by Kellam and Schenck (1980) who found that, root colonization by AMF followed the same trend similar to pre- inoculation with AMF and then, inoculated with Ne that promoted root colonization over co-treatment inoculated simultaneously with Ne and AMF.
Also, in the present investigation, it was proved that, co-toxicity effects for the two co-combined treatments of bacterial isolates + AMF, added either at the same time with M. incognita or 7 days before nematode inoculation resulted in additive or synergistic interaction effects which led to reduction of nematode parameters in eggplant. In this line, Youssef et al. (2015) found that, P. fluorescens+ phosphorine containing Bacillus megaterium caused additive effect on M. incognita infesting green bean under field conditions. Also, El-Nagdi et al. (2023) showed that the combined treatment of Bacillus subtilis or B. pumilus +pomegranate exhibited synergistic action in biocontrolling M. incognita infesting potatoes.
Conclusions and Recommendations
It could be concluded that the consortium treatment with AMF and P. fluorescens significantly decreased root-knot nematode, M. incognita as indicated by number of egg masses, eggs and galls on eggplant more than single treatments. When applied consortium treatments of each bacterial isolate+ AMF, added at the same time with nematode (Ne) inoculation or added at 7 days before Ne inoculation, showed additive or synergistic interaction effects. The tested treatments increased significantly (P ≤ 0.05) plant growth criteria.
Novelty Statement
AMF and P. fluorescens, when they were tested together as combined treatment, they significantly affected root-knot nematode, M. incognita reproduction and fecundity and galls on eggplant more than sole ones. Also, they increased significantly plant growth criteria and showed additive or synergistic interaction effects for nematode reduction on eggplant.
Author’s Contribution
WMAEN, AEAM, and MMAY supervised this work, designed, wrote and executed this manuscript. MMSZ supervised this work, extracted and identified mycorrhizal fungi and reviewed the manuscript. EHT supervised the work, provided the facilities during this work and reviewed the manuscript and NRAS carried out the experiment and examined it in the laboratory. All authors read and approved the final manuscript.
List of abbreviations
AMF, Arbuscular endomycorrhizal fungi; PGPR, Plant growth promoting rhizobacteria; Pf: Pseudomonas fluorescens; Ne, Nematode; CFU, colony forming unit; HCL, hydrochloric acid; KOH, potassium hydroxide; MIRE, Mycorrhizae- infected root extract.
Conflict of interest
The authors have declared no conflict of interest.
References
Baird, R.M., Hodges, N.A. and Denyer, S.P., 2000. Handbook of microbiology quality control: Pharmaceuticals and medical devices. London; New York, NY; Taylor and Francis. pp. 280. https://doi.org/10.4324/9780203305195
El-Nagdi, W.M.A. and Abd-El-Khair, H., 2008. Biological control of Meloidogyne incognita and Rhizoctonia solani in eggplant. Nematol. Medit., 36: 85-92.
El-Nagdi, W.M.A., Youssef, M.M.A., Abd El-Khair, H., Elkelany, U.S., Abd-Elgawad, M.M.M. and Dawood, M.G., 2023. Effect of integration of two bacterial bioagents and a plant residue extract for biocontrolling a root-knot nematode, Meloidogyne incognita infesting potatoes. Egypt. Pharma. J., 22: 67-77. https://doi.org/10.4103/epj.epj_119_22
Gerdemann, J.W. and Nicolson, T.H., 1963. Spores of mycorrhizal endogone species extracted from soil by wet sieving and decanting. Trans. Br. Mycol. Soc., 46(2): 235-244. https://doi.org/10.1016/S0007-1536(63)80079-0
Ghini, R.F., Patrico, R.A., Bettiol W., de Almeida, M.G. and Maia, N.H.A. 2007. Effect of sewage sludge on suppressiveness to soil-borne plant pathogens. Soil Biol. Biochem., 39: 2797–2805. https://doi.org/10.1016/j.soilbio.2007.06.002
Goszczynska, T., Serfontein, J.J. and Serfontein, S., 2000. Introduction to practical phyto-bacteriology. Sponsored by the Swiss Agency for Development and Cooperation (SDC), Switzerland, pp. 83.
Hajra, N., Shahina, F. and Firoza, K., 2013. Biocontrol of root-knot nematode by arbuscular mycorrhizal fungi in Luffa cylindrica. Pak. J. Nematol., 31: 77-84.
Hayder, R.H., Simon, S., Abhilasha, A.L., and Kamaludden 2014. Influence of mycorrhizal fungus and certain rhizobacteria on root-knot-nematode (Meloidogyne incognita) and growth of brinjal (Solanum melongena L). Int. J. Res. Bot., 4: 11-18.
Kellam, M.K. and Schenck, N., 1980. Interactions between a vesicular- arbuscular mycorrhizal fungus and root knot nematode on soyabean. Phytopathology, 70: 291-296. https://doi.org/10.1094/Phyto-70-293
Lelliott, R.A. and Stead, D.E., 1987. Methods on plant pathology volume 2, methods for the diagnosis of bacterial diseases of plants. British society for plant pathology, blackwell scientific publications, Oxford London Edinburgh, Boston, Palo, Alto, Melbourne, pp. 216.
Liu, R., Dai, M., Wu, X., Li, M. and Liu, X., 2012. Suppression of the root-knot nematode [Meloidogyne incognita (Kofoid and White) Chitwood] on tomato by dual inoculation with arbuscular mycorrhizal fungi and plant growth-promoting rhizobacteria. Mycorrhiza, 22: 289–296. https://doi.org/10.1007/s00572-011-0397-8
Mansour, N.A., El-Dafrawi, M.E., Tappozada, A. and Zeid, M.I., 1966. Toxicological studies on the Egyptian cotton leaf worm, Proedenia litura. Potentiation and antagonism of organophosphorus and carbamate insecticides. J. Econ. Entomol., 59: 307-311. https://doi.org/10.1093/jee/59.2.307
Medina, A, Probanza, A., Manero, F.J.G. and Azcón, R., 2003. Interactions of arbuscular mycorrhizal fungi and Bacillus strains and their effects on plant growth, microbial rhizosphere activity (thymidine and leucine incorporation) and fungal biomass (ergosterol and chitin). Appl. Soil Ecol., 22: 15–28. https://doi.org/10.1016/S0929-1393(02)00112-9
Phillips, J.M. and Hayman, J.D., 1970. Improved procedures for clearing roots and staining parasitic and vesicular–arbuscular mycorrhizal fungi for rapid assessment of infection. Trans. Br. Mycol. Soc., 55: 158-161. https://doi.org/10.1016/S0007-1536(70)80110-3
Richer, D.L., 1987. Synergism a patent view. Pest Sci., 19: 309-315. https://doi.org/10.1002/ps.2780190408
Saleh, N.R.A., Mahgoob, A.E.A., Taha, Entesar, H., El-Nagdi, Wafaa, M.A., Youssef, M.M.A. and Zayed, M.M.S., 2020. Effect of certain Pseudomonas fluorescens isolates on the infection of root-knot nematode, Meloidogyne incognita in tomato and eggplant and the plant growth. Arab Univ. J. Agric. Sci., 28(1): 315-327.
Schaad, N.W., 1980. Laboratory guide for identification of plant pathogenic bacteria. Bacteriology Committee of American, Psychopathological Society, Saint Paul, Minnesota, pp. 72.
Schouteden, N., De Waele, D., Panis, B. and Vos, C.M., 2015. Arbuscular mycorrhizal fungi for the biocontrol of plant-parasitic nematodes: A review of the mechanisms involved. Front. Microbiol., 6: 1-12. https://doi.org/10.3389/fmicb.2015.01280
Sharma, S., Kashyap, S., and Vasudevan, P., 2005. In vitro rhizogenesis of Morus alba by mycorrhizal extracts under saline stress. Eur. J. Hortic. Sci., 70: 79-84.
Siddiqui, Z.A. and Akhtar, M.S., 2009. Effects of antagonistic fungi, plant growth-promoting rhizobacteria and arbuscular mycorrhizal fungi alone and in combination on the reproduction of Meloidogyne incognita and growth of tomato. J. Gen. Plant Pathol., 75: 144–153. https://doi.org/10.1007/s10327-009-0154-4
Sikora, R.A. and Fernandez, E., 2005. Nematode parasites of vegetables. In: Luc, M., Sikora, R.A., and Bridge, J. (Eds). Plant parasitic nematodes in subtropical and tropical agriculture. 2nd edition. CABI Publishing. pp. 319-392. https://doi.org/10.1079/9780851997278.0319
Sikora, R.A., Schäfer, K. and Dababat, A., 2007. Modes of action associated with microbially induced in plant suppression of plant-parasitic nematodes. Aust. Plant Pathol., 36: 124–134. https://doi.org/10.1071/AP07008
Talavera, M., Sayadi, S., Chirosa-Rios, M., Salmerón, T., Flor-Peregrin, E. and Verdejo-Lucas, S., 2012. Perception of the impact of root-knot nematode-induced diseases in horticultural protected crops of south-eastern Spain. Nematology, 14: 517–527. https://doi.org/10.1163/156854112X635850
Utobo, E.B., Uma, M. and Nidhi, R., 2016. Climate change scenarios on bioprotction potential of arbuscular mycorrhizal fungi (AMF) in relation to root knot nematode (RKN) on tomato. Am. Eur. J. Agric. Environ. Sci., 16: 518-526.
Vestberg, M., Kukkonen, S., Saari, K., Parikka, P., Huttunen, J., Tainio, L. and Gianinazzi, S., 2004. Microbial inoculation for improving the growth and health of micro propagated strawberry. Appl. Soil Ecol., 27: 243–258. https://doi.org/10.1016/j.apsoil.2004.05.006
Wei, G., Kolepper, J.W. and Tuzum, S., 1996. Induced systemic resistance to encounter diseases and increased plant growth by plant growth promoting bacteria under field conditions. Phytopathology, 86: 221–224. https://doi.org/10.1094/Phyto-86-221
Youssef, M.M.A. and El-Nagdi, W.M.A., 2015. Vesicular arbuscular mycorrhizae: A promising trend for bio controlling plant parasitic nematodes. A review. Sci. Agric., 11: 76-80. https://doi.org/10.15192/PSCP.SA.2015.11.2.7680
Youssef, M.M.A. and Korayem, A.M., 2008. The relationship between eggplant yield and number of galls caused by Meloidogyne incognita and cellular alterations of the infested plants. Plant Prot. Bull., 50: 35-41.
Youssef, M.M.A., El-Ghonaimy, A.M. and El-Nagdi, W.M.A., 2015. Evaluation of some commercial bacterial biofertilizers and isolates against root knot nematode, Meloidogyne incognita infesting green bean, Phaseolus vulgaris. Sci. Agric., 10(1): 49-54. https://doi.org/10.15192/PSCP.SA.2015.10.1.4954
To share on other social networks, click on any share button. What are these?





