Assessment of Genetic Variability in Sugarcane (Saccharum officinarum L.) Genotypes for Better Yield, Quality and Sugarcane Mosaic Virus Resistance in Central Punjab, Pakistan
Research Article
Assessment of Genetic Variability in Sugarcane (Saccharum officinarum L.) Genotypes for Better Yield, Quality and Sugarcane Mosaic Virus Resistance in Central Punjab, Pakistan
Naeem Akhtar1*, Sana Noureen1, Muhammad Asif2, Ahsan Aziz2, Usman Saleem1, Talat Mahmood3, Nadeem Raza4 and Waqas Raza5
1Department of Plant Breeding and Genetics, College of Agriculture, University of Sargodha, Sargodha, Pakistan; 2Department of Agronomy, College of Agriculture, University of Sargodha, Sargodha, Pakistan; 3Department of Plant Breeding and Genetics, Pir Mehr Ali Shah Arid Agriculture University, Rawalpindi, Pakistan; 4Soil Fertility Section, Ayub Agriculture Research Institute, Faisalabad, Pakistan; 5Department of Plant Pathology, College of Agriculture, University of Sargodha, Sargodha, Pakistan.
Abstract | Sugarcane is the most valued sugar crop and main source of sucrose. Sugarcane is economic crop grown in tropical and subtropical areas world widely. It is also used for the production of biofuel and ethanol. Sugarcane yield is decreasing due to biotic and abiotic stresses. Among biotic factors, sugarcane mosaic virus (SCMV) is one of the most devastating factors that causes significant losses of sugarcane production. Here we are reporting the assessment of genetic variation among sugarcane accessions based on morphological parameters, SCMV severity, incidence and resistance/tolerance. The experiment was conducted in College of Agriculture, University of Sargodha, Sargodha, Pakistan using randomized complete design in triplicate. Different morphological parameters were calculated to observe sugarcane production and SCMV susceptibility by determining disease incidence and disease severity by using appropriate statistical software. Results revealed that all sugarcane genotypes performed well, showed significant variability for morphological characters and SCMV tolerance/susceptibility based on phenotypic scoring. Brix percentage is an important parameter as it is a yield contributing trait. Sugarcane accessions SC12 (23.03%) followed by SC11 (22.91%) attained maximum brix percentage while the least brix value 16% was observed in genotype SC31.SC4 displayed resistant to sugarcane mosaic virus but its yield was very poor as compared to others due to its genetic makeup. SC3 exhibited high disease incidence, having less brix percentage but showed maximum cane yield. Genotype SC7 revealed moderate susceptibility but exhibited high yield with maximum brix percentage. Evaluation of all agronomic traits and screening of superior genotypes would help in future sugarcane-breeding program to increase sugar production and development of SCMV resistant cultivars.
Received | June 01, 2023; Accepted | August 31, 2023; Published | September 29, 2023
*Correspondence | Naeem Akhtar, Department of Plant Breeding and Genetics, College of Agriculture, University of Sargodha, Sargodha, Pakistan; Email: waqasraza61@yahoo.com, naeem.siraj@uos.edu.pk
Citation | Akhtar, N., S. Noureen, M. Asif, A. Aziz, U. Saleem, T. Mahmood, N. Raza and W. Raza. 2023. Assessment of genetic variability in sugarcane (Saccharum officinarum L.) genotypes for better yield, quality and sugarcane mosaic virus resistance in central Punjab, Pakistan. Sarhad Journal of Agriculture, 39(3): 773-780.
DOI | https://dx.doi.org/10.17582/journal.sja/2023/39.3.773.780
Keywords | Saccharum officinarum L, SCM, Brix, Stool weight, Disease incidence
Copyright: 2023 by the authors. Licensee ResearchersLinks Ltd, England, UK.
This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Introduction
Sugarcane is high valued cash cropin Pakistan. It contributes 80% in sugarcane production and 40 % in fuel production (Aono et al., 2021). It contributed 0.4% in GDP and 3.4% in agriculture and during 2020-2021, the total production of sugarcane was 81.009 million tones in Pakistan (Economic Survey of Pakistan, 2020-21). Pakistan ranked at 5th position worldwide in sugarcane production (FAO, 2021). After the textile industry sugarcane industry leading at second position in Pakistan (Khan et al., 2021).
Sugarcane is a C4 ratoon crop (Lu et al., 2021). It is the most efficient C4 grown on tropical and subtropical region of Pakistan (Yadav et al., 2013). It belongs to oaceae family and genus Saccharum. Sugarcane has significant importance in manufacturing of different by products such as fuel, bioethanol and cane juice. It is a polyploidy and mainly cultivated for the source of sucrose (Menossiet al., 2008). It is a ratoon crop which is mainly cultivated by means of vegetative method, stalk-containing buds is used for germination purpose (Croft et al., 2008).
There are many factors, which cause potential decrease in sugarcane production including biotic and abiotic factors. The important factors that badly effect the yield of sugarcane are use of improper irrigation method and cultural practices, lack of diseases and pests control and improper fertilizer application (Baloch et al., 2020). Most of the existing sugarcane varieties in Pakistan are susceptible to different diseases like sugarcane mosaic virus, red rot, pokhaboeng, etc. Among diseases, sugarcane mosaic virus (SCMV) is one of the major diseases that causes 6-10% decrease in sugarcane yield (Haider et al., 2011). Sugarcane mosaic virus causes yellowing of leaves, dark patches appear on leaves which causes paleness. It belongs to Potyviridae family and devastating for yield losses. Mosaic Virus affects the plant activities, reducing photosynthetic rate, plant metabolism and sugar percentage (Akbar et al., 2021).
In Pakistan, fuzz (sugarcane seed) production is quite difficult because sugarcane is photothermal sensitive crop and it requires 25-33oC temperature for reproduction stage. In Pakistan, these conditions are available only in few places (Khan et al., 2004). Due to limitation of fuzz production and lack of target-based sugarcane hybridization, the variety development is quite difficult (Khan et al., 2012; Aamer et al., 2018). Regarding improvement of sugarcane genotypes, morphological markers play important role in breeding and selection of superior genotypes from germplasm accessions with high yield contributing traits proved significant in enhancing yield potential (Keerio, 2000). Therefore, to enhance sugarcane yield and quality, improvement should be made on yield contributing traits and diseases resistance of the plant (Khan et al., 2012).
The aim of study was to evaluate sugarcane accessions for better yield in response to sugarcane mosaic virus. The objective of study was to investigate sugarcane mosaic virus resistant genotypes and to screen out superior genotypes from germplasm accessions on the basis of morphological traits for sugarcane improvement and future breeding programs.
Materials and Methods
The experimental material comprised of 72 sugarcane genotypes (Table 1) planted on September 15, 2017 in research area of College of Agriculture, University of Sargodha in randomized complete block design having three replications. A loamy soil was selected and field was well prepared before planting and furrows were developed at a distance 75cm apart. Sugarcane setts having three eye buds were planted in two furrows per replication of plot size 1.5x5 m2and buried with shallow soil. Just after planting sugarcane genotypes, the field was irrigated with canal water. A total of 16 irrigations were applied during the whole year having 3 acre inches water per irrigation. Recommended cultural practices were applied round the year. At proper growth stage(at 7 months’ age), sugarcane germplasm was phenotypically evaluated against sugarcane mosaic virus (SCMV) disease based on naturally appeared disease symptoms on leaves using scoring scale as suggested by Addy et al. (2017). Morphological data of different plant traits were also collected at 12 months’ age to observe performance of sugarcane germplasm for yield contributing and quality traits. Five stoolsper genotype per replication were selected under natural field condition and data were collected for SCMV scoring to observe disease intensity and disease severity by using following formulae:
Viral disease incidence (%)
SCMV incidence was assessed by counting number of symptomatic plants per total observed plants of each variety in each replication as suggested by Addy et al. (2017).
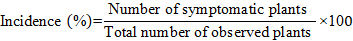
Table 1: List of sugarcane genotypes used for morphological traits and SCMV.
|
S. No. |
Code |
Name of genotype |
S. No. |
Code |
Name of genotype |
|
1 |
SC1 |
SPCG24 |
37 |
SC37 |
CSSG676 |
|
2 |
SC2 |
SPSG28 |
38 |
SC38 |
US384 |
|
3 |
SC3 |
SPSG27 |
39 |
SC39 |
HSF240 |
|
4 |
SC4 |
SPSG26 |
40 |
SC40 |
CSSG2453 |
|
5 |
SC5 |
SSRI4 |
41 |
SC41 |
US133 |
|
6 |
SC6 |
SPSG25 |
42 |
SC42 |
CPS437 |
|
7 |
SC7 |
SSRI1 |
43 |
SC43 |
US127 |
|
8 |
SC8 |
SSRI7 |
44 |
SC44 |
CPPC247 |
|
9 |
SC9 |
SSRI6 |
45 |
SC45 |
CPPC246 |
|
10 |
SC10 |
US718 |
46 |
SC46 |
HSG315 |
|
11 |
SC11 |
SRRI3 |
47 |
SC47 |
CPF248 |
|
12 |
SC12 |
SPSG29 |
48 |
SC48 |
US633 |
|
13 |
SC13 |
India1 |
49 |
SC49 |
XT236 |
|
14 |
SC14 |
Thatha1312 |
50 |
SC50 |
CSSG2402 |
|
15 |
SC15 |
Co0283 |
51 |
SC51 |
CPSG2525 |
|
16 |
SC16 |
MSG502 |
52 |
SC52 |
FST19 |
|
17 |
SC17 |
CPSG2730 |
63 |
SC53 |
MSH2415 |
|
18 |
SC18 |
Aust 134 |
54 |
SC54 |
CP368 |
|
19 |
SC19 |
NIFA01 |
55 |
SC55 |
CPSG2718 |
|
20 |
SC20 |
CSSG33 |
56 |
SC56 |
CPSG3481 |
|
21 |
SC21 |
US832 |
57 |
SC57 |
Co239 |
|
22 |
SC22 |
CSSG23 |
58 |
SC58 |
Co240 |
|
23 |
SC23 |
SCCG32 |
59 |
SC59 |
Co241 |
|
24 |
SC24 |
SPSG27 |
60 |
SC60 |
CPSG2730 |
|
25 |
SC25 |
US272 |
61 |
SC61 |
XT910 |
|
26 |
SC26 |
YT53 |
62 |
SC62 |
SPPC213 |
|
27 |
SC27 |
CSSG25 |
63 |
SC63 |
SPPC220 |
|
28 |
SC28 |
US204 |
64 |
SC64 |
SPF234 |
|
29 |
SC29 |
YT210 |
65 |
SC65 |
SPPC237 |
|
30 |
SC30 |
SSRI2 |
66 |
SC66 |
HSPC240 |
|
31 |
SC31 |
India2 |
67 |
SC67 |
CP67-500 |
|
32 |
SC32 |
MSG1127 |
68 |
SC68 |
SPPC2038 |
|
33 |
SC33 |
XT55 |
69 |
SC69 |
Co 1148 |
|
34 |
SC34 |
US658 |
70 |
SC70 |
NSG59 |
|
35 |
SC35 |
WS130 |
71 |
SC71 |
Laliyan |
|
36 |
SC36 |
US272 |
72 |
SC72 |
SPSG26 |
Viral disease severity (%)
SCMV severity was considered by estimating the percentage leaf area with mosaic symptoms using the following scoring system suggested by Addy et al. (2017).
|
Scoring scale |
Leaf area showing symptoms rating |
|
1 |
No symptoms Immune |
|
2 |
0.1–2.5% Resistant |
|
3 |
2.6–5% Resistant |
|
4 |
5.1–10% Resistant |
|
5 |
10.1–20% Moderately resistant |
|
6 |
20.1–35% Moderately susceptible |
|
7 |
35.1–50% Moderately susceptible |
|
8 |
50.1–75% Susceptible |
|
9 |
75.1–100% Highly susceptible |
Growth and yield data were recorded forcane yield, cane height, cane diameter, leaf area, number of tillers per stool, single cane weight, weight per stool, brix percentage and lodging resistance. Analysis of variance was done by using mean data of all the parameters under study to observe significant differences among cultivars as described by Steel et al. (1997). Tuckey’s range test was used to check the data for similarities and range for each trait. It is also known as Tuckey’s HSD test (Honestly significance difference) it was used to check the genotypes whether they are similar to each other or not. Statistix 8.1 software (Microsoft 365) was used for statistical analysis of data.
Results and Discussion
Evaluation of sugarcane genotypes for sugarcane mosaic virus (SCMV) intensity and severity
Sugarcane mosaic virus (SCMV) is a complex disease of sugarcane and appears on leaves causes chlorosis and yellowing of leaves. Symptoms of disease depends upon the cultivar susceptibility and environment (Haider et al., 2011; Akbar et al., 2021).
Viral disease incidence (%)
Viral tolerance/susceptibility in presented sugarcane genotypes was observed by disease scoring (%) to assess incidence and severity of sugarcane mosaic virus in natural field conditions (Table 3). Analysis of variance exposed highly significant differences among sugarcane genotypes for SCMV incidence (Table 2). This revealed that ample genetic variability was present among sugarcane genotypes for viral incidence. Viral disease scoring suggested that the genotypes SC-16, SC-30, SC 36, SC 44, SC-64, SC-66, SC-71were found highly susceptible while genotypes SC1, SC-3, SC 4, SC-08, SC-07, SC-11, SC-14, SC-25, SC-29, SC-38, SC-46, SC-51, SC-53 and SC-55 were observed as resistant/tolerant against SCMV. The highest incidence of SCMV was observed in genotype SC-16, SC-31, SC-36, SC-39, SC-66, SC-39, SC-66 and SC-70 while SC-4 was the least susceptible genotypes (Figure 1). Our results get support from the findings of Haider et al. (2011), Yasmin et al. (2011) and Addy et al. (2017) who reported similar variations for SCMV disease and other traits among different sugarcane genotypes.
Table 2: Analysis of variances of studied plant parameters in 72 sugarcane genotypes.
|
Variable analyzed |
Sum of squares |
Means squares |
P value |
|
SCMV incidence |
81952.7 |
1154.26 |
0.000 |
|
SCMV severity |
103117 |
1452.35 |
0.000 |
|
Emergence % |
7057.4 |
99.40 |
0.000 |
|
Cane height |
117525 |
1655.3 |
0.000 |
|
Cane diameter |
46.1098 |
064943 |
0.000 |
|
Number of tillers per stool |
286.292 |
4.0323 |
0.000 |
|
Single cane weight |
4124190 |
6.26 |
0.000 |
|
Weight per stool |
231.647 |
3.2626 |
0.000 |
|
Brix % |
197.741 |
2.7851 |
0.000 |
|
Lodging % |
40.862 |
0.5755 |
0.000 |
Note: Highly significant (P<0.01).
Viral disease severity (%)
Regarding SCMV severity analysis of variance indicated that sugarcane genotypes under study differed significantly for viral severity % (Table 2). It showed that enough genetic variation was present among was present among sugarcane genotypes for SCMV severity. Statistical means comparison indicated that the genotype SC16 showed maximum severity. In other word the genotype SC16 was highly susceptible to the viral infection followed by SC 36 and SC-30 whereas genotype SC4 displayed least susceptibility to mosaic virus followed by SC 53 (Figure 2). This revealed that high level of genetic variation prevailed in studied sugarcane genotypes for SCMV susceptibility and tolerance. The results are in accordance with findings of Addy et al. (2017).
Table 3: Sugarcane mosaic virus incidence and severity percentage in 72 sugarcane genotypes.
|
Genotype |
DI (%) |
DS (%) |
Genotype |
DI (%) |
DS (%) |
Genotype |
DI (%) |
DS (%) |
|
SC1 |
20.5 |
10.0 |
SC25 |
40 |
21 |
SC48 |
63.63 |
30 |
|
SC2 |
50 |
31 |
SC26 |
80 |
67 |
SC49 |
68.75 |
45 |
|
SC3 |
54.4 |
42.2 |
SC27 |
85.71 |
70 |
SC50 |
30 |
12 |
|
SC4 |
10 |
4.5 |
SC28 |
87.5 |
72 |
SC51 |
35.29 |
11 |
|
SC5 |
77.77 |
64 |
SC29 |
40 |
20 |
SC52 |
70 |
40 |
|
SC6 |
71.42 |
34 |
SC30 |
83.33 |
74 |
SC53 |
36.36 |
7 |
|
SC7 |
50 |
17 |
SC31 |
100 |
35 |
SC54 |
84.61 |
20 |
|
SC8 |
42.85 |
21 |
SC32 |
50 |
40 |
SC55 |
40 |
15 |
|
SC9 |
57.14 |
45 |
SC33 |
75 |
45 |
SC56 |
63.63 |
34 |
|
SC10 |
83.33 |
48 |
SC34 |
71.42 |
42 |
SC57 |
66.66 |
44 |
|
SC11 |
51.4 |
40.4 |
SC35 |
83.33 |
50 |
SC58 |
53.33 |
25 |
|
SC12 |
75 |
36 |
SC35 |
100 |
75 |
SC59 |
72.72 |
64 |
|
SC13 |
90 |
75 |
SC36 |
70 |
45 |
SC60 |
80 |
45 |
|
SC14 |
48.1 |
35.9 |
SC37 |
44.44 |
14 |
SC61 |
75 |
40 |
|
SC15 |
83.33 |
70 |
SC38 |
50 |
20 |
SC62 |
54.54 |
70 |
|
SC16 |
100 |
80 |
SC39 |
100 |
70 |
SC63 |
73.33 |
40 |
|
SC17 |
87.5 |
52 |
SC40 |
72.72 |
75 |
SC64 |
71.42 |
75 |
|
SC18 |
87.5 |
74 |
SC41 |
90 |
50 |
SC65 |
73.33 |
50 |
|
SC19 |
57.14 |
48 |
SC42 |
76.92 |
45 |
SC66 |
100 |
75 |
|
SC20 |
57.14 |
20 |
SC43 |
75 |
50 |
SC67 |
83.33 |
50 |
|
SC21 |
71.42 |
30 |
SC44 |
91.66 |
74 |
SC68 |
71.42 |
50 |
|
SC22 |
50 |
15 |
SC45 |
53.84 |
15 |
SC69 |
85.71 |
50 |
|
SC23 |
60 |
25 |
SC46 |
42.85 |
15 |
SC70 |
100 |
70 |
|
SC24 |
60 |
25 |
SC47 |
40 |
30 |
SC71 |
83.33 |
75 |
|
SC72 |
90.90 |
75 |
Note: DI= disease intensity% and DS= disease severity %.
Evaluation of sugarcane genotypes for yield contributing and quality traits
Emergence percentage: Emergence is the first stage of plant initiation, which is the most delicate physiological plant stage. Good emergence percentage plays significant role in determining the production performance of sugarcane genotypes. The results revealed that all genotypes displayed highly significant performance for emergence percentage (Table 2). Sugarcane accessions SC8 (79.00 %), SC1 (78.64%) and SC7 (78.00%) gained high mean emergence percentage than others (Figure 3). Similar findings were reported by Tena et al. (2016), and Khaliq et al. (2018).
Cane height (cm): Cane height is an important parameter as cane yield depends on it. Cane height was measured at maturing stage of plant. Analysis of variance showed that all accessions exhibited significant variation for cane height (Table 2). A good range was observed in sugarcane accessions for cane height as SC10 exhibited210cm maximum mean value while SC33 gained 100.67 cm minimum cane height among all genotypes (Figure 4). Our results were similar as reported by Khalid et al. (2014).
Cane diameter (cm): Cane diameter is an important indicator of cane yield as higher the cane dimeter higher will be cane weight. The results of the experiments indicated that significant differences were present among studied sugarcane accessions for cane diameter which showed ample genetic variability for cane diameter (Table 2). Among maximum performance was observed in SC3 (4.43cm) while minimum performance was observed in SC8 i.e., 2.06cm (Figure 5), Alam et al. (2017) and Khan et al. (2018).
Number of tillers per stool: Number of tillers per stool is also an important trait for the yield of sugarcane. All genotypes showed variant results in exhibiting number of tillers per stool as it depended upon genotypic background of every genotypes (Table 2). The mean performance indicated that all genotypes performed well but maximum average performance was observed in SC3 while minimum performance was observed in SC12 (Figure 6). Our results agreed with the findings of Soomro et al. (2006), Mehrab and Abazied (2017) and Khan et al. (2018).
Single cane weight(g): Single cane weight is also one of the most important yield contributing trait. Significant results were obtained after statistical analysis of studied sugarcane accessions (Table 2). Statistical means comparison revealed that SC1 gained maximum weight (1044g) while SC34 (578g) gained less weight then all other genotypes (Figure 7). Our results of analysis supported by the results of Khan et al. (2018) and Tripathi et al. (2017).
Weight per stool (kg): Weight per stool is one of the most important characters of sugarcane genotypes as it contributes directly towards cane yield. High stool population and more weight of sugarcane stoolare indicator of increase in cane yield. ANOVA revealed significant differences among sugarcane genotypes for weight per stool were observed (Table 2). The mean comparisons displayed that the highest weight per stool 7.45Kg was gained by SC7 (Figure 8). Our findings are in compliance with Khan et al. (2018).
Brix percentage: Brix percentage plays significant role in quality of sugarcane. It is used to estimate the soluble solids present in juice of sugarcane. All genotypes exhibited significant genetic variation for brix percentage (Table 2). The highest value for brix percentage was observed in genotype SC12 (23.03%) followed by SC11 (22.91%) while the least brix value 16% was observed in genotype SC31 (Figure 9). Our findings are exactly in compliance with the results of Mehrab and Abazied (2017) and Khan et al. (2018).
Lodging resistance (%): Lodging resistance is an important trait of sugarcane plant because it directly contributes to cane yield. ANOVA Table 2 represented that sugarcane genotypes did not show significant differences among themselves for lodging percentage. This indicated that all studied genotypes showed similar trend for lodging percentage. Mean comparisons showed that there were no statistical differences among all sugarcane genotypes for lodging percentage (Figure 10). Our findings were in contrast with the results of Li et al. (2019).
Conclusions and Recommendations
High level of genetic variation prevailed in studied sugarcane genotypes for morphological traits, sugar recovery and SCMV tolerance. Sugarcane genotypes SC-1, SC-3, SC-11, SC-14, SC-20, SC-24 and SC-40 were found excellent for sugar recovery and cane weight while sugarcane genotypes SC-1, SC-22, SC-04 and SC-38 were observed as resistance/tolerant against SCMV intensity and severity. Furthermore, SC-3, SC-4, SC-20 and SC-33 were found genetically diverse genotypes for studied morphological and quality traits and would be used in sugarcane breeding program to enhance sugar recovery, yield and viral resistance/tolerance in sugarcane cultivars.
Acknowledgements
We are highly thankful to ORIC, University of Sargodha for providing funds to complete this study under project UOS/ORIC/2016/14.
Novelty Statement
Sugarcane mosaic virus is one of the most important disease of sugarcane. This study provides sufficient information about the screening of sugarcane genotypes against SCMV in the field which will be helpful in future for identification and utilization of SCMV free genotypes for in vitro micro-propagation and development of virus free sugarcane cultivars through conventional ways.
Author’s Contribution
Naeem Akhtar: Conducted experiment and wrote manuscript.
Sana Noureen: Helped in data collection and did statistical analysis.
Muhammad Asif: Helped in manuscript write-up.
Ahsan Aziz: Helped in planning and conductance of research.
Usman Saleem: Helped in plagiarism report and proof reading.
Talat Mahmood: Improved language and writing style of the manuscript.
Nadeem Raza: Helped in provision of sugarcane genotypes.
Waqas Raza: Helped in write-up and submission of the revision.
Conflict of interest
The authors have declared no conflict of interest.
References
Aamer, M., M.R. Anwar, G. Mustafa and M. Sohail. 2018. Principal component analysis (pca) of some morphological and quality traits in Sugarcane (Saccharum officinarum L.). J. Nat. Sci. Res., 8(14): 22-26.
Addy, H., A. Wahyudi, A. Sholeh, C. Anugrah, F. Iriyanto, W. Darmanto and B. Sugiharto. 2017. Detection and response of sugarcane against the infection of sugarcane mosaic virus (SCMV) in Indonesia. Agronomy, 7(3): 50. https://doi.org/10.3390/agronomy7030050
Ahmed, M.S. and D.A. Gardezi. 2017. Multivariate analysis in determining morphologically diverse sugarcane genotypes (Saccharum officinarum L.) and their response to flowering at Arja, Azad Kashmir. Sarhad J. Agric., 33(1): 90-102. https://doi.org/10.17582/journal.sja/2017.33.1.90.102
Akbar, S., W. Yao, L. Qin, Y. Yuan, C.A. Powell, B. Chen and M. Zhang. 2021. Comparative analysis of sugar metabolites and their transporters in sugarcane following sugarcane mosaic virus (SCMV) infection. Int. J. Mol. Sci., 22(24): 13574. https://doi.org/10.3390/ijms222413574
Alam, M.N., U.K. Nath, K.M.R. Karim, M.M. Ahmed and R.Y. Mitul. 2017. Genetic variability of exotic sugarcane genotypes. Scientifica, 2017: 1-9. https://doi.org/10.1155/2017/5202913
Aono, A.H., R.J.G. Pimenta, A.L.B. Garcia, F.H. Correr, G.K. Hosaka, M.M. Carrasco and A.P. de Souza. 2021. The wild sugarcane and sorghum kinomes: Insights into expansion, diversification, and expression patterns. Front. Plant Sci., 12. https://doi.org/10.3389/fpls.2021.668623
Baloch, S.M., I.H. Shah, I. Hussain and K. Abdullah. 2002. Low sugar production in Pakistan causes and remedies. Pak. Sugar J., 17(5): 13-14.
Croft, B.J., R.C. Magarey, P.G. Allsopp, M.C. Cox, T.G. Willcox, B.J. Milford and E.S. Wallis. 2008. Sugarcane smut in queensland: Arrival and emergency response. Austral. Plant Pathol., 37(1): 26-34. https://doi.org/10.1071/AP07083
Economy Survey of Pakistan, 2020-21. Federal bureau of statistics. Government of Pakistan.
Food and Agriculture Organization, 2021. FAO statistical year book. Food and Agriculture
organization of United Nations, Rome, Italy.
Haider, M.S., S. Afghan, H. Riaz, M. Tahir, M.A. Javed, N. Rashid and J. Iqbal. 2011. Identification of two Sugarcane mosaic virus (SCMV) variants from naturally infected sugarcane crop in Pakistan. Pak. J. Bot., 43(2): 1157-1162.
Keerio, H.K., 2000. Variety development in sugarcane at PARC Centre (SCRI) Thatta. Pak. Sugar J., 15(6): 70-75.
Khalid, M., H.U. Rahman, M.A. Rabbani and K.A. Farhatullah. 2014. Qualitative and quantitative assessment of newly selected sugarcane varieties. Sarhad J. Agric., 30: 187-191.
Khaliq, A., M. Yasin, M.S. Afzal and N. Ahmed. 2018. Evaluation of different exotic sugarcane genotypes. Russ. J. Agric. Socio-Econ. Sci., 4(76): 296-301. https://doi.org/10.18551/rjoas.2018-04.31
Khan, I.A., S. Bibi, S. Yasmin, A. Khatri, N. Seema and S.A. Abro. 2012. Correlation studies of agronomic traits for higher sugar yield in sugarcane. Pak. J. Bot., 44(3): 969-971.
Khan, I.A., A. Khatri, G.S. Nizamani, M.A. Siddiqui, M.H. Khanzada, N.A. Dahar and M.H. Naqvi. 2004. In-vitro culture studies in sugarcane. Pak. J. Biotechnol., 1(1): 6-10.
Khan, M.A.U., M.T. Aslam, A. Rehman, M. Nawaz, M.J. Khan, M.A. Ayub and R. Ahmad. 2021. Ratooning potential of different promising sugarcane clones under varying trench spacing. J. Innov. Sci., 7(1): 71-77. https://doi.org/10.17582/journal.jis/2021/7.1.71.77
Khan, M.T., I.A. Khan, S. Yasmeen, N. Seema and G.S. Nizamani. 2018. Field evaluation of diverse sugarcane germplasm in agroclimatic conditions of Tandojam, Sindh. Pak. J. Bot., 50(4): 1441-1450.
Li, X., Y.J. Li, Q. Liang, S.H. Lin, Q.Y. Huang, R.Z. Yang and Y.R. Li. 2019. Evaluation of lodging resistance in sugarcane (Saccharum spp. hybrid) germplasm resources. Appl. Ecol. Environ. Res., 17(3): 6107-6116. https://doi.org/10.15666/aeer/1703_61076116
Lu, G., Z. Wang, F. Xu, Y.B. Pan, M.P. Grisham and L. Xu. 2021. Sugarcane mosaic disease: Characteristics, identification and control. Microorganisms, 9(9): 1984. https://doi.org/10.3390/microorganisms9091984
Mehareb, E.M. and S.R. Abazied. 2017. Genetic variability of some promising sugarcane varieties (Saccharum spp.) under harvesting ages for juice quality traits, cane and TSH. Open Access J. Agric. Res., 2(2): 1-14. https://doi.org/10.23880/OAJAR-16000127
Menossi, M., M.C. Silva-Filho, M. Vincentz, M.A. Van-Sluys and G.M. Souza. 2008. Sugarcane functional genomics: Gene discovery for agronomic trait development. Int. J. Plant Genom., 2008: Article ID 458732, 11. https://doi.org/10.1155/2008/458732
Soomro, A.F., S. Junejo, A. Ahmed and M. Aslam. 2006. Evaluation of different promising sugarcane varieties for some quantitative and qualitative attributes under Thatta (Pakistan) conditions. Int. J. Agric. Biol., 8(2): 195-197.
Steel, R.G.D., J.H. Torrie and D.A. Dickey. 1997. Principles and procedures of statistics: A
biometrical approach, 3rd edition. McGraw Hill Book Co., New York.
Tena, E., F. Mekbib and A. Ayana. 2016. Correlation and path coefficient analyses in sugarcane genotypes of Ethiopia. Am. J. Plant Sci., 7(10): 1490-1497. https://doi.org/10.4236/ajps.2016.710141
Tripathi, S., N. Singh, S. Mali, J.R. Naik and S.M. Pritesh. 2017. Sugarcane/ sugarcane juice quality evaluation by FT-NIR spectrometer. Int. J. Curr. Microbiol. Appl. Sci., 6(8): 3025-3032. https://doi.org/10.20546/ijcmas.2017.609.371
Yadav, A.K., R. Abbassi, A. Gupta and M. Dadashzadeh. 2013. Removal of fluoride from aqueous solution and groundwater by wheat straw, sawdust and activated bagasse carbon of sugarcane. Ecol. Eng., 52: 211-218. https://doi.org/10.1016/j.ecoleng.2012.12.069
Yasmin, T., S. Iqbal, A. Farooq, M. Zubair and A. Riaz. 2011. Prevalence, distribution and incidence of major sugarcane infecting viruses in NWFP and Punjab. Pak. J. Phytopathol., 23(1): 24-30.
To share on other social networks, click on any share button. What are these?








