Brood Stock Development, Reproductive Performance and Breeding of African Catfish (Clarias gariepinus) in Pakistan
Research Article
Brood Stock Development, Reproductive Performance and Breeding of African Catfish (Clarias gariepinus) in Pakistan
Muhammad Ramzan Ali1*, Hasina Basharat2, Aziz Ahmed1, Mubeen Fakhar1 and Shamim Akhter2
1Aquaculture and Fisheries Program, National Agriculture Research Centre (NARC), Park Road, Islamabad, Pakistan; 2Department of Zoology, PMAS Arid Agriculture University, Rawalpindi, Pakistan
Abstract | African catfish culture technology is in the phase of experimentation in Pakistan. The present study was conducted to develop a brood stock and to study its reproductive performance and breeding success in culture system of Pakistan. For the brood stock development, one thousand fingerlings of Clarias gariepinus were stocked in earthen ponds. The shooter fish were separated and kept in separate ponds of 0.04-hectare area. The mature 100 males and 100 female brooders fish were selected on basis of morphometric characters. Monthly gonadosomatic index of male and female Clarias gariepinus was lower in male as compare to female fish. Milt volume of male African catfish was directly related to its body weight. It was seen that by increasing weight of male African catfish the milt volume, motility, motility duration and sperm concentration also increased. The relationship of fecundity with total weight was linear and positive, while reverse relationship was observed between absolute fecundity and total weight. The highest percentage of fertilization and hatchability rate was found as 88.74±4.23 and 80.76±3.42, respectively. Which were recorded in females having body weight between 800-900g, respectively. The lowest percentage of fertilization and hatchability rate was 65.23±3.26 and 45.11±2.76 recorded in females having body weight of 1200-1800g, respectively. It was concluded that African catfish can easily be bred through induce breeding in the local environment. In addition, the breeding season of African catfish start in May and ends up in September. A female fish of about 1 kg gave good results in the terms of fertilization rate (%) hatching rate (%) and a handsome number of hatchlings was obtained by the artificial breeding.
Received | March 01, 2024; Accepted | July 13, 2024; Published | August 21, 2024
*Correspondence | Muhammad Ramzan Ali, 1Aquaculture and Fisheries Program, National Agriculture Research Centre (NARC), Park Road, Islamabad, Pakistan; Email: [email protected]
Citation | Ali, M.R., H. Basharat., A. Ahmed., M. Fakhar and Shamim Akhter. 2024. Brood stock development, reproductive performance and breeding of African catfish (Clarias gariepinus) in Pakistan. Sarhad Journal of Agriculture, 40(3): 988-997.
DOI | https://dx.doi.org/10.17582/journal.sja/2024/40.3.988.997
Keywords | African catfish, Brood stock development, Reproductive performance, Fertilization rate, Arterial breeding, Milt quality
Copyright: 2024 by the authors. Licensee ResearchersLinks Ltd, England, UK.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Introduction
The African catfish (Clarias gariepinus) was introduced in Pakistan as it can be stocked at higher densities and its per hectare production is about 16-40 tons. In addition, they have high consumer preference due to fewer bones, good meat, adaptability to wide range of environmental conditions and disease resistance (Basharat et al. 2020) For the successful introduction of an exotic species to any system, local seed production is an important factor. The techniques of induced breeding have contributed significantly towards breeding success of a species and expansion of aquaculture during recent decades. Induced spawning of exotic and local carps has become a common practice in Pakistan. A large number of hatcheries have been established in the private and public sector for the artificial breeding of exotic species (Bhuiyan et al., 2013). These techniques have enabled the farmers to gain profitability by breeding and raising the species that do not naturally reproduce in captivity. These techniques are also helpful for manipulation of the reproduction timing to suit production cycles. Due to the difference of environmental or culture conditions (e.g difference of water temperature or substrate type), some species do not breed in captive conditions. These conditions may induce stress or may not provide the signals required to complete the reproduction in fish (Mittelmark and Kapuscinski, 1991). Induced breeding has solved the shortage problem of good quality seed having uniformity in size that is free from diseases, pests and parasites at the time of stocking in ponds for large-scale commercial culture of numerous fish species (Marimuthu et al., 2011).
For the artificial or semi artificial breeding, healthy and sexually ripe fish are needed. The brood fish can be raised on farms or obtained from the spawning ground in natural waters just prior to the spawning season. In African catfish gonads, are matured usually in the rainy season. Photoperiodicity and annual changes in water temperature influence processes of maturation of African catfish, a rise in water level due to rainfall also activate spawning process (Degraaf et al., 1995).
Evaluation of the commercial potential of stock, practical culture and actual management of the fishery can be evaluated through biological parameters of the reproductive potential i.e., fecundity (Gomez-Marquez et al., 2003; Doha and Hye, 1970). Effective fisheries management involving practical aquaculture is dependent on precise evaluation of fecundity for understanding the capability of fish populations to recover (Tracey et al., 2007). The evaluation of the potential of egg production can be possible through study of fecundity and its relation to female size (Chondar, 1977). Dadzie et al. (2000) reported that the ovaries of reproductively ripened females, quickly gain size just before the breeding season, and regular seasonal changes are observed in weight of gonads specifically the ovaries. Therefore, the study of relationship between body weights with gonadal weight, (gonadosomatic index) is used as a technique for determining the spawning season of any fish species (Ahirrao, 2002; Shankar and Kulkarni, 2005).
In Pakistan African catfish seed is being imported from Thailand and the cost of imported seed is on high side. African catfish culture technology is in the phase of experimentation in Pakistan and becoming popular among the fish farmers. The major problem of African catfish culture in the country is non availability of its seed. In order to introduce the culture of this fish, there is a dire need to produce African catfish seed locally, this will ensure the availability of seed to the fish farmers throughout the year on reduced cost. For the mass seed production of African catfish at local level, complete artificial reproduction technology package is desirable. The goal of the present study was to develop a brood stock and to study its reproductive performance in culture system of Pakistan.
Materials and Methods
Selection of brood fish and Gonadosomatic index (GSI)
The imported fish from Thailand (April, 2015) was kept in earthen ponds and raceways. For the brood stock development, one thousand fingerlings of African catfish were stocked in earthen ponds. The fish were fed on artificial feeding of 35% crude protein level at 4 % fish biomass daily. The growth data were collected on monthly basis and amount of feed was adjusted accordingly. The shooter fish were separated and kept in separate ponds of 0.04-hectare area. The water quality of stocked ponds was monitored regularly on fortnightly basis. The mature 100 males and 100 female brooders fish were selected on basis of morphometric characters.
For the determination of gonadosomatic index, at least three fish of good health status, were collected from ponds after every month during the whole year. These fish were first weighed to record their total body weight by using digital balance. Fish were dissected to remove the gonads for study of gonadosomatic index. The individual fish and its gonads were weighed and calculation of its gonadosomatic index was done by the formula (Chellappa et al., 2010):
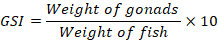
Reproductive performance of male African catfish
For the study of reproductive performance of male African catfish, the mature male brooders fish were selected on basis of morphometric characters and were divided into four groups based upon their sizes. At least three males from each group were used to study the milt quality parameters.
Milt collection
Mature male was injected with ovaprim (Syndel laboratories B, Canada) at a rate 0.25ml/kg body weight. Males were dissected after 13 hours of injection and their testes were removed surgically and cleaned from blood before milt collection. Perforations were made on the lobes of testes with the help of clean needle. Milt was squeezed out by pressing the testes. Milt volume was measured with plastic syringe in ml. The milt was studied further to assess following sperm quality parameters.
Sperm concentration: Concentration of spermatozoa was determined according to standard method by using Neubauer haemo-cytometer. By using micropipette 2 μL drop of 30-fold diluted semen sample with 9% NaCl was loaded on haemo-cytometer. Pipette tip was placed on V-shaped groove of haemo-cytometer while loading the sample. The sample was allowed to settle the sperm concentration and measured by taking counts in central counting square using binocular light microscope at 40X magnification attached with viewable camera.
Sperm motility: Motility of sperm was evaluated immediately after milt collection by dilution of 0.1 µl milt with 20 µl of fresh water, under light microscope at 100x magnification. The sperm that were seen moving on glass slide were assessed motile and expressed in percentage. Sperm that were appeared still were considered non motile.
Motility duration: The time period from which sperm get activated till 100% of spermatozoa become non-motile, is known as motility duration. When sperm motility was estimated, motility duration was calculated at the same time by keeping the sample at laboratory conditions. After every 1-minute sample was checked to observe the percentage motility until all spermatozoa became still.
Reproductive performance of female African catfish
Fecundity, relative fecundity and absolute fecundity of African catfish was calculated to check the reproductive performance of female African catfish (Qasim and Qayyum, 1963). The fish was wiped with a dry clean towel, a staggered cut was given carefully with sharp surgical blades. For careful removal of the ovaries, the lateral side muscles were completely opened. The weight of fish was recorded without internal organs and ovaries (denoted weight as Wb). Ovary was also weighed and denoted as “Wo”. An approximately 2.0 cm piece of ovary was weighed and its weight was recorded as Ws, then it was placed in small beaker and the number of eggs was counted as “S”. Relative and absolute fecundity was calculated using following formula:
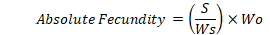
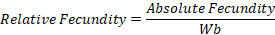
Artificial breeding
One mature male and two female fish were selected for the breeding purpose. After weighing, females were administered with the dose of artificially synthesized hormone ovaprim at the rate of 0.5 ml/kg for induced ovulation. No dose was given to the males. Females were released into circular tanks after injection.
Water temperature was maintained at 28-30 0C with continuous water exchange. Ovulation occurred after 8-10 hours after injection. Eggs were stripped completely by gently applying pressure on abdomen of female. After stripping, weight of egg mass and female fish was recorded again. Male fish were anesthetized and sacrificed for removal of testes. Testes were preserved in 0.9% saline solution. Milt was obtained by lacerating testes with scissor; milt (2ml milt) was mixed with stripped eggs (2ml of saline solution for 1g eggs).
Sperm activation was initiated by adding equal volume of fresh water as per saline solution added. Fertilization occurred within 2-3 minutes and eggs started appearing brown and become sticky. Eggs were mixed with milt by using clean bird feather. Eggs were incubated on nylon bags substrate which was prepared by cutting feed storage bags into equal halves. Pieces of nylon bags were spread in circular tanks by fixing ends by placing bricks at corners. Regular water supply was maintained with 28±1ºC. Tanks were covered with wooden board to avoid direct sunshine. Hatching occurs after 24-26 hours. Hatching rate was determined as described below.
Fertilization and hatching rate
For the study of fertilization and hatching rates, mature female fish were divided into three groups based upon their sizes. The number of eggs released by female was found out by deducting the weight of female before spawning from weight of female after spawning and then by multiplying with 700 as 1g of African catfish contains 700 eggs approximately. Fertilization rate was determined when eggs reached 4-8 cell stage of development. For determination of fertilization rate, eggs were collected from circular tank in which eggs were incubated by spreading them on a nylon mesh used as hatching substrate under regulated water flow until hatching occurs. The total number of eggs were counted in 1 g sample. Fertilized and unfertilized eggs were counted separately under stereo microscope (OPTIKA). The percentage fertilization was estimated as:
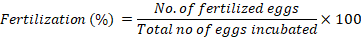
Eggs were shifted back to their original batch for incubation. Several hours after the incubation of these fertilized eggs, hatching began. The hatched larvae were counted carefully in the batch while un-hatched eggs were discarded; the percentage hatchability was estimated thus:
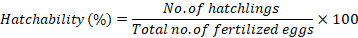
Data analysis
The data obtained was entered into MS Excel sheet, and further calculations were made by using MS Excel 2010. Data was presented in the form of suitable graphics using MS Excel. The significance among different parameters was checked by ANOVA (analysis of variance) and Duncan’s Multiple Range Test using SPSS software. Relationship among different parameters was determined by correlation and simple regression using MS Excel.
Results and Discussion
Identification of fish is an important tool for fish breeding. A clear sexual dimorphism was observed in African catfish. Male catfish were smaller and narrower than female catfish with a clear genital papilla. The head of male catfish was narrower and stouter than the head female catfish. The average length of females was on larger than males. Females were identified more clearly with rounded pinkish vent. The head of female fish was wide and broad. Similar features for identification of male and female African catfish and other clariid catfishes as previously described by Haylor and Oyegunwa (1993); Madu and Aluka (1999). Growth rates, for example, can vary by sex, as females grow faster and attain larger sizes than males (Henderson et al., 2003).
Gonadosomatic index is a tool to determine the sexual maturity of fish in relation to testes and ovary development. It is also beneficial to find out the pre spawning, actual spawning and post spawning stages of the fish species. Monthly gonadosomatic index of male and female African catfish during this study is shown in Figure 1. The value of GSI was lower in male as compare to female fish. Kime (1995) reported that as ovaries are larger than testis so GSI values are greater for females compared to males. Males also have less well-defined stages of gonadal maturation.
For both sexes, variation in Gonadosomatic index (GSI) values were seen among different months. Gonadosomatic index (GSI) values for both sexes of African catfish start decreasing from October till January. The smaller values of gonadosomatic index in winter season is related to diminution of gonadal stuffs. The same conclusion is also drawn from researches for various populations of catfishes in Africa (Willoughby and Tweddle, 1978; Dadzie and Okach, 1989) as well as Ethiopia (Elias and Dadebo, 2000;
Table 1: Mean (±S.D) of selected parameters of African catfish milt quality. Means with different letters differ significantly (P<0.05).
|
Parameters |
Group 1 |
Group II |
Group III |
Group IV |
|
Body weight |
770.25±2.23d |
832.56±3.10c |
912.80±1.56b |
1250.10±4.38a |
|
Volume of milt(ml) |
0.8±0.12d |
1.0±0.04c |
1.30±0.22b |
2.20±0.06a |
|
Progressive Motility (%) |
60.0±1.0c |
70.0±1.20b |
75.0±1.02a |
75.0±0.56a |
|
Concentration of sperm (106 mL-1) |
48.7±0.08d |
73.8±0.04c |
87.3±0.04b |
94.4±0.02a |
|
Sperm motility duration (seconds) |
300.0±0.32d |
360.0±0.66c |
420.0±0.44b |
480.0±0.29a |
Daba and Meseret, 2004). Gonadosomatic index (GSI) of African catfish start increasing; from February and the peak values of GSI were found from April to August. The results of the gonadosomatic index (GSI) of African catfish in present study, were in agreement with the findings of Emam and Abughrien (2014) who demonstrated peak values of GSI of Clarias lazera during spring and summer (spawning season) and showed the lowermost value during winter (resting season). The degeneration of testicular and ovarian tissues occurred during winter declaring it as inactive season for gonadal activities of the catfish. Restoration of both testis and ovaries to fully matured structure started again during springtime and continued during summer where the testis showed enlarged seminiferous lobules with all the spermatogenic cells and spermatozoa. Thus, both spring and summer were reflected as spawning seasons of the catfish.
The breeding potential of male broodstock of fish can easily be assessed by determining the milt qualitative parameters. The selected parameters of African catfish milt quality are presented in Table 1. During this study, it was observed that milt volume of male African catfish is directly related to its body weight. There was a highly significant difference of milt volume obtained from male fish of different sizes. The highest milt volume was recorded by fish of group IV followed by the Group III, II and Group I, respectively. A positive correlation between body size (length and weight) and volume of milt were found in rainbow trout and Atlantic salmon (Gjerde, 1984). A substantial effect of the brood stock age was seen on the sperm quality (Vuthiphandchai and Zohar, 1999). A better sperm quality was observed for three-year-old striped bass than the 1-or 2-year-old fish reared in captive conditions. Similar findings were also reported by Okoye et al. (2018), testicular weight and semen volume was significantly lower of less fish mean weight than those of higher fish body weight.
The milt quality parameters of African catfish i.e., sperm concentration (106 ml-1), progressive motility (%) and sperm motility duration (seconds) also differed significantly among the four fish groups. It was seen that by increasing weight of male African catfish, the milt volume sperm motility, motility duration and sperm concentration also increase. Rurangwa et al. (2004) stated that milt quality mostly depends on qualitative parameters of milt i.e., milt volume, composition of seminal fluid, sperm density and sperm motility. Hajirezaee et al. (2010) had reported that the milt quality parameters (i.e., sperm production, spermatozoa motility and seminal fluid composition) are influenced by several factors including brooders biological features (length, weight, age, rearing conditions nourishment and animal welfare).
The motility duration in the present study ranged from 300 to 480 seconds. The values of motility duration in present study, are higher than reported by Odedeyi et al. (2014) who reported 62 secs, 70 secs, 72 secs, 70 secs and 70 secs in fish groups based upon their weight. All parameters of male reproductive performance differed significantly from one group of fish to another in present study (P<0.05). The results could be variable as even among the individuals of the same species, semen quality also varies considerably (Piironen, 1985). In present study, sperm motility decreases with the passage of time at room temperature. Galo et al. (2014) also reported a negative pattern of sperm motility duration with the storage time of milt in Amazon catfish. According to Sahinoz et al. (2007) Hippoglossus hippoglossus, and studying Mastacembelus mastacembelus species shows variation in semen quality at each phase within the reproductive season.
The number of eggs possessed by a gravid female fish is defined as fecundity, a key feature of fish culture related with the average propagative characteristics of fish. The relationship of fecundity with total weight was linear and positive, while reverse relationship was observed between absolute fecundity and total weight (Figure 2 and Figure 3).
In most of the catfish species, the total number and weight of eggs spawned are positively correlated with weight of female (Broussard and Stickney, 1981; Nguenga et al., 2000). Ondhoro and Mwanja (2014) reported mean number of eggs produced per kilogram brood stock fed with Kajjansi 35%, Ugachick 35% and Tende Innovative Farm feeds as 45,375±13,399, 51,477±16,266 and 57,075± 18,922, respectively. According to Ozdilek and Jones (2014) the fecundity of African catfish in the River Asi was related more linearly than exponentially to fish weight, age and gonad weight. As per linear expressions, 49,000 eggs were produced by a 40 cm long female while about 36,310 eggs by a 37.46 cm female (the medium length of the spawning population) and about 316,891 eggs by the largest female which was more than the probable value from the linear equation. Similarly, about 4,483 eggs were produced by the small sized female (910.4g) whereas, on the average greater no of eggs were produced by females of larger size weighing around 4020.6g.
Fecundity in African catfish from Lakes Kariba, Mcllwaine and Kyle, improved exponentially in relation to total length, while in a linear correlation was reported between total length and fecundity. Same Spawning behavior was observed in Oreochromis niloticus and Clarias lazera (Clay, 1979). The intraspecific variations were observed in the fecundity of African catfish living in the River Asi, as seen in those of other Clarias populations.
African catfish females with average weight ranged from 680 to 1800g were bred artificially by using synthetic ovaprim at the rate of 0.5ml/kg. At temperature range of 28-30 oC, latency time period was observed as 7-9 hours. Previously, Adebayo (2006) has reported 8-12 hours as latency period at 22.5-31.0 oC. The latency period and humidity/temperature are factors of prime importance in success of artificial propagation of C. gariepinus through induced breeding under controlled environmental conditions (Agbebei et al. 2013). The data on percentage of fertilization, ovulation rate and hatchability rate, is presented in Table 2. The highest percentage of fertilization and hatchability rate was found to be 88.74±4.23 and 80.76±3.42, respectively which were recorded in females having body weight between 800-900g. The lowest percentage of fertilization and hatchability rate was 65.23±3.26 and 45.11±2.76, respectively recorded in females having body weight of 1200-1800g. Our results are in agreement with the studies of Degraff et al. (1995) who stated that variation in size of brood stock can lead to the difference in fertilization and hatching rates. Changadeya et al. (2003) revealed that small fish had increased hatchability compared to large ones. Our results are in disagreement as they are negatively correlated with the weight of females (Ataguba et al. 2013).
The percentage of fertilized eggs or hatched larvae varies in African catfish and other fin fish and depend on many factors. These factors include the fish origin, stimulation type with reference to environmental conditions and hormone used, proper time of gonadal harvest and conditions for storage, management of feeding for the spawning specimens and several other factors (Nowosad et al., 2014, 2016; 2018; Kristan et al., 2018). For example, less than 60% developing embryos were reported in African catfish in studies con-
Table 2: Fertilization rate and hatching rate in Ovaprim induced African catfish with varying body weights.
|
Parameters |
Group I |
Group II |
Group III |
|
Body weight (g) |
680-720 |
800-900 |
1200-1800 |
|
No of eggs released |
71,400±48,023.2c |
74,822±52,102.2b |
140,700±81,270.2a |
|
Fertilization rate (%) |
83.25±2.78b |
88.74±4.23a |
65.23±3.26c |
|
Hatching rate (%) |
78.52±2.67b |
80.76±3.42a |
45.11±2.73c |
Means with different letters differ significantly (P<0.05).
ducted by Brzuska et al. (2004). In a study conducted by Muller et al. (2002), average survival rate of 61% (range 43.7%-72.4%) and in a study of Samarin et al. (2019), 81%-88% of survival rate was reported. Apparently, body weight of female African catfish did not affect the survival rate of hatchlings. It was also estimated about 70% hatchling survived after yolk absorbing stage of first three days after that period survival rate became stabilized. However, de Graaf et al. (1995) reported only 41.5% survival rate at this stage. Opiyo et al. (2017) reported 68.13, 36.12 and 23.28% survival rate in three different strains of African catfish. The survival rate of African catfish larvae reported by Hawarry et al. (2016) ranged from 55.85% to 89.91%, using LH–RHa plus and GnRHa for induced breeding.
Conclusions and Recommendations
From this study, it was concluded that African catfish can easily be bred through induce breeding (the most important factor for the introduction of new species) in the local environment. The breeding season of African catfish start in May and ends up in September. A female fish of about 1 kg gave good results in the terms of fertilization rate (%) hatching rate (%) and a handsome number of hatchlings was obtained by the artificial breeding.
Novelty Statement
African catfish culture technology is in the phase of experimentation in Pakistan and becoming popular among the fish farmers. In order to introduce the culture of this fish and to ensure the availability of seed to the fish farmers locally, the present study was conducted to study its reproductive performance and artificial breeding in Pakistan.
Authors’ Contribution
Muhammad Ramzan Ali and Shamim Akhter: Supervised research, help in experimental setup and data analysis and manuscript writing.
Hasina Basharat: She performed this research study and draft paper.
Aziz Ahmed: Helped in experimental setup and data collection.
Mubeen Fakhar: Helped lab analysis, reviewed and edited the manuscript.
Conflict of interest
The authors have declared no conflict of interest.
References
Adebayo, O.T. 2006. Reproductive performance of African clariid catfish Clarias gariepinus broodstock on varying maternal stress. J. Fish. Int., 1(1): 17-20.
Agbebi, O.T., T.G. Ogunmuyiwa and S.M. Herbert. 2013. Effect of dietary garlic source on feed utilization, growth and Histopathology of the African catfish (Clarias gariepinus). J. Agric. Sci., 5(5), 26. https://doi.org/10.5539/jas.v5n5p26
Ahirrao, S.D. 2002. Status of gonads in relation to total length (TL) and gonadosomatic index (GSI) in freshwater spiny eel (pisces) Mastacembelus armatus (Lacepede) from Marathwada region Maharashtra. J. Aquat. Biol., 17(2), 55-57
Ataguba, G.A., V.T. Okomoda and M.Chukwuemeka. 2013. Relationship between broodstock weight combination and spawning success in African catfish (Clarias gariepinus). Croat. J. fish., 71: 176-181. https://doi.org/10.14798/71.4.694
Basharat, H., M.R. Ali., M.M. Shahid., A. Ahmed and S. Akhter. 2020. Introduction of African catfish (Clarias gariepinus) in aquaculture system of Pakistan: Its transportation, acclimatization and cannibalism study. Pak. J. Agric. Sci., 56(6): 1645-1652.
Bhuiyan A.S., J. Akhter and S.M. Al-Noor. 2013. Efficacy of Two Inducing Agents, PG and DOM+SGNRH on the Induced Breeding of the Major Carp, Kalibaus (Labeo calbasu). Our Nature. 11(1): 17-24. https://doi.org/10.3126/on.v11i1.8239
Broussard, J.M.C. and R.R, Stickney. 1981. Evaluation of reproductive characters for four strains of channel catfish. Trans. Am. Fish. Soc., 110: 502-506. https://doi.org/10.1577/1548-8659(1981)110%3C502:EORCFF%3E2.0.CO;2
Brzuska, E. 2004. Artificial spawning of carp (Cyprinus carpio L.); differences between the effects of reproduction in females of Hungarian, Polish and French origin treated with carp pituitary homogenate or [D-Tle6, ProNHEt9] GnRH (Lecirelin). Aquac. Res., 35(14): 1318-1327. https://doi.org/10.1111/j.1365-2109.2004.01153.x
Changadeya, W., L.B. Malekano and A.J.D Ambali. 2003. Potential of genetics for aquaculture development in Africa. NAGA, World Fish Centre Quarterly, 26(3): 31-35.
Chellappa, S., J.T. Lima, A. Aruijo and N.T. Chellappa. 2010. Ovarian development and spawning of Serra Spanish mackerel in coastal waters of Northeastern Brazil. Braz. J. Biol., 70(2):451-456. https://doi.org/10.1590/S1519-69842010005000012
Chondar, S.L. 1977. Fecundity and its role in racial studies of Gudusia chapra (Pisces: Clupeidae). In Proc. Indian Acad. Sci., Sec-B, 86(4): 245-25. https://doi.org/10.1007/BF03050969
Clay, D. 1979. Sexual maturity and fecundity of the African catfish (Clarias gariepinus) with an observation on the spawning behavior of the Nile catfish (Clarias lazera). Zool. J. Linn. Soc., 65: 351-365. https://doi.org/10.1111/j.1096-3642.1979.tb01100.x
Daba, T., and T. Meseret. 2004. Reproductive biology and feeding habits of Clarias gariepinus in Lake Zeway, Ethiopia. Proceedings of the 13th national Conference of Ethiopian Society of Animal Production, Addis Ababa, Ethiopia. (pp. 199-208).
Dadzie, S., and J.O. Okach.1989. The reproductive biology of a siluroid catfish, Bagrus docmak (Cypriniformes: Bagridae) in the Winam Gulf of Lake Victoria. J. Afr. Zool., 103:143-154.
Dadzie, S., F. Abou-Seedo and T. Al-Shallal. 2000. Reproductive biology of the silver pomfret, Pampus argenteus (Euphrasen), in Kuwait waters. J. Appl. Ichthyol., 16(6): 247-253. https://doi.org/10.1046/j.1439-0426.2000.00237.x
Degraaf, G.J., F. Galemoni and B. Banzoussi.1995. Artificial reproduction and fingerling production of the African catfish, Clarias gariepinus (Burchell 1822), in protected and unprotected ponds. Aquac. Res., 26(4): 233-242. https://doi.org/10.1111/j.1365-2109.1995.tb00908.x
Doha, S. and M.A. Hye.1970. Fecundity of Padma River Hilsha, Hilsha ilisha (Hamilton). Pak. J. Sci., 22: 176-178.
Elias and Dadabo. 2000. Reproductive biology and feeding habits of the catfish Clarias gariepinus Burchell (Pisces: Clariidae) in Lake Hawassa, Ethiopia. Ethiop. J. Sci., 23: 231-246. https://doi.org/10.4314/sinet.v23i2.18168
Emam, M.A. and B. Abughrien. 2014. Seasonal histological changes in gonads of the catfish (Clarias lazera). Aquaculture, 5: 101-112. https://doi.org/10.4172/2150-3508.1000087
Galo, J.M., J.D.P. Streit., J.A. Povh., D.C. Fornari., E.K.D. Resende., D.D. Oliveira and R.P. Ribeiro. 2014. Sperm quality of the Amazon catfish Leiarius marmoratus (Gill, 1870) after cold storage. Braz. J. Biol., 74(4): 933-938. https://doi.org/10.1590/1519-6984.00313
Gjerde, B. 1984. Variation in semen production of farmed Atlantic salmon and rainbow trout. Aquaculture, 40(2): 109-114. https://doi.org/10.1016/0044-8486(84)90349-1
Gomez-Marquez, J.L., B. Pena-Mendoza., I.H. Salgado-Ugarte and M. Guzman-Arroyo. 2003. Reproductive aspects of Oreochromis niloticus (Perciformes: Cichlidae) at Coatetelco lake, Morelos, Mexico. Revista de Biologia Tropical, 51(1): 221-228.
Hajirezaee, S., B.M. Amiri and A. Mirvaghefi. 2010. Fish milt quality and major factors influencing the milt quality parameters: A review. Afr. J. Biotechnol., 9(54): 9148-9154.
Hawarry, N.W., H.A. Soliman and M.S. Ramy. 2016 Breeding response and larval quality of African catfish (Clarias gariepinus, Burchell 1822) using different hormones/hormonal analogues with dopamine antagonist. Egypt. J. Aquat. Res., 42(2): 231-239. https://doi.org/10.1016/j.ejar.2016.06.003
Haylor, G. and O. Oyegunwa. 1993. Onset of airbreathing and development of accessory breathing organs in relation to temperature in African Catfish (Claria gariepinus Burchell). Aquac. Res., 24: 253-260. https://doi.org/10.1111/j.1365-2109.1993.tb00548.x
Henderson, T.A., P.T. Saunders., A. Moffett-King., N.P. Groome and H.O. Critchley. 2003. Steroid receptor expression in uterine natural killer cells. J. Clin. Endocrinol. Metab., 88(1): 440-449. https://doi.org/10.1210/jc.2002-021174
Kime, D.E. 1995. The effects of pollution on reproduction in fish. Fish Biol., 5: 52-95. https://doi.org/10.1007/BF01103366
Kristan, J., D. Zarski., M. Blecha., T. Policar., O. Malinovskyi., A.M. Samarin and D. Kucharczyk. 2018. Fertilizing ability of gametes at different post-activation times and the sperm-oocyte ratio in the artificial reproduction of pikeperch Sander lucioperca. Aquac. Res., 49(4), 1383-1388. https://doi.org/10.1111/are.13570
Marimuthu, K., R. Umah., S. Muralikrishnan., R. Xavier, and S. Kathiresan. 2011. Effect of different feed application rate on growth, survival and cannibalism of African catfish, Clarias gariepinus fingerlings. Emir. J. Food Agric., 23(4): 330-337.
Mittelmark, J. and A. Kapuscunski. 1991. Minnesota Sea Grant: Induced reproduction in fish. http://www.scagrant.umn.edu/aquaculture/induced_fish_reproduction.
Madu, C.T. and P.O. Aluko. 1999. Hybrid mud catfish production: Comparative growth and survival of hybrids and putative parents. Proceedings of the 12th Annual Conference of the Biotechnology Society of Nigeria, (BSN’99), Nigeria, pp: 89-94.
Muller, T., B. Kucska., H. Laszlo., A. Ittzes., B. Urbanyi., C. Blake., C. Guti., B. Csorbai., B. Kovacs and T. Szabo. 2002. Successful, induced propagation of African catfish (Clarias gariepinus) by ovarian lavage with sperm and hormone mixture. Aquaculture, 485: 197-200. https://doi.org/10.1016/j.aquaculture.2017.11.051
Nguenga, D., G.G. Teugels and F. Ollevier. 2000. Fertilization, hatching, survival and growth rates in reciprocal crosses of two strains of an African catfish Heterobranchus longifilis under controlled hatchery conditions. Aquac. Res., 31, 565-573. https://doi.org/10.1046/j.1365-2109.2000.00468.x
Nowosad, J., D. Kucharczyk., K. Targonska., E. Wyszomirska., R. Chwaluczy and K. Kupren. 2016. The synergistic effect of temperature and hormonal stimulation on spawning efficiency of Common Barbel, Barbus barbus L. Turk. J. Fish. Aquat. Sci., 16(3): 517-524. https://doi.org/10.4194/1303-2712-v16_3_04
Nowosad, J., M. Sikora and D Kucharczyk. 2018. Survival rates and the occurrence of larval malformations, including Siamese twins, following fertilization of post-ovulatory aged oocytes in ide Leuciscus idus. Dis. Aquat. Organ., 127(3): 237-242. https://doi.org/10.3354/dao03208
Nowosad, J., K. Targorska., R. Chwaluczyk., R. Kaszubowski and D. Kucharczyk. 2014. Effect of temperature on the effectiveness of artificial reproduction of dace (Cyprinidae Leuciscus leuciscus L) under laboratory and field conditions. J. Therm. Biol., 45: 62-68. https://doi.org/10.1016/j.jtherbio.2014.07.011
Odedeyi, D.O., F.A. Gbore and O. Ademoye. 2014. Influence of the length of time after hormonal stimulation on the milt quality of African Catfish Clarias gariepinus Brood Stock. Aust. j. basic appl. sci., 8(1): 407-412.
Okoye, C.N., A.F. Udoumoh., U.M. Igwebuike and C.T. Okereke. 2018. Ovarian morphology and development of 3 to 8 months old African catfish, Clarias gariepinus. Comp. Clin. Path., 27(4):87-891. https://doi.org/10.1007/s00580-018-2678-5
Ondhoro, C.C. and T.M. Mwanja. 2014. Basic steps in the production of the African catfish seed. http://hdl.handle.net/1834/35266
Opiyo, M.A., P. Orina and C. H. Karisa. 2017. Fecundity, growth parameters and survival rate of three African Catfish (Clarias Gariepinus) strains under hatchery conditions. J. Aquacult. Eng. Fish. Res., 3(2): 75-81. https://doi.org/10.3153/JAEFR17010
Ozdilek, S.Y. and R.I Jones. 2014. The diet composition and trophic position of introduced Prussian carp Carassius gibelio (Bloch, 1782) and native fish species in a Turkish river. Turk. J. Fish. Aquat. Sci., 14(3): 769-776.
Piironen, J. 1985. Variation in the properties of milt from the Finnish land locked salmon (Salmo salar sebago Girard) during a spawning season. Aquaculture, 48(3-4), 337-350. https://doi.org/10.1016/0044-8486(85)90136-X
Qasim, S.Z., and A. Qayyum. 1963. Fecundities of some freshwater fish. The Proceedings of the National Academy of Sciences, India, 29: pp. 373-382.
Rurangwa, E., D.E. Kime., F. Ollevier, and J.P. Nash. 2004. The measurement of sperm motility and factors affecting sperm quality in cultured fish. Aquaculture, 234(1-4), 1-28. https://doi.org/10.1016/j.aquaculture.2003.12.006
Sahinoz, E., F. Aral, and Z. Dogu. 2007. Changes in Mesopotamian spiny eel, Mastacembelus mastacembelus (Bank & Solender in Russell, 1794) (Mastacembelidae) milt quality during a spawning period. Theriogenology, 67(4): 848-854. https://doi.org/10.1016/j.theriogenology.2006.11.001
Samarin, A., S. Azadeh., B. Miroslav., K. Jiri., P. Tomas. 2019. In vitro storage of pikeperch (Sander lucioperca) eggs. Aquac. Inter., 27(4), 1037-1044. https://doi.org/10.1016/j.theriogenology.2006.11.001
Shankar, D. S., and R.S. Kulkarni. 2005. Changes in tissue cholesterol and serum cortisol level during four reproductive phases of the male freshwater fish, Notopterus notopterus. J. Environ. Biol., 26(4): 701-704.
Tracey, S.R., J.M. Lyle and M. Haddon. 2007. Reproductive biology and per-recruit analyses of striped trumpeter (Latris lineata) from Tasmania, Australia: implications for management. Fish. Res., 84(3): 358-367. https://doi.org/10.1016/j.fishres.2006.11.025
Vuthiphandchai, V. and Y. Zohar. 1999. Age-related sperm quality of captive striped bass Morone saxatilis. J. World Aquac. Soc., 30(1): 65-72. https://doi.org/10.1111/j.1749-7345.1999.tb00318.x
Willoughby, N.G. and D. Tweddle. 1978. The ecology of the catfish Clarias gariepinus and Clarias gamensis in the Shire Valley, Malawi. J. Zool., 186: 507-534. https://doi.org/10.1111/j.1469-7998.1978.tb03936.x
To share on other social networks, click on any share button. What are these?







