Antimicrobial and Anti-Prostate Cancer Activity of Turmeric (Curcuma longa L.) and Black Pepper (Piper nigrum L.) used in Typical Pakistani Cuisine
Antimicrobial and Anti-Prostate Cancer Activity of Turmeric (Curcuma longa L.) and Black Pepper (Piper nigrum L.) used in Typical Pakistani Cuisine
Saba Irshad1,*, Ayesha Ashfaq1, Ammara Muazzam1 and Abida Yasmeen2
1Institute of Biochemistry and Biotechnology, University of the Punjab, Lahore, Quaid-i-Azam Campus-54590, Pakistan
2Department of Chemistry, Lahore College for Women University, Lahore, Pakistan
ABSTRACT
Plant extracts have been used as an active antimicrobial compound since long. The present study was intended to validate the antimicrobial, antidiarrheal and anticancer activity of turmeric (Curcuma longa L.) and black pepper (Piper nigrum L.), used in Pakistani cuisine to enrich flavour and to treat infectious wounds. Their crude ethanol and methanol extracts were tested against five important bacterial and fungal strains. Both extracts showed maximum antibacterial activity against B. subtilis, E. coli and S. aureus. All alcoholic extracts were competent enough to reduce the growth rate of M. canis and A. flavus up to 35%, with no inhibitory influence on Candida species and F. solani. These extracts fully inhibit growth of PC3 cells. Moreover, partially purified lectin, from Curcuma longa L. with a molecular weight of 17.3 KDa might be useful to treat patients as a considerably cheap herbal drug which can be prescribed to poor people efficiently at an affordable cost.
Article Information
Received 22 March 2017
Revised 28 April 2017
Accepted 12 May 2017
Available online 25 August 2017
Authors’ Contribution
SI planned and supervised the research project. AA did all the practical work. AM prepared the Manuscript. AY helped in anticancer assays.
Key words
PC3 cells, Spice, Curcumin, Piperine, Lectin.
DOI: http://dx.doi.org/10.17582/journal.pjz/2017.49.5.1665.1669
* Corresponding author: [email protected]
0030-9923/2017/0005-1665 $ 9.00/0
Copyright 2017 Zoological Society of Pakistan
Introduction
Herbs and spices have been used since ancient times as natural remedies for the treatment of a variety of disorders. Naturally occurring drugs, antimicrobial agents and natural food preservatives have gained increased attention nowadays. Both scientists as well as consumers are apprehensive to trail increased resistance of the antibiotics against pathogens along with teratogenic and carcinogenic after effects of food additives (Fischbach, 2009).
In the work reported here, two well-known spices, turmeric and black pepper have been used for the assessment of their biological activities against a wide range of prokaryotic and eukaryotic species. The plant extracts were prepared by using different solvents to identify the best solvent with desired antimicrobial activity. The active constituents of turmeric are curcumin and lectin; curcumin initiates apoptosis of cancerous cells without affecting the normal cells, while lectin has been reported to have antibacterial, antifungal and α-glucosidase inhibitory activity (Petnual et al., 2010).
Lectins are proteins, with no immunogenic origin, that interact with glycoproteins, glycolipids and other polysaccharides resulting in the formation of stable glycol-conjugates on cell surface and in solutions. These specific characteristics affirm lectin as a defensive constituent of plants against insects, microorganisms and mammalian predators (George et al., 2011). Lectins have also been extensively used as a probe for the isolation of different sugars types, thus aiding in immunological studies (Sharon and Lis, 2002).
Black pepper and its components exhibit antioxidant and free radical scavenging properties. Piperine, the active constituent of black pepper, has been shown to prevent the formation of reactive oxygen species and to reduce the resulting oxidative damage, suggesting it may be useful for anti-cancer treatment (Srinivasan, 2007; Ahmad et al., 2016). Black pepper also exhibited immunomodulatory and anti-inflammatory effects. Its phytochemicals have gained much attention in recent years because of their obvious effect against p-glycoproteins and as a detoxifying agent (Meghwal and Goswami, 2013).
The present study was aimed to evaluate the antibacterial, antifungal, antidiarrheal and anticancer activities of turmeric (Piper nigrum L.) and black pepper (Curcuma longa L.) extracts.
Materials and methods
Sample collection and extract preparation
Black pepper seeds (Piper nigrum L.) and turmeric rhizome (Curcuma longa L.) were purchased from local market of Lahore and Hafizabad. Species were identified by a taxonomist from the Department of Botany, University of the Punjab, Lahore, Pakistan. Ethanol, methanol and aqueous extracts were prepared by dissolving 25 g of turmeric and black pepper powder in 100 ml of ethanol, methanol and water (25 % w/v) in individual flasks. The mixture was kept at room temperature for seven days. On the eighth day it was filtered through sterilized filter paper (Whatman No. 1) and evaporated in water bath until 5 ml extract was left in each flask. Extracts were stored at 4 oC.
Antibacterial activity assay
The antibacterial assay of turmeric and black pepper was accessed by agar well diffusion method against Escherichia coli, Bacillus subtilis, Corynebacterium, Staphylococcus aureus and Pseudomonas aeruginosa. Luria broth (LB) agar (0.5 % yeast extract, 1 % tryptone and 1 % sodium chloride and 2% agar) was prepared, autoclaved and poured in sterilized petri plates. After solidification, 100 μl of each bacterial culture was spread on the surface of individual plates. Wells of 5 mm diameter were made using a sterilized borer and 100 μl of each of the prepared extracts (1 mg/ml diluted in water) was poured into it. Water, ethanol and methanol were used as negative control and 30 μl ampicillin (100 ppm) was used as a positive control. The plates were incubated at 37 oC for 18 h and the diameters of the zone of inhibition were measured (mm).
Antifungal activity assay
Antifungal activity of turmeric and black pepper extract was evaluated against Candida albicans, Aspergillus flavus, Microsporum canis, Fusarium solani and Candida glabrata by agar tube dilution method (Therese et al., 2006). The Sabouraud Dextrose Agar (SDA) was prepared in screw cap tubes and autoclaved. 100 µl of each extract (1 mg/ml diluted in water) was added to individual tubes. The tubes were left in slanting position till solidification. Fungal inoculums were cut into pieces of 4 mm diameter by sterilized blade and placed in each tube accordingly over the surface of media. Miconazole 1mg/ml was used as positive control. Tubes were incubated at 27 oC for 7 days. The growth inhibition was calculated by the following formula:
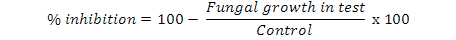
Antidiarrheal activity
The study of antidiarrheal effect was carried out by using white albino mice, with body weight of 34 to 37 grams. The diarrhea was induced by giving them castor oil. The antidiarrheal effect of black pepper (Piper nigrum L.) was assessed by oral administration of ethanol or methanol extracts. The body weight of mice was measured at regular intervals for normalization of weight loss.
Anticancer assay using prostate cancer cells (PC3)
The human PC3 cell line was used in this study. The cells were grown and maintained in a CO2 (5 %) humidified incubator at 37°C. RPMI-1640 medium supplemented with fetal bovine serum (FBS 10 %), and penicillin and streptomycin, (100 μg/ml each) was used for cell cultures. Approximately 10000 cells (from log phase cultures) were plated in 100 μl of RPMI-164 medium (supplemented with 10 % FBS) per well in 96 well culture plates (Sigma culture wear). The proliferative response of the different types of extracts (Ethanol, Methanol and Aqueous extracts of Curcuma longa L. and Piper nigrum L.) were determined by MTT assay (Stockert et al., 2012).
MTT colorimetric assay
A colorimetric MTT assay using 3-(4,5- dimethylthiazoyl)-2, 5-diphenyltetrazolium bromide (MTT) was carried out by using PC3 cells. Cells were plated in micro culture plates both in the presence and absence of the different types of extracts and incubated in a humidified CO2 incubator at 37°C. After 24 or 48h of incubation, 10 μl of MTT (5 mg/ml concentration), was added to each well. The plates were again incubated at 37°C for 4 h. 100 μl solubilization solution (containing DMSO and Sorenson buffer) was added in each well. The plates were read on an ELISA reader at a wavelength of 570 nm. The optical density (OD) was noted and average for each group of replicates and the standard deviation was calculated. The cell growth inhibition was calculated using the formula (Stockert et al., 2012):
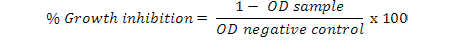
Results and discussion
Antibacterial activity
The antibacterial activities of turmeric (Curcuma longa L.) and black pepper (Piper nigrum L.) extract were tested against B. subtilis, C. bacterium, E. coli, P. aeruginosa and S. aureus by using agar well diffusion method. The penetration of the active contents of the extracts and lectin in nutrient agar was compared to ampicillin, which used as positive control because of the similarity of β- lactam ring present in ampicillin and ring structures of amino acids.
The ethanol extract of turmeric displayed the highest zone of inhibition (11 mm) against B. subtilis followed by E. coli and S. aureus that were 10 mm and 9 mm, respectively. The methanol extract of turmeric displayed maximum activity against B. subtilis (9 mm) followed by 8 mm for E. coli and S. aureus (Fig. 1). It was concluded that B. subtilis is significantly more sensitive against both ethanol and methanol extracts of turmeric while P. aeruginosa is least sensitive.
The B. subtilis and E. coli were more sensitive to the ethanol extract of black pepper followed by S. aureus, C. bacterium and P. aeruginosa. The methanol extract of black pepper displayed highest zone of inhibition against B. subtilis, E. coli and S. aureus (8 mm) followed by (7 mm) E. coli and P. aeruginosa.
The results are in accordance with previous studies as ethanol extract of turmeric has been previously reported to have antibacterial activity against different strains (Naz et al., 2010; Mukhtar and Ghori, 2012; Darout et al., 2000).
Antifungal activity
Antifungal activity assay of methanol and ethanol extracts of turmeric and black pepper against C. glabrata, C. albicans, A. flavus, M. canis and F. solani was carried out by using agar tube dilution method.
M. canis and A. flavus was inhibited by all extracts to some extent (Fig. 2). The methanol and ethanol extracts of turmeric showed 25% and 35% inhibition against A. flavus while methanol and ethanol extract of black pepper exhibited 30% and 35% inhibition, respectively. No activity was observed against F.solani and Candida species.
It has been previously reported that alcohol extracts of turmeric have antifungal activity against different fungal strains (Petnual et al., 2010). In contrast, the extract of black pepper was effective against F. oxysporum and A. niger (Rani et al., 2013). Its volatile oil has 100% inhibitory efficiency against different fungal strains (Singh et al., 2004) and in comparison to ordinary available antibiotics, the ethanol extract showed inhibitory activity of 25mm, 21mm and 16mm in the presence of S. aureus, B. cereus, and C. albicans, respectively (Sharon and Lis, 2001).
Antidiarrheal effect
Antidiarrheal effect of crude ethanol and methanol extracts of black pepper was evaluated by measuring the body weight of mice before and after administration. The body weight of positive control increased 0.5 g after 24 h, as 1 mg of the standard drug against diarrhea lopium (Loperamide, 30mg/kg) was injected as positive control, which protected the mice against the diarrheal effect caused by castor oil and mice remained healthy. Saline solution was used for the negative control mice, which had a body weight reduction by 0.7 while for test mice, 0.4 g weight was reduced in methanol crude extract and 0.3 g was reduced in ethanol crude extract. These results indicate a smaller weight loss in case of test mice as compared to negative controls.
The aqueous extract of black pepper was tested for its anti-diarrheal affect by inducing food born diarrhea and was found to have good anti- diarrheal effect that is directly related to anti-motility and anti-secretary activities in mice (Shamkuwar et al., 2012). Experiments using black pepper extract suggest that the anti-secretory, anti-motility and anti- diarrheal activities possessed by crude extract might be due to the presence of carbohydrates and alkaloids. Its active content piperine has been previously reported to prevent diarrhea induced by various diets (e.g., oil and chemicals) and reduce intestinal fluid secretion in mice (Reshmi et al., 2010).
Inhibition of proliferation of PC3 cells
The effect of different alcoholic extracts on the proliferation of PC3 cells was studied and it was observed that ethanol extracts caused maximum inhibition. Turmeric showed 60 % inhibition followed by black pepper i.e., 47.8 % (Fig. 3). The methanol extracts of turmeric and black pepper
These results correlate with the study of Unnikrisshnan and Kuttanin (Unnikrishnan and Kuttan, 1990) where they demonstrated that alcoholic crude extracts of spices have more cytotoxic and anti-inflammatory activities than aqueous mixtures. The reason behind this mechanism might be the solubility or stability of biological active content in ethanol as compared to methanol or distilled water.
Liu et al., (2010) reported that piperine obtained from Piper nigrum suppressed TNF-induced NF-kappa B activation thus inhibiting the progression of cancerous cells. The results of this study was correlated with the study of Roy et al. (2002), where they found ethanol crude extract of Piper nigrum as a good anticancer agent that inhibit tumor cell proliferation (e.g., Ehrlich ascites tumor and Doltons lymphoma cells) and can also minimize different mutations (e.g., ethyl-carbamte induced mutation) in Drosophila (El Hamss et al., 2003; Sunila and Kuttan, 2004; Hirata et al., 2007; Damanhouri and Ahmad, 2014).
Conclusion
This work attempts to signify the importance of Curcuma longa L. and Piper nigrum L. in pharmacology and therapeutics. We have demonstrated that spice-derived constituents have significant antibacterial, antifungal and antidiarrheal control and may be useful for the treatment of prostate cancer. These findings can serve as a revolutionary breakthrough in molecular biology after purification of active phytochemicals of dietary ethnomedicinal spices. They could be made available as cheap herbal drugs affordable to the poor people of rural areas, holding great promise in the efforts to control hazardous maladies.
Acknowledgements
The authors are thankful to the H.E.J., Research Institute of Chemistry, University of Karachi, Karachi, Pakistan, and Institute of Biochemistry and Biotechnology, University of the Punjab, Lahore, Pakistan, for providing the research facilities to carry out this study. Permission from the ethics committee of the Institute of Biochemistry and Biotechnology was taken and all efforts were employed to minimize pain and discomfort to the animal model while conducting these experiments.
Statement of conflict of interest
All authors declare that there is no conflict of interests regarding the publication of this article. Otherwise, we should mention any conflict of interest in this section of the manuscript.
References
Ahmad, N., Abbasi, B.H. and Fazal, H., 2016. Effect of different in vitro culture extracts of black pepper (Piper nigrum L.) on toxic metabolites-producing strains. Toxicol. Ind. Hlth., 32: 500-506. https://doi.org/10.1177/0748233713505126
Damanhouri, Z.A. and Ahmad, A., 2014. A Review on therapeutic potential of Piper nigrum L. (Black Pepper): The king of spices. Med. Aromat. Pl., 3: 1-6. https://doi.org/10.4172/2167-0412.1000161
Darout, I.A., Christy, A.A., Skaug, N.I.L.S. and Egeberg, P.K., 2000. Identification and quantification of some potentially antimicrobial anionic components in miswak extract. Indian J. Pharmacol., 32: 11-14.
El Hamss, R., Idaomar, M., Alonso-Moraga, A. and Serrano, A.M., 2003. Antimutagenic properties of bell and black peppers. Fd. Chem. Toxicol., 41: 41-47. https://doi.org/10.1016/S0278-6915(02)00216-8
Fischbach, M.A., 2009. Antibiotics from microbes: converging to kill. Curr. Opin. Microbiol., 12: 520-527. https://doi.org/10.1016/j.mib.2009.07.002
George, O., Solscheid, C., Bertolo, E. and Lisgarten, D., 2011. Extraction and purification of the lectin found in the tubers of Eranthis hyemalis (winter aconite). J. Integr. OMICS, 1: 268-272.
Hirata, N., Tokunaga, M., Naruto, S., Iinuma, M. and Matsuda, H., 2007. Testosterone 5α-reductase inhibitory active constituents of Piper nigrum leaf. Biol. Pharm. Bull., 30: 2402-2405. https://doi.org/10.1248/bpb.30.2402
Liu, Y., Yadev, V.R., Aggarwal, B.B. and Nair, M.G., 2010. Inhibitory effects of black pepper (Piper nigrum) extracts and compounds on human tumor cell proliferation, cyclooxygenase enzymes, lipid peroxidation and nuclear transcription factor-kappa-B. Nat. Prod. Commun., 5: 1253-1257.
Meghwal, M. and Goswami, T.K., 2013. Piper nigrum and piperine: an update. Phytother. Res., 27: 1121-1130. https://doi.org/10.1002/ptr.4972
Mukhtar, S. and Ghori, I., 2012. Antibacterial activity of aqueous and ethanolic extracts of garlic, cinnamon and turmeric against Escherichia coli ATCC 25922 and Bacillus subtillus 3256. Int. J. appl. Biol. Pharmacol. Tech., 3: 131-136.
Naz, S., Jabeen, S., Ilyas, S., Manzoor, F., Aslam, F. and Ali, A., 2010. Antibacterial activity of Curcuma longa varieties against different strains of bacteria. Pak. J. Bot., 42: 455-62.
Petnual, P., Sangvanich, P. and Karnchanatat, A.A., 2010. Lectin from the rhizomes of turmeric (Curcuma longa L.) and its antifungal, antibacterial, and α-glucosidase inhibitory activities. Fd. Sci. Biotechnol., 19: 907-916. https://doi.org/10.1007/s10068-010-0128-5
Rani, S.S.K., Saxena, N. and Udaysree, N., 2013. Antimicrobial activity of black pepper (Piper nigrum L.). Global J. Pharmacol., 7: 87-90.
Reshmi, S.K., Sathya, E. and Devi, P.S., 2010. Isolation of piperdine from Piper nigrum and its antiproliferative activity. J. med. Pl. Res., 4: 1535-1546.
Roy, M., Chakraborty, S., Siddiqi, M. and Bhattacharya, R.K., 2002. Induction of apoptosis in tumor cells by natural phenolic compounds. Asian Pac. J. Cancer Prev., 3: 61-67.
Shamkuwar, P.B., Shahi, S.R. and Jadhav, S.T., 2012. Evaluation of antidiarrhoeal effect of Black pepper (Piper nigrum L.). Asian J. Pl. Sci. Res., 2: 48-53.
Sharon, N. and Lis, H., 2001. How proteins bind carbohydrates: lessons from legume lectins. J. Agric. Fd. Chem., 50: 6586-6591. https://doi.org/10.1021/jf020190s
Singh, G., Marimuthu, P., Catalan, C. and Delampasona, M.P., 2004. Chemical, antioxidant and antifungal activities of volatile oil of black pepper and its acetone extract. J. Sci. Fd. Agric., 84: 1878-1884. https://doi.org/10.1002/jsfa.1863
Srinivasan, K., 2007. Black pepper and its pungent principle-piperine: a review of diverse physiological effects. Crit. Rev. Fd. Sci. Nutr., 47: 735-748. https://doi.org/10.1080/10408390601062054
Stockert, J.C., Blázquez-Castro, A., Cañete, M., Horobin, R.W. and Villanueva, Á., 2012. MTT assay for cell viability: Intracellular localization of the formazan product is in lipid droplets. Acta Histochem., 114: 785-796. https://doi.org/10.1016/j.acthis.2012.01.006
Sunila, E.S. and Kuttan, G., 2004. Immunomodulatory and antitumor activity of Piper longum Linn. and piperine. J. Ethnopharmacol., 90: 339-346. https://doi.org/10.1016/j.jep.2003.10.016
Therese, K.L., Bagyalakshmi, R., Madhavan, H.N. and Deepa, P., 2006. In-vitro susceptibility testing by agar dilution method to determine the minimum inhibitory concentrations of amphotericin B, fluconazole and ketoconazole against ocular fungal isolates. Indian J. med. Microbiol., 24: 273-279. https://doi.org/10.4103/0255-0857.29386
Unnikrishnan, M.C. and Kuttan, R., 1990. Tumour reducing and anticarcinogenic activity of selected spices. Cancer Lett., 51: 85-89. https://doi.org/10.1016/0304-3835(90)90235-P
To share on other social networks, click on any share button. What are these?










