Application of Water Quality and Pollution Tolerance Indexes as Effective Tools for River Management
Application of Water Quality and Pollution Tolerance Indexes as Effective Tools for River Management
Joyce Osarogie Odigie*
Department of Animal and Environmental Biology, Faculty of Life Sciences, University of Benin, Edo State, Nigeria.
Abstract | High levels of anthropogenic activities alters freshwater bodies and thus depreciating water quality. Water quality (WQI) and Pollution tolerance indexes (PTI) are effective biomonitoring tools being applied in river management by freshwater ecologists. Obueniyomo River is the main water supply channel for the inhabitants of Agemokpa village in Ovia East Local Government Area of Edo State, as they are fully dependent on it for agricultural and domestic use, bringing its ecological integrity to question. These anthropogenic effects result in the deteriorating quality of the water, which hampers the distribution of benthic macroinvertebrates and may be hazardous to the health of the inhabitant that consumes it on a daily basis. The goal of this study was to assess the effectiveness of WQI and PTI indexes as biomonitoring tools in river management. We studied Obueniyomo River to evaluate human influence on the river and aquatic biota from March 2016- February 2018. Three stations upstream, mid-stream and downstream were selected. The data from the three study sites were subjected to statistical and biodiversity analysis with the extracted data for water quality analyzed via Weighted Arithmetic Index and pollution tolerance Index via pollution sensitive group. The dominant benthos group was Chironomidae: making up 20.99%, 18.47% and 16.65% of the benthic community. The results of WQI calculated across the three study stations were above the 100 benchmark. However, the PTI values recorded at the three study stations were less than 10, correlating with WQI indicating pollution, making the water unsuitable for human consumption and aquatic life.
Article History
Received: August 29, 2018
Revised: Febraury 05, 2019
Accepted: July 12, 2019
Published: September 05, 2019
Keywords
Biomonitoring studies, Macroinvertebrates, Pollution tolerance index, River management, Water quality index
Corresponding Author
Odigie Osarogie Joyce
To cite this article:
Odigie, J.O., 2019. Application of water quality and pollution tolerance indexes as effective tools for river management. Punjab Univ. J. Zool., 34(2): 105-113. https://dx.doi.org/10.17582/journal.pujz/2019.34.1.105.113
Introduction
Water is an indispensable resource for survival as it contains minerals that are significant for human and aquatic life. Lentic and lotic ecosystems are the earth’s utmost freshwater resources that provide innumerable benefits especially for industrial, agricultural (irrigation) and local purposes. Other important socio-economic benefits includes, “tourism and recreation”, which are traditionally and artistically significant worldwide (Dirican, 2015). However, environmental dilapidation due to increased populace and fiscal expansion deepens global degeneration in biodiversity and the environmental functionality of freshwater ecosystems (Deborde et al., 2016). These upsurge in human land use such as deforestation, agriculture, land alterations, modification of stream channels, and undue nutrient input, endangers stream habitats (McGoff et al., 2013; Odigie, 2014). Furthermore, anthropogenic disruptions of the ecological reliability of freshwater ecosystems causes a decrease in primary productivity, alteration of stream trophic structure and modification of stream channel dynamics, thus, leading to the reduction of sediment stability (Walsh et al., 2002).
In Nigeria, the pollution of water ways by organic discharges is a genuine danger to inland water ways (Arimoro et al., 2007). In the past, pollution of waterbodies was a trivial issue because human populations were negligible as inhabitants either lived in dispersed societies, where reduced wastes discarded into rivers were subjected to self-purification (Olomukoro and Azubuike, 2009). Rural settlements are now a recent haven for the illegal dumping of untreated wastes from the urban centers. Also, unrestrained agronomy, unwarranted use of fertilizers, and pesticides destroys the ecological balance in riverine ecosystems (Olomukoro and Azubuike, 2009).
According to Camur-Elipek et al. (2006) alterations in benthic community structure are widely used in pollution assessment studies. Several assessments and biomonitoring strategies has been advocated by ecologists to evaluate the biotic quality of stream ecosystems as this strategy will go long way in aiding human sustainability and the ecological demands for fresh waters. These, however, have paved ways for the emergence of new approaches that creates wide-ranging investigations on the general state of freshwater biomes. Such studies include; Karrouch et al. (2017), Kebede et al. (2010), Odigie (2014), Olomukoro and Dirisu (2014), Ouyang (2005), Sharifah-Aisyah et al. (2015) and Sing et al. (2004).
The application of PTI for biomonitoring studies in tropical rainforest rivers in Edo state has been scarcely studied apart from Olomukoro and Dirisu (2014) who applied PTI to check the pollution status of Edion and Omodo Rivers in Agbede wetlands and reported moderate pollution levels in both water bodies. This dearth of information on the application of PTI and WQI in dense rainforest rivers situated in rural communities of Edo state was the driving force of this research. The current study seeks to provide basic information that will bridge the gap on the efficacy of biomonitoring applications in River management, thus, taking into account the environmental data and benthic macroinvertebrates structure in freshwater ecosystems using Obueniyomo River as a baseline.
Materials and Methods
Study area
Obueniyomo River is situated in Agemokopa village (Ovia East LGA) about 60km from Benin City, Edo State, Nigeria. The river takes its source from Odighi Reserve and meanders through a dense tropical rainforest and villages (Ugboke, Ago-Arowele, Aburime) into Ovia River. It lies between Latitude 005˚ 34.445’ E and Longitude 06˚ 37.477’ N at an altitude of 40m above sea level (Figure 1). The study area has a tropical climate with heavy rainfall. Heavy rain is known in Agemokpa village with the climate similar to Benin City, although during the two years sampling period, the community experienced prolonged dry season and a slight delay in rainfall. The size of the Obueniyomo River which runs through major settlements is approximately 1500 meters. The wet seasons at Agemokpa produces a river regime of peak flow from August to early November and low movement from December to April. The rainy season last for 9 months (March to November) with a mean annual rainfall from 1300-2500mm. The dorminant plant species within ten meters’ distance around the river includes; Achyranthes aspera (Devil’s Horsewhip), Acroceras zizanoides (Oat Grass), Alchornea cordifolia (English Christmas Bush), Alstonia boonei (Cheese Wood), Anthocleista vogelii (English Cabbage Tree), Asystasia gangetica (Chinese Violet), Axonopus compressus (Carpet Grass), Bambusa vulgaris (Bamboo), Baphia nitida (Camwood), Calopogonium mucunoides (Calopo), Cercestris afzelii (Mbembei), Chromolaena odorata (Camphor Grass), Cissus araloides (Monkey Plum), Colocasia esculenta (Cocoyam) and Combretum racemosum (English Christmas Rose).
Sampled stations
Three sampling stations along the stretch of Obueniyomo River were selected for this study. Site selection was driven by; the objectives of the study, anthropogenic impacted sites, biota, number of microhabitats represented in the system under investigation and somewhat by logistics. Also, some characteristics such as flow velocity, canopy cover, and depth of overlying water as well as substrate type and size.
Station 1: This is a stretch upstream of Obueniyomo River sited close to the source. It lies between Latitude 005˚ 35.203’ E and Longitude 06 ˚ 37.740’ N at an altitude of 51 meters above sea level. The water is clear and sediment muddy. There is a dense canopy of forest trees around the station. This site experience moderate anthropogenic activities as paths are created with wood to access the dense forest for timber. The main pollutants of this site are from rainfall runoff and decomposition of plant materials.
Station 2: This station is located midstream of Obueniyomo River. It lies between Latitude 005 ˚ 34.820’ E and Longitude 06 ˚ 37. 777’ N at an altitude of 36 meters above sea level. The water is also clear and sediment a bit muddy. There are fewer forest trees around the station but ferns, mosses, and grasses dominate this station. Less anthropogenic activities occur on this site, thus, the pollutants are mainly decomposed aquatic macrophytes.
Station 3: It lies between Latitude 005˚ 34.512 E and Longitude 06˚ 37.422’ N at an altitude of 39 meters above sea level. The station is located close to the bridge and major road of Agemokpa village. There are intense anthropogenic activities in this station; as farmers return from farms and children from school, they go straight to the river for a swim or bath in the cool water. Washing of farm implements and motorbikes are common around this site. In addition, peeled cassava is commonly seen immersed in the water
for fermentation and pulping. Also, lather from clothes washing and white precipitate from cassava washing was observed floating on the surface of the water.
Benthic macrofauna
Sampling of macroinvertebrates: Composite samples for benthic macrofauna was collected with the use of Ekman grab (0.023m2; 15.2 × 15.2 cm cutting edge; 5.5 kg) made by Hydrobios earlier used by Ogbeibu and Oribhabor (2002) and Olomukoro (2008). Three hauls were collected, sieved and stored in a pre-labelled plastic container. It was then fixed with 10% formalin and 0.1% Rose Bengal dye for further benthic macroinvertebrates analysis in the laboratory.
Sorting
In the laboratory, the fixed samples with the benthic macrofauna was poured into a white enamel tray for sorting. For improved sorting; adequate volume of water was added to the sorting dish to improve visibility. Large benthos was picked with forceps while smaller were collected with the use of a pipette. Also, Optical dissecting microscope (Model 570, USA) with ×2 and ×4 magnifications was also used to aid proper sorting.
Preservation
The sorted benthos samples were preserved in 4% formalin for identification and counting.
Identification and Counting
Optical dissecting microscope (Model 570, USA) with ×2 and ×4 magnification and a Binocular Light Microscope® (Olympus, China) were used for identification and counting of benthos in the laboratory. Furthermore, the grouping of the benthic macroinvertebrates to the lowest taxonomic group was achieved through the aid of relevant identification manuals and guides.
Determination of water quality index
Weighted Arithmetic Index method by Cude (2001) was applied for water quality calculation. Different water quality constituents are multiplied by weighting a factor which included the aggregating factor by applying the arithmetic mean to evaluate the quality of the water. The rating scale (Qi) for every single factor was calculated using.
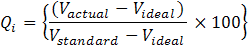
Qi = Quality rating of ith parameter for a total of n water quality parameters; V actual = Actual value of the water quality parameter obtained from laboratory analysis; V ideal = Ideal value of the water quality parameter obtained from the standard tables; Therefore, Videal for pH = 7, DO V ideal = 14.6 mg/L while other parameters = 0; Vstandard = Recommended Federal Ministry of Environment permissible; limits standard of water quality parameter.
The quality rating scale (Qi) is therefore calculated as follows:
Wi = 1/Si Where, Wi = Relative (unit) weight for nth parameter
Si= Standard permissible value for nth parameter; 1= Proportionality constant.
The total WQI was calculated by aggregating the quality rating with the unit weight linearly by the relative (unit) weight (Wi) calculated by a value inversely proportional to the recommended standard (Si) for the analogous factor using the following equation:
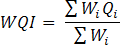
Where Qi = Quality rating and Wi = Relative weight
The results obtained were compared using water quality ratings benchmark of 100 by Ramakrishniah et al. (2009).
Determination of pollution tolerance index
Calculation of Pollution tolerance Index (PTI) by utilizing computational methods has been described previously by Olumokoro and Dirisu (2014). Three (3) macroinvertebrates groups were allocated into the pollution sensitive group (Ephemeroptera, Trichoptera, Coleoptera and Plecoptera), facultative or tolerant group (Diptera, Chironomidae, Zygoptera and Decapoda) and highly tolerant or pollution tolerant group (Mollusca, Oligochaetes and Hirudinea). These were summed to get the PTI standards for the three stations and the same application considered in spatial variations. Values acquired were matched with established standards by Bonada et al. (2006), “23 and above = Unpolluted water, 17-22 = good, 11-16 =fair and < 10 =poor”.
Ecological Data
The ecological data obtained from the water, sediment and benthos sampling were subjected to various statistical analyses using Microsoft Excel (2016), SPSS version 23.0 and Paleontological Statistics version 3.0. Fauna diversity of the macrobenthic community was determined using Margalef’s Index, Shannon- Weiner Index (H), Evenness Index (E), Simpson’s Dominance Index (C), (Ogbeibu, 2005; Olomukoro and Victor, 2001).
Results and Discussion
Benthos group
In this study, the benthic macrofauna recorded from the three stations accounted for relatively high species richness in stations 1 and 2 where less anthropogenic activities occurred. Species richness recorded high values of 2.6450 in station 2 with station 3 recording low values of 1.9902 in the study sites. Higher diversity were observed in station 1 (1.0920 and 6.9820) with a reduction in station 2 (1.0770 and 6.7320). However, the evenness index in our study showed that the benthic macrofauna of Obueniyomo River were evenly distributed in station 3 (0.9294) than the other two stations. Furthermore, benthic macrofauna were dominant in stations 1 and 2 which recored the same values of 0.1030 with station 3 recording least dorminace (0.9294) . Also, number of species present, as well as the abundance of each species, recorded maximum values in station 1 (0.1030) and 2 (10.100) in the study months. This shows that stations 1 and 2 recorded the highest number of species as well as maximum abundance of benthos, with the reverse occurring in station 3 (Table 1). The dominant benthos group observed in this study was Chironomidae which is a pollution tolerant taxa and accounted for 20.99% in station 1, 18.47% in station 2 and 16.65% in station 3 respectively. However, the dominant group was closely followed by Decapoda (12.71% station 1, 13.25% station 2 and 10.75% station 3), Coleoptera (8.83% station 1, 11.58% station 2 and 12.95% station 3) and Diptera (10.10% station 1, 9.61% station 2 and 9.43% station 3). The least dominant groups observed were Nematoda (0.46% station 1, 0.45% station 2 and 0.26% station 3), Hymenoptera (0.46% station 1, 1.06% station 2 and 1.50% station 3), Orthoptera, (0.46% station 1, 0.00% station 2 and 0.00% station 3) and Lepidoptera (0.00% station 1, 0.23% station 2 and 0.23% station 3) in the study months. The relative percentage composition of the benthos assemblages from the three stations shows that incessant anthropogenic activities ranging from logging, farming and domestic inputs into the water body increased the sedimentation rate of river, leading to high levels of organic pollution that served as the leading factor contributing to the dominance of Chironomidae in the three study sites. Chironomidae are considered as favourable bioindicators of water quality and an exceptional factor especially in the assessment of freshwater effluence in an aquatic ecosystem (Table 2 and Figure 2).
Table 1: Species richness of the three stations from March 2016 – February 2018.
|
Species |
Station 1 |
Station 2 |
Station 3 |
|
Species Richness Index (d) |
2.5901 |
2.6450 |
1.9902 |
|
Shannon Wiener Index (H) |
1.0920 |
1.0770 |
1.0930 |
|
Shannon’s Index (HI) |
6.9820 |
6.7320 |
6.7640 |
|
Evenness Index (EI) |
0.8394 |
0.8275 |
0.9294 |
|
Simpson’s Dominance Index (C) |
0.1030 |
0.1030 |
0.0950 |
|
Simpson’s Index (D) |
0.1030 |
0.1020 |
0.0940 |
|
Simpson’s Index (DI) |
8.5470 |
10.100 |
4.6080 |
|
Equitability (J) |
0.8394 |
0.8275 |
0.9294 |
Table 2: Relative percentage composition of the benthic macroinvertebrates in the study stations.
|
TAXA |
Station 1 |
Station 2 |
Station 3 |
|
Nematoda |
0.46 |
0.45 |
0.26 |
|
Oligochaeta |
8.34 |
4.69 |
7.22 |
|
Hirudinea |
1.43 |
1.06 |
3.26 |
|
Decapoda |
12.71 |
13.25 |
10.75 |
|
Arachnida |
4.37 |
4.09 |
3.88 |
|
Plecoptera |
1.43 |
0.68 |
0.00 |
|
Ephemeroptera |
7.76 |
10.52 |
10.75 |
|
Odonata |
7.56 |
5.68 |
2.56 |
|
Hemiptera |
4.24 |
7.34 |
5.99 |
|
Lepidoptera |
0.00 |
0.23 |
0.23 |
|
Trichoptera |
5.08 |
6.13 |
5.46 |
|
Coleoptera |
8.83 |
11.58 |
12.95 |
|
Diptera |
10.10 |
9.61 |
9.43 |
|
Chironomidae |
20.99 |
18.47 |
16.65 |
|
Hymenoptera |
0.46 |
1.06 |
1.50 |
|
Orthoptera |
0.46 |
0.00 |
0.00 |
|
Mollusca |
3.39 |
1.44 |
4.58 |
|
Amphibian |
1.30 |
1.06 |
3.35 |
|
Unidentified benthic larvae |
1.69 |
2.65 |
1.41 |
Water quality
In this study, the results of WQI calculated across the three study stations showed slightly elevated levels in
Table 3: Water quality index and pollution tolerance index of Obueniyomo River.
|
|
Stations 1 |
Stations 2 |
Stations 3 |
|
|
|
± SD |
± SD |
± SD |
Bench Mark |
|
(Min-Max) |
(Min-Max) |
(Min-Max) |
||
|
WQI |
155.12±231.66 |
117.40±176.89 |
412.47±1403.07 |
100 |
|
25.98-1186.99) |
(20.56-364.91) |
(25.34-6986.74) |
||
|
PTI |
5.56 |
6.44 |
5.52 |
Ratings |
|
< 10 = POOR |
stations 1 (155.12±231.66) and 2 (177.40±176.89) indicating pollution and poor water quality. However, station 3 (412.47±1403.07) was above 300 signifying an elevated polluted state and unsuitability for human consumption, although the water from this river remains the major source for drinking and domestic use by the inhabitants of the rural community (Table 3). Furthermore, the spatial and monthly variations of WQI in Obueniyomo River showed peak levels from the months of August-October 2017 in station 3 and September- November in station 1 with constant levels observed at other study months in the three stations (Figure 3).
Pollution tolerance index
The values of unpolluted water that ranges from 23 and above is termed excellent. In addition, a value ranging from 17-22 is termed good and polluted water range from 11-16 is termed fair, while from < 10 is poor. In Obueniyomo river, the EPT (Ephemeroptera, Plecoptera and Trichoptera) group were not as abundant as Chironomidae with is a pollution tolerant species which recorded the highest occurrence at the three study stations. These results serve as pointer to the pollution status of Obueniyomo River. The summary of the results of PTI of the three stations of Obueniyomo River revealed that the values recorded at the study station 1 was 5.56, while stations 2 and 3 recorded 6.44 and 5.52 respectively. The PTI values recorded at the three study stations were less than 10, which indicated pollution. The findings of the PTI and WQI of Obueniyomo River highly correlated, which makes the status of the water body to be of high risk to the inhabitants of Agemokpa community (Table 3).
Omoigberale et al. (2014) asserted that “the water quality of a river at any point reflects several factors that includes; lithology of the basin, atmospheric inputs, climatic conditions and anthropogenic inputs”. In the present study, the WQI from the three stations were above the 100-bench mark indicating poor water quality which was attributed to the increased organic pollution of the water body from anthropogenic, agricultural and allochthonous materials thereby reducing the oxygen level of the water body. These factors attributed the abundance of Chironomidae which is a pollution tolerant species whose abundance in aquatic ecosystem is indicative of high levels of organic pollution. In similar studies that corroborated with the results from Obueniyomo River, Sharma et al. (2011) recorded like results in Behlol Nullah tributary of River Tawi, thus strongly suggesting poor condition of riverine system due to increased population of Chironomidae. In the same vein, Ayobahan et al. (2014) assessed the impact of anthropogenic activities on the water quality of Benin River and detected variations in the physicochemical parameters between the study sites with intense human activities which elevated the pollution levels of sampled sites. The WQI of the stations was very poor for (201 - 300) human consumption which was similar to the results obtained in Obueniyomo River. However, the EPT (Ephemeroptera, Plecoptera and Trichoptera) group were not as abundant as Chironomidae which recorded the highest percentage representation in the study stations, indicating elevated stress levels of the river from organic pollution. Also, the low faunal abundance in the three sites and the presence of the pollution tolerant group were indications that study stations are ecologically unhealthy sites and this is similar with the corresponding reports of Akaahan (2014) in Benue River and Olomukoro and Dirisu (2012) in Edion River. The relative abundance of this benthos group has been highlighted by Wallace and Hynes (1981); who stressed that abundance of substrates and pH levels in the selected sited may also have favored the dominance of Chironomidae taxa at the three sites studied. In addition, Guimarães et al. (2009), used WQI to characterize urban streams using benthic macrofauna community metrics, recording the dominance of Chironomidae which occurred in high percentage in all streams. They asserted that, the dominance of Chironomidae in almost all sampling sites in both periods may also have favored the absence of differences among streams and between periods using Shannon-Wiener diversity (H’) and Pielou evenness (J’) metrics, as the very high values of abundance, which may have masked possible differences in the community. Ribeiro and Uieda (2005) suggested that the removal of the benthos group Chironomidae from the analysis could probably show other patterns, thus highlighting the importance of Chironomidae in structuring aquatic communities. Furthermore, the spatial and monthly variations of WQI in Obueniyomo River and the peak levels observed from the months of August-October 2017 in station 3 and September- November in station 1 with constant levels observed at other study months agreed with Patil et al. (2012) who asserted that the physicochemical parameters of water are important as it projects the exact clue of the status of any waterbody when compared with the results and standard values obtained.
Nonetheless, benthos diversities in specific aquatic ecosystems have been applied by numerous researchers to establish the ecological state of the aquatic environments that is been studied. The applied methods have been corroborated by benthic ecologists as a significant tools that is dependable, and globally acceptable as a standard for biomonitoring studies (Olomukoro and Dirisu, 2014). In the present study, the PTI values for Stations 1, 2 and 3 recorded a range of 5.56, 6.44 and 5.52 which was less than 10 and correlated with WQI, thus indicative that the water body is high in pollution. Although the PTIs obtained in the current study differ greatly from those recorded in the assessment of the health status of some rivers, streams, lakes and ponds; Andem et al. (2015), Olomukoro and Dirisu (2014) and Ghosh and Biswas (2017). The characteristic differences in the fauna of the aquatic populations from these studies were the leading factor for the bias recorded in Obueniyomo River. In station 1(upstream) and 2 (mid-stream) the species richness recorded, resulted in a more stable community structure. In compares to station 3 (downstream) where intense anthropogenic activities occurred, the benthic biota were affected and resulted to an unstable ecosystem. These observations compared to the result of Sharma et al. (2008) when they assessed the ecological status of some streams & rivers in Hindu-Kush Himalayan (HKH) region of India and reported that increased anthropogenic disturbances especially downstream of Behzat stream, threatened the diversity of benthic organisms and community structure of the waterbody. In addition, numerous studies have pointed to the fact that the richness in macrofauna aids the detection of ecological reactions due to their sensitivity to manifold disturbances (Friberg et al., 2011). This occurs owing to an inordinate impact of challenges happening between tropical streams ecosystem (Feio et al., 2015). However, the paucity of data on the nomenclature of faunal groups, reduced effectiveness of biotic indices, alterations in community structure, disparity in purposeful methods, and periodic variant have amplified the interest in studying tropical streams using benthic macrofauna in this part of the world (Deborde et al., 2016). Further studies on the clustering of the benthic fauna according to their tolerance levels in less studied rural streams and rivers had been advocated by freshwater ecologists as it will provide a clearer picture on the tolerance rate of benthic macroinvertebrates to different sources of pollution mainly from anthropogenic activities.
Conclusion
In this study, the pollution sensitive taxa Ephemeroptera, Plecoptera and Trichoptera recorded low diversity, while the pollution tolerant taxa Chironomidae recorded the highest diversity in the three study stations signifying the reduced water quality status and the low PTI levels of the waterbody. This hydrobiological investigation elaborates emphasis on the applications of water quality and pollution tolerance indexes in monitoring the ecology of macroinvertebrates fauna and the physicochemical characteristics of freshwater bodies, as these two factors remain the major components of ecological biomonitoring in river management.
Acknowledgements
This publication is part of the author’s doctoral thesis and I wish to acknowledge my supervisor Prof J.O Olomukoro for his useful advice and criticism during the course of this research work. Also, Mr. Festus Arijode who worked tirelessly with me in the field.
Conflict of Interest
The Author declare that there are no conflict of interest regarding the publication of this article.
References
Akaahan, T.J.A., 2014. Studies on benthic fauna as bioindicators of pollution in River Benue at Makurdi, Benue State, Nigeria. Int. Res. J. Environ. Sci., 3: 33-38.
Andem, A.B., Esenowo, I.K. and Bassey, D.O., 2015. Application of biotic indices and pollution tolerance index in assessing macro-invertebrate assemblage of Ediba River, Cross River State, Nigeria. Environ. Anal. Toxicol. S7: 007. https://doi.org/10.4172/2161-0525.S7-007
Arimoro, F.O., Ikomi, R.B. and Efemuna, E., 2007. Macroinvertebrate community patterns and diversity in relation to water quality status of River Ase, Niger Delta, Nigeria. J. Fish. Aquat. Sci., 2: 337-344. http://dx.doi.org/10.3923/jfas.2007.337.344
Ayobahan, S.U., Ezenwa, I.M., Orogun, E.E., Uriri, J.E. and Wemimo, I.J., 2014. Assessment of anthropogenic activities on water quality of Benin River. J. Appl. Sci. Environ. Manage., 18:629-636. https://doi.org/10.4314/jasem.v18i4.11
Bonada, N., Prat, N., Resh, V. H. and Statzner, B., 2006. Developments in aquatic insect biomonitoring: A comparative analysis of recent approaches. Ann. Rev. Entomol., 51:495-523. https://doi.org/10.1146/annurev.ento.51.110104.151124
Camur-Elipeka, B., Arslan, N., Kirgiz, T. and Oterler, B., 2006. Benthic macrofauna in Tunca River (Turkey) and their relationships with environmental variables. Acta hydrochim. Hydrobiol., 34: 360 – 366. https://doi.org/10.1002/aheh.200500631
Cude, C., 2001. Oregon Water quality index: a tool for evaluating water quality management effectiveness. J. Am. Water Res. Assoc., 37: 125-137.
Deborde, D.D.D., Hernandez, M.B.M. and Magbanua, F.S., 2016. Benthic macroinvertebrate community as indicator of stream health: effects of land use on stream benthic macroinvertebrates. Sci. Diliman, 28: 5-25.
Dirican, S., 2015. Assessment of water quality using physico-chemical parameters of Çamlıgöze Dam Lake in Sivas, Turkey. Ecologia, 5:1-7. https://doi.org/10.3923/ecologia.2015.1.7
Feio, M.J., Ferreira, W.R., Macedo, D.R., Eller, A.P., Alves, C.B.M., França, J.S. and Callisto, M., 2015. Defining and testing targets for the recovery of tropical streams based on macroinvertebrate communities and abiotic conditions. River Res. Applic., 31:70-84. https://doi.org/10.1002/rra.2716
Findlay, S., Quinn, J.M., Hickey, C.W., Burrell, G. and Downes, M., 2001. Effects of land use and riparian flow path on delivery of dissolved organic carbon to streams. Limnol. Oceanogra., 46: 345-355. https://doi.org/10.4319/lo.2001.46.2.0345
Friberg, N., Bonada, N., Bradley, D.C., Dunbar, M.J., Edwards, F.K., Grey, J., Hayes, R.B., Hildrew, A.G., Lamouroux, N. and Trimmer, M., 2011. Biomonitoring of human impacts in freshwater ecosystems: the good, the bad and the ugly. Adv. Ecol. Res., 44:1-68. https://doi.org/10.1016/B978-0-12-374794-5.00001-8
Ghosh, D. and Biswas, K.J., 2017. Efficiency of Pollution Tolerance Index (PTI) of macroinvertebrates in detecting aquatic pollution in an oxbow lake in India. Univ. Sci., 22: 237-261. https://doi.org/10.11144/Javeriana.SC22-3.eopt
Guimarães, R.M., Facure, K.G., Pavanin, L.A. and Jacobucci, G.B., 2009. Water quality characterization of urban streams using benthic macrofauna community metrics. Acta Limno. Brassellie, 21: 217-226.
Henley, W.F., Patterson, M.A., Neves, R.J. and Lemly, A.D., 2000. Effects of sedimentation and turbidity on lotic food webs: A concise review for natural resource managers. Rev. Fish. Sci., 8:125-139. https://doi.org/10.1080/10641260091129198
Karrouch, L., Chahlaoui, A. and Essahale, A., 2017. Anthropogenic impacts on the distribution and biodiversity of benthic macroinvertebrates and water quality of the Boufekrane River, Meknes, Morocco. J. Geosci. Environ. Protn., 5:173-195. https://doi.org/10.4236/gep.2017.57014
Kebede, Y., Kebede, T, Assefa, F. and Amsalu, A., 2010. Environmental impact of coffee processing effluent on the ecological integrity of rivers found in Gomma Woreda of Jimma Zone, Ethiopia. Ecohydro. Hydrobiol., 10:259-270. https://doi.org/10.2478/v10104-011-0019-2
Mcgoff, E., Solimini, A.G., Pusch, M.T., Jurca, T. and Sandin, L., 2013. Does lake habitat alteration and land-use pressure homogenize European littoral macroinvertebrate communities? J. Appl. Ecol., 50:1010-1018. https://doi.org/10.1111/1365-2664.12106
Odigie, J.O., 2014. Harmful effects of wastewater disposal into water bodies: a case review of the Ikpoba River, Benin City, Nigeria. Trop. Freshwat. Bio., 23: 87 – 101. https://doi.org/10.4314/tfb.v23i1.5
Ogbeibu, A.E. and Oribhabor, B.J., 2002. Ecological impact of River impoundment using benthic macrofauna as indicators. Water Res., 36: 2427-2436. https://doi.org/10.1016/S0043-1354(01)00489-4
Ogbeibu, A.E., 2005. Biostatistics: a practical approach to research and data handling. Mindex Publishing Co. Ltd., Benin City, Nigeria. pp. 171-173.
Olomukoro, J.O. and Dirisu, A., 2012. Macrofauna community of a post-lindane treated stream flowing through derived savannah in Southern Nigeria. Trop. Freshwat. Bio., 21:67-82. https://doi.org/10.4314/tfb.v21i1.6
Olomukoro, J.O. and Dirisu, A., 2014. Macroinvertebrate community and pollution tolerance index in Edion and Omodo Rivers in derived savannah wetlands in Southern Nigeria. Jordan J. Biol. Sci., 7:19 -24. https://doi.org/10.12816/0008208
Olomukoro, J.O. and Victor, R., 2001. The distributional relationship between the macrobenthic invertebrate fauna and particulate organic matter in a small tropical stream. Trop. J. Environ. Sci. Health, 2:58 – 64.
Olomukoro, J.O., 2008. Factors influencing the benthic macrofauna of erosional biotope in Warri River, Nigeria. Biosci. Res. Commun., 20: 18-23.
Olomukoro, J.O. and Azubuike, C.N., 2009. Heavy metals and macroinvertebrate communities in bottom sediment of Ekpan Creek, Warri, Nigeria. Jordan J. Biol. Sci., 2:1–8.
Omoigberale, M.O., Oboh, I.P., Erhunmwunse, N.O., Ezenwa, I.M. and Omoruyi, S.O., 2014. An assessment of the trace metal contents of Owan River, Edo State, Nigeria. Eur. Int. J. Sci. Technol., 3: 88-98.
Ougang, Y., 2005. Evaluation of river water quality monitoring stations by principal component analysis. Water Res., 39:2621-2635. https://doi.org/10.1016/j.watres.2005.04.024
Patil, P.N., Sawant, D.V. and Deshmukh, R.N., 2012. Physico-chemical parameters for testing of water: A review. Int. J. Environ. Sci., 3:1194-1207.
Ramakrishniah, C.R., Sadashivaiah, C. and Ranganna, G., 2009. Assessment of water quality index for the groundwater in Tumkur Taluk. J. Chem., 6:523-530. https://doi.org/10.1155/2009/757424
Ribeiro, L.O. and Uieda, V.S., 2005. Estrutura da comunidade de macroinvertebrados bentônicos de um riacho de serra em itatinga, São Paulo, Brasil. Rev. Brasil Zool., 22: 613-618. https://doi.org/10.1590/S0101-81752005000300013
Sharifah-Aisyah, S.O.A., Aweng, E.R.A, Razak, W.A. and Ahmad, A.K.B., 2015. Preliminary study on benthic macroinvertebrates distribution and assemblages at Lata Meraung Waterfall, Pahang, Malaysia. J. Teknologi (Sci. Engine.), 72:1-7. https://doi.org/10.11113/jt.v72.3929
Sharma, K.K., Langer, S. and Sharma, R., 2011. Water quality and macrobenthic invertebrate fauna of Behlol Nullah, Jammu (Jand K). Ecol. Scan., 5:111-115.
Sharma, M.P., Sharma, S., Goel, V., Sharma, P. and Kumar, A., 2008. Water quality assessment of Ninglad stream using benthic macrofauna. Life Sci. J., 5: 67-72.
Singh, K., Malik, A., Mohan, D. and Sinha, S., 2004. Multivariate statistical techniques for the evaluation of spatial and temporal variations in water quality of Gomti River (India) - A case study. Water Res., 38:3980-3992. https://doi.org/10.1016/j.watres.2004.06.011
Wallace, R.B. and Hynes, H.B.N., 1981. The effect of chemical treatment against black fly larvae on the fauna of running waters. In: Laird M (Eds.), Black Flies, the future for biological methods in integrated control, Acad. Press, London, pp. 327 – 358.
Walsh, C.J., Gooderham, J.P.R., Grace, M.R., Sdraulig, S., Rosyidi, M.I. and Lelono, A., 2002. The relative influence of diffuse and point-source disturbances on a small upland stream in East Java Indonesia: a preliminary investigation. Hydrobiologia, 487: 183–192. https://doi.org/10.1023/A:1022990822198
To share on other social networks, click on any share button. What are these?








